Abstract
Significance: In early gestation, fetal skin wounds undergo regeneration and healing without a scar. This phenomenon is intrinsic to early fetal skin but disappears during late gestation. Adult wounds undergo repair via a fibroproliferative response that leads to incomplete regeneration of the original tissue and a resultant scar. This outcome can have devastating effects for patients and is a significant financial burden to the healthcare system.
Recent Advances: Studies have demonstrated the possible role of several stem cells in wound healing. In particular, epidermal stem cells and mesenchymal stem cells have been implicated in wound repair and regeneration. Recently, stem cells with adult epidermal stem cell markers have been found in fetal skin dermis. These cells are thought to play a role in scarless fetal wound healing.
Critical Issues: Despite numerous studies on scarless fetal wound healing, the exact mechanism is still largely unknown. Although inflammation is greatly reduced, the stem cell profile of regenerating fetal skin wounds remains unknown. Without a detailed understanding of stem cell differences between fetal and adult wounds, the ability to prevent or treat both normal and pathologic excessive scarring, in the form of keloids and hypertrophic scars, is limited.
Future Directions: Further studies on differences between fetal and adult skin-specific stem cells may elucidate the mechanism of scarless wound healing in the early fetus. With this knowledge, the potential to reduce scarring in adult wounds may be achieved.

H. Peter Lorenz, MD
Scope and Significance
Fetal skin wounds heal without scars in a process closely resembling regeneration. Despite intensive studies, the precise mechanism of scarless fetal wound healing is still unknown. Numerous stem cells have been implicated in postnatal wound healing and, in particular, epidermal stem cells and mesenchymal stem cells (MSCs) have been studied in the wound and as potential cell-based regenerative therapies. Recently, a new stem cell has been identified in the fetal dermis and blood as a possible player in scarless wound healing. Here, we discuss the current research on stem cells as related to the fetal wound.
Translational Relevance
Ongoing research aims at differentiating fetal scarless and adult scarring wound-healing mechanisms. Although recent advances have identified key differences in the composition of fetal and adult wounds, the mechanism that results in regeneration versus repair is not yet fully understood. By understanding the role of stem cells in fetal wounds, the process of scarless wound healing may be deciphered. The ultimate goal of this research is the development of cell-based therapies for tissue regeneration applications.
Clinical Relevance
The clinical burden of scarring can be devastating. Scars can restrict growth in the pediatric population, impair movement across joints, and result in disturbing psychological and social consequences in locations such as the face. Humans can also develop pathological scars, such as keloids and hypertrophic scars, which arise when wound healing proceeds rampantly in excess. The result is an enlarged and symptomatic scar that is raised, cosmetically unpleasing, and associated with pain and/or itching.1
Discussion of Findings and Relevant Literature
Fetal wound healing
Wound healing requires the complex interplay of numerous biological pathways and cell types, and the result of this process depends on the developmental age of the organism. Interestingly, wounds occurring in early to mid-gestational fetal skin have been shown to heal via regeneration without the formation of a scar.2 These wounds heal rapidly and are characterized by a complete regeneration of dermal and epithelial tissue, including an identical collagen pattern as uninjured tissue, as well as the complete regeneration of epidermal appendages (Figs. 1 and 2).3 This phenomenon has been demonstrated across many mammalian species, as well as in an ex vivo fetal skin model.4–7 Conversely, adult wounds heal by a fibroproliferative response that emphasizes repair over regeneration. This results in the formation of scar tissue, which is composed largely of an unorganized dense collagen meshwork. Scar tissue is also characterized by the loss of epidermal appendages (hair follicles and sebaceous glands) and a flattened epidermis without rete ridges (Figs. 3 and 4).3 Scar tissue is fundamentally different from uninjured skin and has a tensile strength that is less than 80% of its original form.8,9
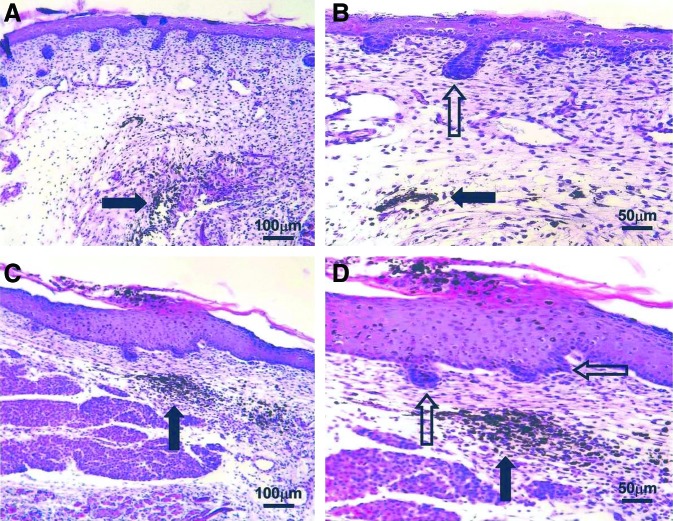
Figure 1.
Hematoxylin and eosin staining of scarless E16.5 fetal wounds. Black arrows indicate India ink tattoo made at the time of wounding to demonstrate scarless wound location. Healed wounds (A, C) at 72 h (100×). The epidermal appendage (developing hair follicles) pattern shows numerous appendages directly in the healed wound. Magnified views of the same wounds (B, D) showing epidermal appendages (open arrows) within the wound site (200×). No inflammatory infiltrate is present. Reprinted with permission from Beanes et al.3 E16.5 is embryonic day 16.5, with day 0.5 defined as the morning after nocturnal conception. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 2.
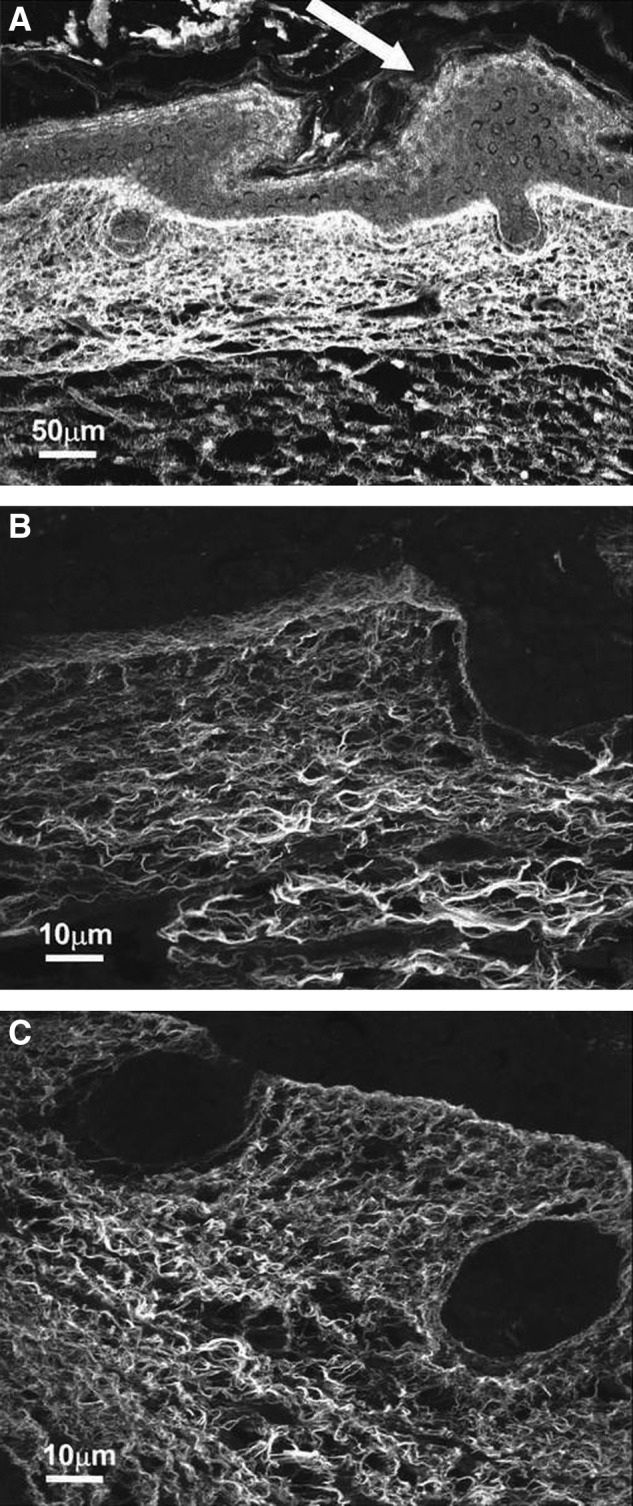
Confocal microscopy of scarless E16.5 fetal wounds. Collagen fibers are stained with Sirius red and appear white. (A) Healed wound skin harvested at 72 h (200×). The epidermis is thickened at the wound site (white arrow). The collagen fiber is reticular and unchanged from the surrounding dermis. (B) Healed wound skin harvested at 72 h under a higher magnification (1,000×). The collagen fibers are thin and closely approximating each other with little interfiber space. The fibers are arranged in a wispy reticular pattern. (C) Nonwounded E19.5 fetal skin is shown at the same magnification as in the (B) (1,000×). The dermal collagen fiber pattern is identical to the center image. Reprinted with permission from Beanes et al.3
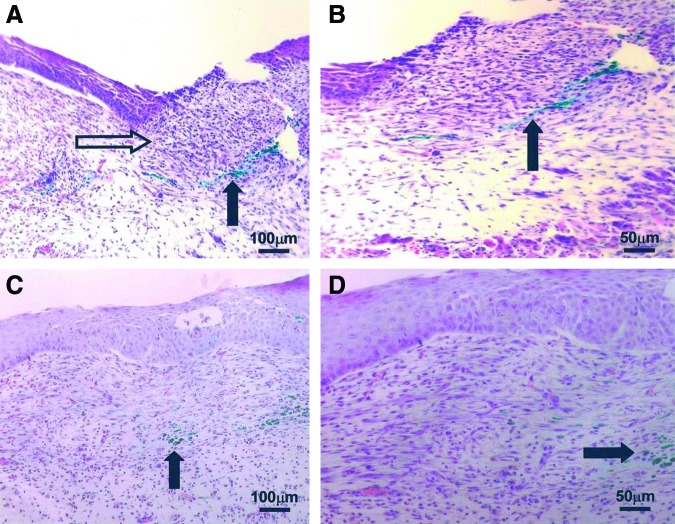
Figure 3.
Hematoxylin and eosin staining of scarring E18.5 fetal wounds. Black arrows indicate green vital dye tattoo made at the time of wounding. (A) Wound at 24 h (100×) remains open and an inflammatory cell infiltrate is present (open arrow). (B) Magnified view (200×) of the same incompletely healed wound at 24 h. (C) Wound at 72 h (100×) has no epidermal appendages, consistent with adult-type repair and scar formation. (D) Magnified view (200×) of the wound shown in the (C) demonstrating an inflammatory cell infiltrate and lack of dermal appendages. Reprinted with permission from Beanes et al.3 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 4.
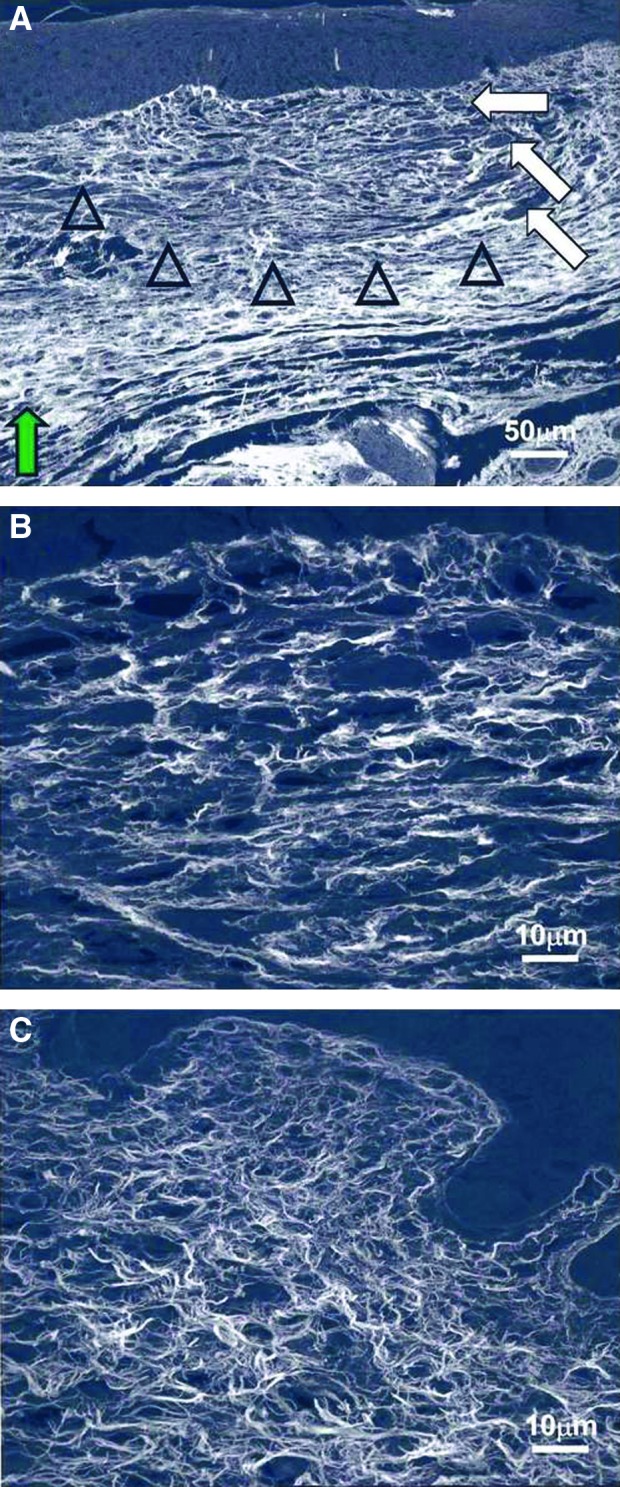
Confocal microscopy of scarring E18.5 fetal wounds. Collagen fibers are stained with Sirius red and appear white. (A) Healed wound skin harvested at 72 h (200×). The dermal collagen pattern (open arrowheads) is different from the surrounding nonwounded dermis (green arrow). The fibers are less densely compacted, and no epidermal appendages are present. Neovascularization is shown with the white arrows. (B) Healed wound at 72 h at a higher magnification (1,000×). The collagen fibers are thicker but with greater interfiber spaces compared with nonwounded dermis. (C) Nonwounded skin at 21.5 days of gestational age (1,000×). When compared with wound collagen fibers (B), nonwounded dermal collagen fibers are thinner, with less interfiber space. Reprinted with permission from Beanes et al.3 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The translational implications of scarless wound healing have generated interest in the underlying mechanism of this response, and thus far, both human and animal models of fetal wounding have been developed in which complete regeneration of dermal and epidermal tissues is observed.4–6 Through this work, gestational age has been found to play a critical role in the transition from scarless wound healing to the postnatal scarring phenotype in the developing fetus.10 Not surprisingly, the timing of this transition is species specific, occurring in humans at least after 24 weeks of gestation, whereas in mice and rats, scarring begins on embryonic day 18.5 (E18.5) and 17.5 (E17.5), respectively (with day 0.5 defined as the morning after nocturnal conception).11,12 Gestational age is not the sole determinant of the healing phenotype. The regeneration-scar transition has also been shown to be influenced by wound size, with larger wounds requiring repair at an earlier gestational age to achieve scarless healing.10 Another critical question related to the scarless phenotype is the relative contributions of the fetal skin and intrauterine environment to this process. Although scarless wound healing was once attributed to the tissue's environmental conditions, studies have ever since demonstrated that the intrauterine environment is neither necessary nor sufficient for the scarless phenotype.13
Further illustrating the complexity of this process, research has also demonstrated that fetal wounds differ from adult wounds in inflammatory responses, extracellular matrix (ECM) components, growth factor expression and responses, and profiles of gene expression.14 Despite this tremendous increase in knowledge gained over the past decades, the precise mechanisms regulating scarless fetal wound healing remain unknown. Nevertheless, recent research on fetal skin stem cells as they pertain to the fetal wound is promising to elucidate scarless wound healing mechanisms and provide cell-based regenerative therapeutics in the future.
Epidermal stem cells
Mammalian skin is a complex structure that consists of the epidermis, dermis, and the epidermal appendages, with the epidermis and dermis separated by a basement membrane. The epidermis is composed of several cell layers, of which the basal layer lies on the basement membrane. The basal keratinocyte layer stratifies and differentiates to give rise to the spinous layer, granular layer, and the acellular stratum corneum.
Epidermal development
The epidermis is derived from the ectoderm during embryonic development. In mice, a single epidermal layer is present from E9.5 to E12.5, before the arrival of mesenchymal cells that populate the dermis and instruct the formation of skin appendages.15 Stratification of the epidermis occurs between E12.5 and E15.5, a process that is usually completed by E17.5, when the epidermis consists of an inner layer of basal cells with proliferative potential and layers of terminally differentiating suprabasal cells.15 The epidermal appendages extend into the dermis.
Growth and homeostasis
In postnatal epidermis, homeostasis is maintained by epidermal stem cells that replace keratinocytes which are lost through normal differentiation, and turnover or through cell death after injury. Current evidence supports the existence of multiple stem cells with restricted lineage in cutaneous epithelium.16 These cells are located in distinct niches: in the basal layer of the epidermis adjacent to the basement membrane, in the bulge region of the hair follicle, at the base of the sebaceous gland, and in the upper isthmus (a region between the bulge and the sebaceous gland).17–19
The current paradigm that explains the growth and homeostasis of the epidermis divides the interfollicular epidermis into discrete proliferative units known as epidermal proliferating units (EPU).20 This model proposes that there is one self-renewing basal stem cell per EPU and that the other basal cells are transiently amplifying (TA) cells.19 True epidermal stem cells are hypothesized to persist for the life of the organism, whereas TA cells have a more limited lifespan and divide several times before exiting the basal layer and terminally differentiating.
Stem cells of the epidermis
A population of multipotent stem cells that are capable of generating all of the components of the epidermis are the hair follicle stem cells. They reside in a specialized microenvironment known as the bulge, which forms a part of the outer root sheath.21,22 Under normal homeostatic conditions, these cells do not contribute to the maintenance of the interfollicular epidermis, but can transform into epidermal stem cells as a consequence of trauma.23 With injury, the bulge cells can rapidly migrate upward and repair the epidermis in vivo.23
Despite the significant increase in our understanding of postnatal epidermal stem cells and their niche microenvironments, our knowledge of epidermal stem cells in fetal skin, and their function during scarless fetal wound healing remains largely unknown. Studies have shown that postnatal epidermal stem cells from aged mice show similar plasticity compared with neonatal epidermal stem cells.24 Given the significant structural differences between fetal and postnatal skin, with fetal skin lacking mature hair follicles and bulges, it is likely that the origin and location of stem cells in fetal skin are different from those in adult skin.25 Nevertheless, recent work in this field indicates that fetal skin stem cell function may still be one of the key mechanisms underlying scarless wound healing.
Epidermal stem cell division
Another key difference between fetal and postnatal skin that may impact scarless wound healing is the change in the pattern of stem cell division and the orientation of the mitotic spindle during skin development. During the early stages of embryonic development, cell divisions are mostly symmetric and parallel to the basement membrane, which allows for growth and maintenance of the surface of the developing embryo as a single layer.15 During stratification, the stem cells need to form the suprabasal layers, and therefore begin to undergo asymmetric division. This results in the proportion of asymmetric cell divisions rising from 0% at E12.5, to ∼70% between E14.5 and E17.5 in the fetal epidermis.15,26 Interestingly, while this timing correlates with the scarless to scarring transition, further work is needed to determine a correlative versus causative relationship.
Stem cells of the dermis
Most of the research examining skin stem cells and their role in healing has focused on epidermal stem cells. Although much information exists on the role of the ECM in the dermis during wound healing, very little information is known about dermal-derived stem cells. One population of mesenchymal cells in the skin, known as dermal papilla (DP) cells, is the focus of intense interest because the DP not only regulates hair follicle development and growth, but is also thought to be a reservoir of multipotent stem cells.27 A population of cells known as skin-derived progenitor (SKPs) cells has recently been shown to have the potential to differentiate in vitro into neurons, glia, smooth muscle cells, adipocytes, and other cell types. The SKP cells originate from the DP.28,29 The SKP cells also display functional properties predicted of a dermal stem cell, such as contributing to dermal matrix maintenance, wound healing, hair follicle morphogenesis,28 and have even been isolated during embryogenesis.29 Nevertheless, whether SKP cells have a function during fetal wound healing is unknown and requires further study.
Mesenchymal stem cells
More traditionally defined MSCs are hypothesized to contribute to fetal wound healing,30,31 although this remains speculative. MSCs are subsets of multipotent stromal cells that can be isolated from various adult organs and tissues, including bone marrow, lung, brain, liver, and skeletal muscle.32 These cells are thought to be perivascular in nature,33 and are defined, in part, by their capacity for plastic adherence under standard culture conditions as well as their in vitro differentiation capacity to form osteoblasts, adipocytes, and chondroblasts.34
Bone marrow mesenchymal stem cells
Of these adult-derived cells, bone marrow MSCs (BM-MSCs), in particular, have been extensively studied, and are thought to both mobilize and home to sites of injury, due in large part to interactions of the cytokine stromal cell-derived factor 1 (SDF-1) with its cellular receptor, chemokine (C-X-C motif) receptor 4, or CXCR-4.35 Once at the injury site, these cells contribute to tissue repair and regeneration mainly through paracrine effects (release of growth factors such as hepatocyte growth factor, epidermal growth factor, and vascular endothelial growth factor) and modulation of the immune response (such as antagonism of the pro-inflammatory cytokine interleukin 1 [IL-1] and production of the anti-inflammatory cytokine interleukin 10 [IL-10]). However, engraftment and direct cellular differentiation may also occur.35 Evidence that BM-MSCs participate in postnatal skin development and homeostasis exists.36 Given this regenerative profile, efficacy of adult-derived MSCs when applied to a variety of preclinical injury models, including cutaneous wounds, has been shown. However, a more rigorous evaluation of these cells in the clinical setting is needed.35,36
Mesenchymal stem cells and the fetal environment
MSCs have been isolated from various fetal extra-embryonic tissues and fluids, including the placenta, umbilical cord, amniotic membrane, amniotic fluid, and umbilical cord blood,37 as well as from more traditional sources such as the fetal bone marrow.38 In fact, these cells possess multiple theoretical advantages over adult-derived MSCs, including a closer ontogenic relationship to embryonic stem cells, which results in a greater plasticity and lower immunogenicity.37,39,40 Another advantage is their potential for increased clinical availability, as extra-embryonic sources in particular are abundant and typically discarded after birth. In addition, both fetal and extra-embryonic MSCs have been shown to possess a similar, or even enhanced, capacity to support neovascularization and tissue regeneration in vivo as compared with adult-derived MSCs.39,40–43
Despite this regenerative potential, whether MSCs, from any source, actively participate in the scarless fetal wound healing process remains unclear due to limited studies. However, circumstantial evidence supports this possibility, as xenografted human BM-MSCs have not only shown the ability to survive long term within fetal sheep wounds, but also undergo site specific differentiation into multiple cell types, including chondrocytes, epithelial cells, and cardiac and skeletal muscle.31 Nevertheless, the use of adult-derived BM-MSCs in this work does not accurately reflect the MSC phenotype likely to be involved in fetal regeneration.
Amniotic fluid mesenchymal stem cells
A more representative evaluation of the role of MSCs in fetal wound healing requires the study of cells that are actually present in the fetal environment, such as the recently completed work using autologous transplantation of labeled amniotic fluid-derived MSCs (aMSCs).30 In this two-part study, lamb fetuses were first given incisional and open resection wounds in parallel with an autologous aMSC transfer, with the wounds harvested 7–14 days later for characterization of cell recruitment and differentiation. Extending on the previously described findings with human BM-MSCs, the labeled aMSCs in this study displayed the ability to home to wounds, and to heal by both primary and secondary intention. Furthermore, these cells displayed a capacity for in situ differentiation, forming cells that were indistinguishable from surrounding chondrocytes.
In the second phase of this work, researchers attempted to isolate the relative contribution of aMSCs to fetal healing by using a similar cell transfer strategy on fetuses receiving a set of size-matched skin wounds that were either left open or covered with a cell-impermeable membrane, and analyzing wounds for closure rates and ECM composition.30 Wounds that were not accessible to aMSCs displayed significantly slower rates of healing with concurrently decreased elastin levels, suggesting a beneficial contribution of these native cells to the fetal wound. Nevertheless, all wounds eventually closed, with no significant differences observed in the levels of other ECM components characteristic of fetal healing,44 suggesting that while extra-embryonic MSCs may enhance fetal healing, they are not absolutely necessary for its occurrence.
While limited, this body of work on MSCs is in accordance with previous studies demonstrating that components intrinsic to the fetal skin are most important for the scarless healing phenotype.44 Though the presence of an MSC population in the fetal skin or elsewhere that is a crucial contributor to the scarless phenotype is possible, the more likely mechanism is that MSCs from multiple sources contribute to scarless fetal wound healing, but none may be critical for its occurrence. Nonetheless, these cells remain a promising therapeutic source for cell-based regenerative applications.
Fetal skin stem cells
A new fetal skin stem cell was recently identified in fetal and adult mice, as well as in adult humans, that has been implicated in fetal scarless wound healing. The cell surface expression profile of these cells closely resembles that of stem cells, and although they are found circulating in the blood, they were identified in fetal dermis and epidermis. During embryonic development, they are distributed in many tissues and likely contribute to differentiation.45
These fetal skin dermal cells were discovered as a group of cells that heavily express E-cadherin. They have been identified in the dermis of E14.5 to E18.5 fetal mice, the bone marrow of adult mice, and the blood of E16.5, 4-week-old, and 34-week-old mice, as well as adult human blood (Fig. 5). The ratio of these cells in E16.5 fetal mouse blood is more than 20 times greater than that of adult mouse blood.45 The increased number of these circulating cells in scarless healing animals supports their possible function in fetal skin regeneration.
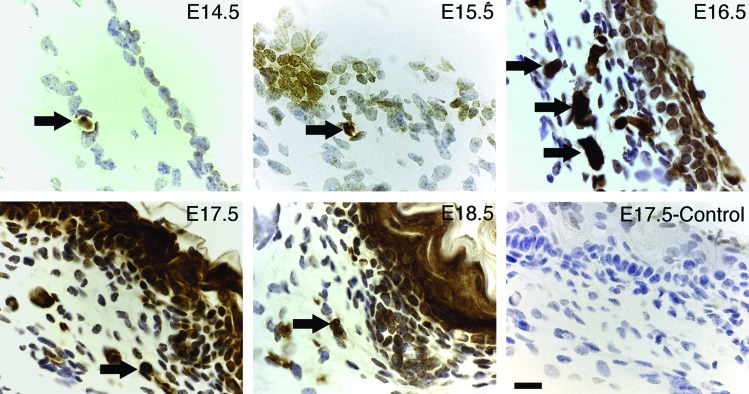
Figure 5.
Localization of E-cadherin-positive fetal cells. Dorsal skin was collected from E14.5 to E18.5 fetal mice, and the expression of E-cadherin was analyzed by immunohistochemistry. The control section was stained without a primary antibody. Arrows indicate E-cadherin-positive cells, with the highest number of dermal E-cadherin cells occurring in E16.5. By E18.5, E-cadherin was expressed strongly in epithelial cells. Scale bar=20 μm. Reprinted with permission from Kong et al.45 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Cell surface expression
Fetal skin stem cells demonstrate rapid proliferation in vitro. Although cell surface expression profiles have been shown to change during culture, freshly isolated cells possess a cell surface expression profile similar to epidermal stem cells. They express E-cadherin, integrin-β1, CD184, CD34, CD13low, and Sca-1low, but not CD45, CD44, and CD117. In addition, these cells express embryonic stem cell transcription markers Oct-4, Nanog, and Sox-2, and the germ plasm-specific marker VASA.45,46 These data suggest that fetal skin stem cells may play a crucial role in embryonic and epidermal development.
Postnatal wound healing
The effect of fetal skin stem cells on postnatal wound repair was examined.45 In this work, wounds were generated on adult mice and fetal cells were injected through the tail vein. Significantly less scar and faster healing was observed in mice treated with fetal cells versus control (Fig. 6). The treated wounds also expressed less type-I collagen and smooth muscle actin in the interstitium. When green fluorescent protein (GFP)-labeled cells were injected into wounded adult mice, the fetal cells showed the ability to migrate to the wound, differentiate into dermal cells, and express fibroblast growth factor-2, an antagonist to fibrosis. These experiments demonstrate the strong affinity of fetal skin cells to injured tissue and the ability to reduce scar.
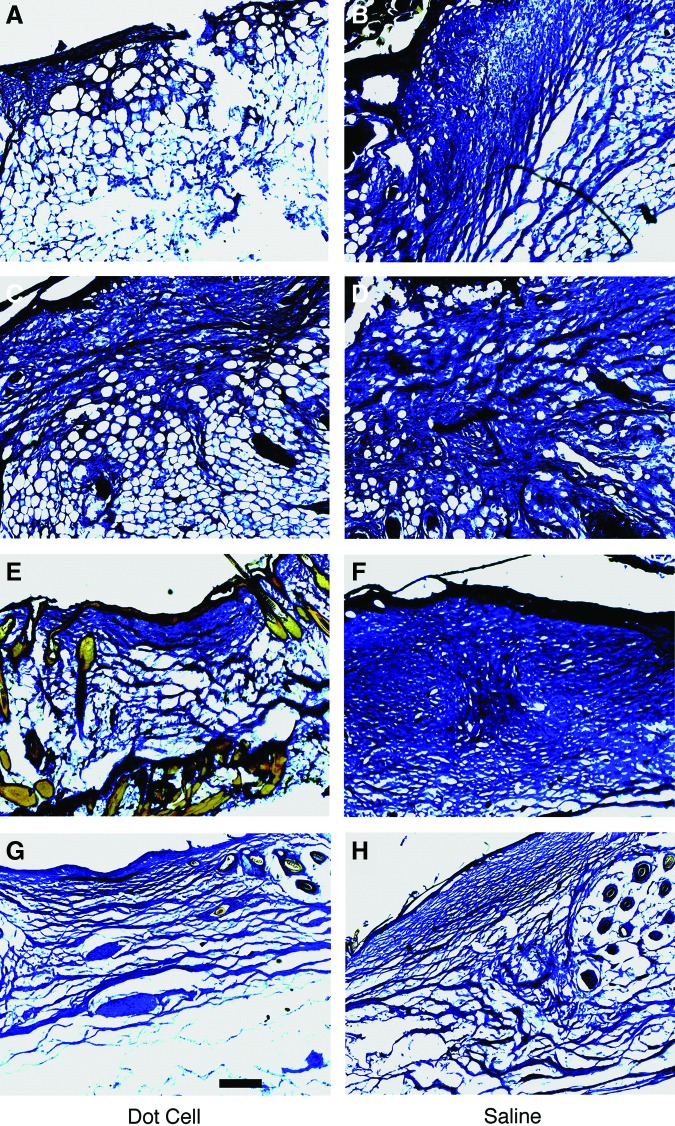
Figure 6.
Wound repair function of fetal E-cadherin cells. Five hundred thousand freshly sorted fetal E-cadherin cells in 100 μL saline were injected by tail vein into mice with fresh dorsal full-thickness skin wounds. Wounds were collected on day 5 (A), day 7 (C), day 15 (E), and day 20 (G). Wounds from saline control mice were collected on the same days (B, D, F, H). Fibrotic tissue expression in the wounds was examined using trichrome stain, and images were taken using conventional bright field light microscopy. Significantly less scar tissue was observed in the fetal E-cadherin cell transplanted group. Scale bar=200 μm. Reprinted with permission from Kong et al.45 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Further wound-healing experiments were performed utilizing in vitro expanded fetal stem cells on slow healing diabetic mice.46 The results showed smaller wound surfaces as well as newly grown hair at the scar periphery of fetal cell-transplanted mice. These data suggest that the fetal cells can not only reduce scarring, but also increase re-epithelialization and hair growth.
Fetal wound healing
Despite the current studies on fetal skin stem cells, the exact mechanism for scarless repair is yet unclear. In vivo experimentation has shown that transplanted fetal cells will migrate to injured tissue and promote regeneration. One mechanism for the ability of fetal cells to migrate to wound sites may be due to its cell surface marker CD184 (CXCR-4), which is the receptor for SDF-1. As previously discussed, SDF-1 is known to regulate the migration of MSCs to sites of injury, and fetal cells may be recruited through similar cytokine–receptor interactions.
The recent discovery of fetal skin stem cells adds another dimension to the complex mechanism of scarless fetal wound healing. Although the ability of these cells to promote regeneration over repair via transplantation in the postnatal wound has been demonstrated, the exact mechanisms are largely unknown. In addition, their exact role in scarless fetal wound healing has yet to be elucidated. Furthermore, it is unclear whether an inherent capacity for regeneration is merely switched off during gestation. A recent investigation of cardiac regeneration in zebrafish and neonatal mice demonstrate that the majority of cardiomyocytes within regenerated tissue originate from pre-existing cardiomyocytes.47,48 These studies suggest that the role of stem cells in regeneration is limited. Studies are currently ongoing to further explain these processes.
Conclusion
Scar formation is a common, unwanted consequence of full-thickness wound healing. While adult wound healing involves the production of scar tissue that is characterized by an abnormal organization of the neodermis, fetal wounds have the remarkable ability of undergoing regeneration and healing in a scarless manner. Despite extensive investigation of scarless fetal wound healing, the exact mechanisms of how this regenerative process takes place remain unknown. Several studies have shown that inflammation is a key difference between fetal and adult skin wound healing. Cell-based therapeutic applications stimulating endogenous progenitor cells or supplying exogenous ones have become a prime translational goal. Epidermal stem cells, MSCs, and fetal skin stem cells have been implicated in fetal and adult wound healing. However, neither the percentage nor the residing anatomical niches when they contribute to scarless wound healing is known. Ultimately, a better understanding of the role that stem cells have in fetal wound healing will hopefully enable potential cell-based intervention in the adult wound-healing milieu to decrease the amount of scar formation.
Take-Home Messages.
• In early gestation, fetal wounds undergo regeneration and heal without scar formation.
• The exact mechanism of scarless fetal wound healing is still unknown.
• Epidermal stem cells and MSCs have been implicated in fetal wound healing.
• With further research and a better understanding of stem cells in the fetal wound, cell-based therapies for regenerative applications may be possible.
Abbreviations and Acronyms
- aMSC
amniotic fluid-derived MSC
- BM-MSC
bone marrow-derived MSC
- CXCR-4
chemokine (C-X-C motif) receptor 4
- DP
dermal papilla
- ECM
extracellular matrix
- EPU
epidermal proliferating unit
- GFP
green fluorescent protein
- IL-1
interleukin 1
- IL-10
interleukin 10
- MSC
mesenchymal stem cell
- SDF-1
stromal cell-derived factor 1
- SKP
skin-derived progenitor
- TA
transiently amplifying
Acknowledgments and Funding Sources
The authors thank Ingrid Lai and William Shu for their generous gift support of this work.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Michael Hu is a surgery resident at the University of Hawai'i who is currently undergoing a postdoctoral fellowship at Stanford. His interests include stem cell biology and regenerative medicine. He is pursuing a career in craniofacial plastic surgery. Robert Rennert is a medical student from The Ohio State University who is conducting research in tissue engineering. Adrian McArdle is a plastic surgery resident in Ireland who is interested in the stem cell niche environment. Michael Chung is a medical student from the University of Kentucky with interest in plastic surgery. Graham Walmsley is pursuing a joint MD/PhD at Stanford and is interested in stem cell biology. Michael Longaker is a Professor of Surgery and Bioengineering at Stanford. He is the Director of Research for the Program in Regenerative Medicine, Children's Surgical Research, and Division of Plastic and Reconstructive Surgery. His extensive research experience includes wound healing, tissue engineering, and developmental/stem cell biology. H. Peter Lorenz is a Professor and Chief of Plastic Surgery at the Lucile Packard Children's Hospital at Stanford. His clinical interests are in craniofacial surgery, pediatric plastic surgery, and reconstructive and cosmetic surgery. His laboratory group is studying mechanisms underlying scarless skin healing and the function of progenitor cells during wound repair/regeneration.
References
- 1.Shih B, Garside E, McGrouther DA, and Bayat A: Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen 2010; 18: 139. [DOI] [PubMed] [Google Scholar]
- 2.Rowlatt U: Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 1979; 381: 353. [DOI] [PubMed] [Google Scholar]
- 3.Beanes SR, Hu FY, Soo C, et al. : Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plast Reconstr Surg 2002; 109: 160. [DOI] [PubMed] [Google Scholar]
- 4.Adzick NS. and Longaker MT: Animal models for the study of fetal tissue repair. J Surg Res 1991; 51: 216. [DOI] [PubMed] [Google Scholar]
- 5.Adzick NS. and Longaker MT: Scarless fetal healing: therapeutic implications. Ann Surg 1992; 215: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenz HP, Longaker MT, Perkocha LA, et al. : Scarless wound repair: a human fetal skin model. Development 1992; 114: 253. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz HP, Whitby DJ, Longaker MT, and Adzick NS: Fetal wound healing: the ontogeny of scar formation in the non-human primate. Ann Surg 1993; 217: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer AJ. and Clark RA: Cutaneous wound healing. N Engl J Med 1999; 341: 738. [DOI] [PubMed] [Google Scholar]
- 9.Gurtner GC, Werner S, Barrandon Y, and Longaker MT: Wound repair and regeneration. Nature 2008; 453: 314. [DOI] [PubMed] [Google Scholar]
- 10.Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS: Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg 1997; 32: 411. [DOI] [PubMed] [Google Scholar]
- 11.Ihara S, Motobayashi Y, Nagao E, and Kistler A: Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development 1990; 110: 671. [DOI] [PubMed] [Google Scholar]
- 12.Colwell AS, Krummel TM, Longaker MT, and Lorenz HP: An in vivo mouse excisional wound model of scarless healing. Plast Reconstr Surg 2006; 117: 2292. [DOI] [PubMed] [Google Scholar]
- 13.Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS: Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 1994; 219: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo DD, Zimmermann AS, Nauta A, Longaker MT, and Lorenz HP: Scarless fetal skin wound healing update. Birth Defects Res C Embryo Today 2012; 96: 237. [DOI] [PubMed] [Google Scholar]
- 15.Blanpain C. and Fuchs E: Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009; 10: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghazizadeh S. and Taichman LB: Multiple classes of stem cells in cutaneous epithelium: A lineage analysis of adult mouse skin. EMBO J 2001; 20: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijhof JG, Braun KM, Giangreco A, et al. : The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 2006; 133: 3027. [DOI] [PubMed] [Google Scholar]
- 18.Cotsarelis G, Sun TT, and Lavker RM: Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990; 61: 1329. [DOI] [PubMed] [Google Scholar]
- 19.Potten CS: The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet 1974; 7: 77. [DOI] [PubMed] [Google Scholar]
- 20.Potten CS. and Morris RJ: Epithelial stem cells in vivo. J Cell Sci Suppl 1988; 10: 45. [DOI] [PubMed] [Google Scholar]
- 21.Oshima H, Rochat A, Kedzia C, Kobayashi K, and Barrandon Y: Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001; 104: 233. [DOI] [PubMed] [Google Scholar]
- 22.Taylor G, Lehrer MS, Jensen PJ, Sun TT, and Lavker RM: Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000; 102: 451. [DOI] [PubMed] [Google Scholar]
- 23.Levy V, Lindon C, Zheng Y, Harfe BD, and Morgan BA: Epidermal stem cells arise from the hair follicle after wounding. FASEB J 2007; 21: 1358. [DOI] [PubMed] [Google Scholar]
- 24.Liang L, Chinnathambi S, Stern M, Tomanek-Chalkley A, Manuel TD, Bickenbach JR: As epidermal stem cells age they do not substantially change their characteristics. J Investig Dermatol Symp Proc 2004; 9: 229. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan EP, Longaker MT, and Lorenz HP: Fetal skin wound healing. Adv Clin Chem 2009; 48: 137. [DOI] [PubMed] [Google Scholar]
- 26.Lechler T. and Fuchs E: Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005; 437: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driskell RR, Clavel C, Rendl M, and Watt FM: Hair follicle dermal papilla cells at a glance. J Cell Sci 2011; 124: 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biernaskie J, Paris M, Morozova O, et al. : SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 2009; 5: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes KJ, McKenzie IA, Mill P, et al. : A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 2004; 6: 1082. [DOI] [PubMed] [Google Scholar]
- 30.Klein JD, Turner CG, Steigman SA, et al. : Amniotic mesenchymal stem cells enhance normal fetal wound healing. Stem Cells Dev 2011; 20: 969. [DOI] [PubMed] [Google Scholar]
- 31.Mackenzie TC. and Flake AW: Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis 2001; 27: 601. [DOI] [PubMed] [Google Scholar]
- 32.da Silva Meirelles L, Chagastelles PC, and Nardi NB: Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006; 119: 2204. [DOI] [PubMed] [Google Scholar]
- 33.Crisan M, Yap S, Casteilla L, et al. : A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3: 301. [DOI] [PubMed] [Google Scholar]
- 34.Pittenger MF, Mackay AM, Beck SC, et al. : Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143. [DOI] [PubMed] [Google Scholar]
- 35.Rennert RC, Sorkin M, Garg RK, and Gurtner GC: Stem cell recruitment after injury: lessons for regenerative medicine. Regen Med 2012; 7: 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen JS, Wong VW, and Gurtner GC: Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol 2012; 3: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek A. and Bersinger NA: Human placental stem cells: biomedical potential and clinical relevance. J Stem Cells 2011; 6: 75. [PubMed] [Google Scholar]
- 38.Chan J, Waddington SN, O'Donoghue K, et al. : Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells 2007; 25: 875. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Huang S, Feng C, and Fu X: Umbilical cord-derived mesenchymal stem cells: strategies, challenges, and potential for cutaneous regeneration. Front Med 2012; 6: 41. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZY, Teoh SH, Hui JH, Fisk NM, Choolani M, Chan JK: The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials 2012; 33: 2656. [DOI] [PubMed] [Google Scholar]
- 41.Skardal A, Mack D, Kapetanovic E, et al. : Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 2012; 1: 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZY, Teoh SH, Chong MS, et al. : Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells 2009; 27: 126. [DOI] [PubMed] [Google Scholar]
- 43.Zhang ZY, Teoh SH, Chong MS, et al. : Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials 2010; 31: 608. [DOI] [PubMed] [Google Scholar]
- 44.Leung A, Crombleholme TM, and Keswani SG: Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr 2012; 24: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong W, Li S, Longaker MT, and Lorenz HP: Blood-derived small Dot cells reduce scar in wound healing. Exp Cell Res 2008; 314: 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong W, Li S, and Lorenz HP: Germ plasm-like Dot cells maintain their wound regenerative function after in vitro expansion. Clin Exp Pharmacol Physiol 2010; 37: e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porrello ER, Mahmoud AI, Simpson E, et al. : Transient regenerative potential of the neonatal mouse heart. Science 2011; 331: 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kikuchi K. and Poss KD: Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol 2012; 28: 719. [DOI] [PMC free article] [PubMed] [Google Scholar]