Abstract
Significance: During gestation, fetal skin progresses from a single layer derived from ectoderm to a complex, multi-layer tissue with the stratum corneum (SC) as the outermost layer. Innate immunity is a conferred complex process involving a balance of pro- and anti-inflammatory cytokines, structural proteins, and specific antigen-presenting cells. The SC is a part of the innate immune system as an impermeable physical barrier containing anti-microbial lipids and host defense proteins. Postnatally, the epidermis continually replenishes itself, provides a protective barrier, and repairs injuries.
Recent Advances: Vernix caseosa protects the fetus during gestation and facilitates development of the SC in the aqueous uterine environment. The anti-infective, hydrating, acidification, and wound-healing properties post birth provide insights for the development of strategies that facilitate SC maturation and repair in the premature infant.
Critical Issues: Reduction of infant mortality is a global health priority. Premature infants have an incompetent skin barrier putting them at risk for irritant exposure, skin compromise and life-threatening infections. Effective interventions to accelerate skin barrier maturation are compelling.
Future Directions: Investigations to determine the ontogeny of barrier maturation, that is, SC structure, composition, cohesiveness, permeability, susceptibility to injury, and microflora, as a function of gestational age are essential. Clinicians need to know when the premature skin barrier becomes fully competent and comparable to healthy newborn skin. This will guide the development of innovative strategies for optimizing skin barrier development.

Marty O. Visscher, PhD
Scope and Significance
During the third trimester of pregnancy, a remarkable process of epidermal differentiation culminates in the formation of the stratum corneum (SC), a sophisticated innate immune interface. The full-term infant relies on the protective skin barrier during transition to a cold, dry, microbe-rich extrauterine environment at birth. Functions include (1) barrier to water loss and irritants, (2) infection control and immunosurveillance, (3) resilience to mechanical trauma, (4) sensation and tactile discrimination, (5) thermal regulation, and (6) acid mantle formation (Table 1). They are conferred by an exquisite, multi-layered epidermis that overlays the dermis, a derivative of mesoderm. This review emphasizes the importance of the outermost layer, the SC, the first line of defense. A wound can be defined as a “breach of the integument, beginning at the SC” or any change that disrupts the normal structure and homeostasis.
Table 1.
Human skin structure and function
| Function | Skin Structure |
|---|---|
| Barrier | |
| Physical (irritants) | Stratum corneum and epidermis |
| Ultraviolet radiation | Melanocytes (epidermis) |
| Immunological | Langerhans cells (epidermis) |
| Resilient foundation | Dermis |
| Sensation | Sensory nerves (epidermis and dermis) |
| Tactile discrimination | Stratum corneum and sensory nerves |
| Thermal regulation | Eccrine sweat glands (dermis) |
| Blood supply (dermis) | |
| Adipose fat below dermis | |
| Acid mantle formation | Stratum corneum and epidermis |
Translational Relevance
We review skin ontogeny in the context of fetal development and birth. Skin barrier maturation in the premature infant differs from that of the full-term baby. The premature infant has a poorly formed, incompetent skin barrier that is ill-equipped to handle environmental stressors. A comprehensive understanding of skin barrier formation in both infant groups is expected to guide the development of effective strategies to minimize the consequences of an immature epidermis.
Clinical Relevance
Reduction in infant mortality and morbidity is a global health priority. Compared with term infants, premature infants are at increased risk for infection due to an incompetent epidermal barrier. Nosocomial infection is a leading cause of premature mortality. Implementation of evidence-based care bundles for catheter insertion and maintenance among very premature infants in 24 neonatal intensive care units (NICUs) significantly reduced catheter-related bloodstream infections, but the decrease was much less than expected.1 Poor skin integrity is now believed to be a major predisposing factor for neonatal sepsis. Prevention and mitigation of premature skin compromise are essential.
Discussion
Fetal skin development
Human fetal skin is derived from ectoderm during embryogenesis. The initial single layer undergoes programmatic differentiation to form a highly sophisticated stratified epithelium. By the time of birth, human skin is capable of performing multiple functions that are necessary for survival in a relatively dry environment. Most importantly, the fully formed epidermis is designed to continually replenish itself, provide a protective barrier against environmental insults, and repair tissue injuries.
Ectoderm becomes a single epithelial layer when Wnt signaling blocks ectoderm from responding to fibroblast growth factors (FGFs) but it responds to bone morphogenic proteins (BMPs).2 Epidermal cells express Wnts. The Wnt responsive cells are influenced by mesenchymal cells and, via FGF and BMP inhibition, produce the hair placode. The cells that do not respond to Wnts become epidermal cells via BMP, FGF, and Notch signaling. By 4 weeks post conceptual age, the skin consists of two layers, the periderm and a basal layer.3 Eight stages of epidermal differentiation occur from ∼5 to 26 weeks of gestation.4 The periderm protects the developing epidermis from amniotic fluid and manages glucose uptake. Melanocytes start appearing in the basal layer from 5 to 8 weeks gestation.
By 8–11 weeks, three epidermal layers have appeared. Proliferation and maturation of keratinocytes from the basal layer generates the spinous cell layer between the basal layer and the periderm.5 The transcriptional regulator p63 and the canonical Notch pathway signal basal cells to form spinous cells in the stratification process.6 Hair germination occurs from 9 to 14 weeks. From 12 to 16 weeks, periderm, spinous cells (two layers), basal cells, desmosomes, hair follicles, and mesenchymal cells of the hair bulb are found. From 14 to 17 weeks, the upper spinous cells are flattened and there are 6–8 basal layers for each periderm layer. By 16–23 weeks, there are 4–5 epidermal layers. Keratin-containing squame regions appear within the interfollicular space along with a discrete waxy material. The granular layer appears at the hair follicle but not within the interfollicular regions. About week 23, the periderm is no longer present. Polygonal, keratinized cells are seen along the hair follicle and the interfollicular spaces. The terminally differentiated outermost layer, the SC, occurs at 23 weeks but may be only a few layers thick. The epidermis is fully keratinized by 26 weeks with one basal layer, 2–3 spinous layers, a granular layer (with keratohyalin granules), and 5–6 layers of SC (Figs. 1 and 2). The SC forms around the fetal hair follicle at 18–19 weeks and along the hair canal by week 21.7 This folliculocentric pattern of organized intrauterine epidermal maturation supports an important role for the hair follicle in the initiation of barrier formation. Cornification of the interfollicular epidermis occurs in a programmatic fashion from head (initially, week 23) to toe and dorsal ventral (week 25, abdomen) across the fetus.8
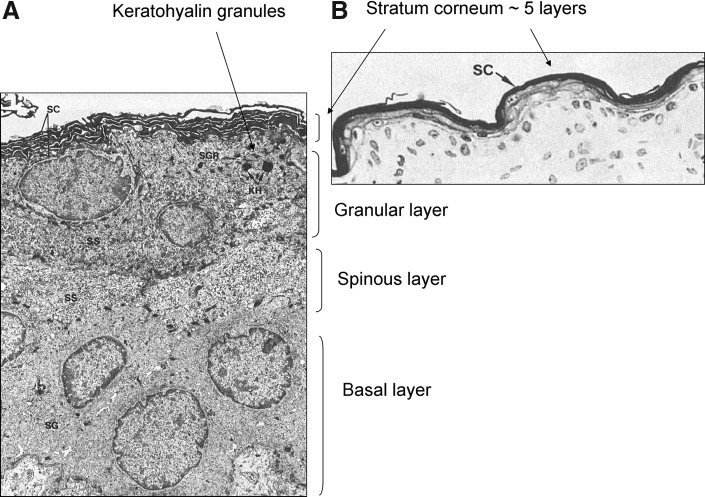
Figure 1.
Electron micrographs of skin samples from a premature infant at 26 weeks of gestation.4 (A) The image shows the basal, spinous, and granular layers and a few layers of SC. The keratohyalin granules in the upper granular layer can be seen. (B) The stratum corneum is five layers thick at this time. Used with permission from Holbrook and Odland.4 SC, stratum corneum.
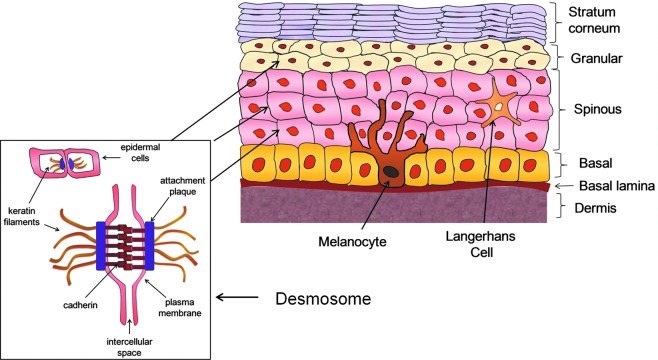
Figure 2.
Skin structure and function. The human epidermis evolves during gestation to form a well-designed, structurally sophisticated, integrated, cohesive, and protective tissue composed of four major layers, that is, basal, spinous, granular, and SC (cornified). Within the spinous and granular layers, the cells are attached to each other via desmosomes, protein-based structures that interconnect the layers of the epidermal barrier. A wound can be defined as a “breach of the integument, beginning at the SC” and/or any change that disrupts the normal structure and homeostasis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Epidermal Langerhans cells (LCs) are a part of the innate immune system to protect against infection. In the fetal mouse, LC precursors appear in the dermis on day 14, the epidermis on day 18, and differentiate to LCs.9 Epidermal keratinocytes signal LC proliferation.9 Between postnatal days 4 and 7, rapid LC proliferation occurs (density of 1,000/mm2) to establish a network within the skin. In humans, LCs appear and express antigens by 6 weeks of gestation.10 The regulatory cytokine transforming growth factor beta 1 plays a role in LC development11 and is detected in the spinous layers at 9 weeks of gestation.12 LC precursors emerge after gestational weeks 11 and 13, as indicated by the presence of specific trans-membrane cell receptors Langerin and CD1c, respectively.12 The cell density is consistent during early development but increases in activity by 12 weeks. Mast cells are observed only after 11 weeks of gestation and then increase in the second trimester.13 Currently, the impact of premature birth, that is, when the epidermis is not fully formed, on LC proliferation is unknown but of clinical importance in late-onset sepsis.
The dermis is beneath the epidermis and derived from the mesoderm. It provides mechanical structure via a network of connective tissue, which includes the proteins collagen and elastin. Hair follicles, sebaceous glands, sensory nerves, and vasculature are within the dermis. Collagen is a major structural protein. Just below the epidermis is the basal lamina, composed of extracellular matrix proteins including integrin and laminin.
Epidermal structure and function
Proteins
The integrity of the stratified epidermis is conferred by specific keratinocyte proteins. The keratins are fibrous intermediate filament proteins. They can be acidic or basic and are aggregated into microfibrils. Keratins 5 and 14 are found in the proliferating basal layer. Keratins 1, 2, 9, and 10 are in the suprabasal differentiating layers (i.e., spinous, granular; Figs. 2 and 3).14 Within the spinous and granular layers, the cells are attached to each other via desmosomes containing the proteins desmogleins and desmoplakin. The desmosomes interconnect the layers of the epidermal barrier to provide structural integrity (Fig. 2). Involucrin is a precursor of the SC envelope and occurs in the spinous, granular, and SC layers. Transglutaminases, positioned in the spinous and granular layers, are calcium-dependent enzymes that cross-link proteins during the initial phases of SC cell envelope formation. Epidermal basal, spinous, and granular cells have phospholipid membranes. The envelopes of SC cells are insoluble, highly cross-linked structures of 5–20 nm thickness that enhance the mechanical resilience. The envelope is composed of a ceramide-containing bilayer that is covalently attached to involucrin, envoplankin, and periplankin on the cell surface. Loricrin is a major protein component and it combines with small proline-rich proteins to produce a scaffold onto which other structural proteins, for example, keratin filaments, can attach.14 Filaggrin is derived from profilaggrin in the keratohyalin granules (granular layer) and aggregates the SC keratin filaments. It later undergoes proteolysis to form a mixture of small molecules known as natural moisturizing factor (NMF). NMF is responsible for keeping water associated with the SC, thereby facilitating skin hydration, plasticity (flexibility), and normal cell loss from the surface after apoptosis.
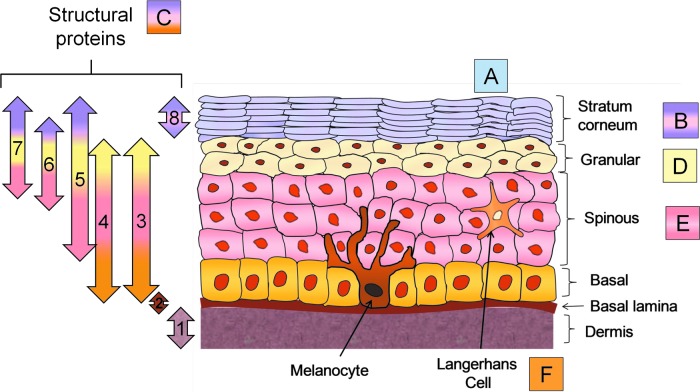
Figure 3.
Forms of epidermal innate immunity. The skin provides innate immunity via several mechanisms. Structural proteins throughout the skin (C) form the highly structured physical barrier and include collagen in the dermis (1), integrin (2), transglutaminases (3), desmoplankin (4), keratin 1,10 (5), involucrin (6), filaggrin (7), and loricrin (8). Sebum, sweat, and fatty acids on the skin surface (A) provide antimicrobials. The SC (B) contributes structural integrity via barrier lipids, corneodesomosones, and the antimicrobials lysozyme and lactoferrin. Lipids in the lamellar bodies (D) produce SC lipids. Antimicrobial and wound repair properties are conferred by the cytokines and proteins of the differentiating keratinocytes (E). Langerhans cells (LCs) (F) defend the organism if the SC barrier is breached. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Lipids
Lipids are synthesized in the nucleated epidermal cells and stored in lamellar bodies in the granular layer (Fig. 4). Lamellar bodies have polar lipids, glycospingolipids, free sterols (cholesterol), phospholipids, enzymes, and human β-defensin. Between 16 and 22 weeks, membranes of the lamellar bodies fuse with the plasma membrane and release the lipids into the space between the cells at the interface between the granular layer and the SC.15 Under the action of the lamellar body enzymes (lipases, proteases, and glycosidases), the polar lipids are converted to nonpolar lipids, phospholipidases hydrolyze phospholipids to fatty acids and glycerin, and glycospingolipids (glucosylceramide, sphingomyelin) are degraded to form ceramides. The result is a regular array of lipid bilayers alternating with water between the corneocytes. The lipid bilayers constitute the major barrier to water loss. The composition is relatively constant from weeks 8 to 16.16 By week 19, the fraction of sterol esters/wax esters is substantially higher, and triglyceride levels increase. The sterol ester/wax esters and ceramides increase between 14–17 and 20–28 weeks and fatty acids decrease (Fig. 5).17 Cholesterol levels are higher in fetal and infant epidermis compared with adults.
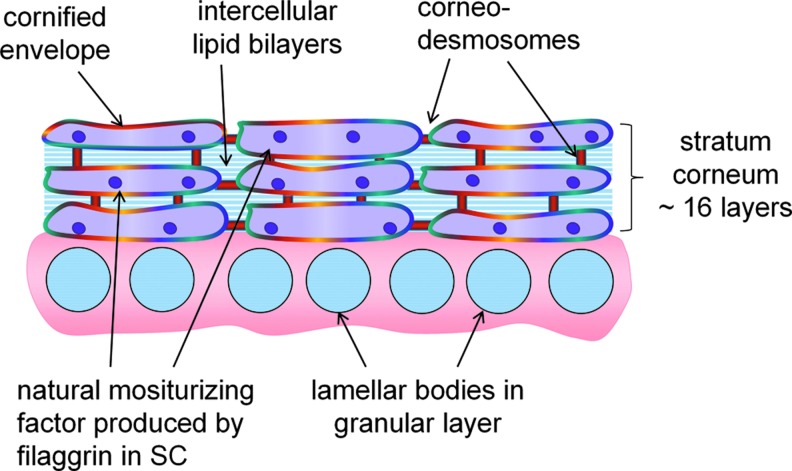
Figure 4.
SC architecture. During the final trimester, a remarkable process of epidermal differentiation culminates in formation of the SC. The SC is in direct contact with the environment, making it the first line of defense and consists of ∼16 corneocyte cell layers that are embedded in the lipid matrix. Lipids are synthesized in the nucleated cells of the epidermis and stored in lamellar bodies in the spinous layer. They are secreted from the lamellar bodies into the intercellular spaces at the interface between the granular layer and the SC. The thickness is 10–40 μm, varying with anatomical location. The corneocytes are interconnected within and between layers via corneodesmosomes. The protein filaggrin undergoes proteolysis to form NMF, which is responsible for SC hydration, water-handling properties, and plasticity. By design, the SC structure is mechanically tough and difficult to penetrate from the outside. NMF, natural moisturizing factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 5.
Epidermal lipid composition during gestation. (A) The epidermal lipid composition is relatively constant from week 8 through week 16.16 By week 19, the fraction of sterol esters/wax esters is substantially higher, and triglyceride levels increase. The sterol esters and wax esters increase between 14–17 and 20–28 weeks. (B) Sterol esters/wax esters and ceramides increase, and fatty acids decrease over the period.17
SC architecture
The SC consists of ∼16 cell (corneocyte) layers embedded in the lipid matrix and a thickness of 10–40 μm, varying with anatomical location. The corneocytes are interconnected within and between layers via corneodesmosomes (Fig. 4). SC enzymes degrade the corneodesmosomes, enabling the cells to detach (desquamate) from the skin surface. By design, the SC is mechanically tough and difficult to penetrate. It is the barrier to water loss but simultaneously allows normal water vapor, that is, transepidermal water, to pass. Transpeidermal water loss (TEWL) is measured experimentally as a rate as g/m2/h, and values are 4–8 g/m2/h in healthy, intact full-term infant SC. Under normal conditions, that is, homeostasis, basal keratinocyte proliferation occurs at the same rate as loss of SC cells from the surface. Epidermal self-renewal is facilitated by stem cells in the interfollicular epidermis, hair follicles, and sebaceous glands.18
Epidermal innate immunity
The skin provides innate immunity via a complex balance of pro- and anti-inflammatory cytokines, structural proteins, lipids, specific antigen-presenting cells, and a direct, impermeable physical barrier (SC; Fig. 3). After puberty, the sebaceous glands secrete a lipid mixture (sebum) that is composed of squalene, triglycerides, and wax monoesters onto the skin surface. The triglycerides are hydrolyzed to form sapienic and lauric fatty acids, and both have antibacterial properties.19 The antimicrobial peptide dermicidin, produced in the eccrine glands, is consistently found at high levels on the skin with sweating.20 Dermicidin causes keratinocytes to secrete cytokines and chemokines, presumably to activate an immune response.21 Keratinocytes undergoing differentiation make two classes of antimicrobial peptides, human beta defensins (HBD) and cathelicidins.19 Their levels increase markedly with epidermal damage (wounding) or infection. HBD2 and LL-37 (human catelicidin), a degradation product of catelicidin (hCAP18), are associated with the lamellar body lipids in the granular layer.22 The antimicrobial proteins lysozyme and lactoferrin are present in newborn SC at levels that are five times higher than in adults.23 The acid SC is a part of the innate immune system as it facilitates bacterial homeostasis, skin colonization and inhibits pathogenic bacteria.24
Full-term neonatal skin
Full-term neonatal skin is well developed and functional at birth, despite being exposed to water and amniotic fluid for 9 months. The epidermis is thick, and the SC layers are well formed. How does the full-term infant develop an excellent barrier while in water? During epidermal tissue development, for example in cell culture systems, the SC does not form if the epidermis is covered with water. Once the SC has formed, continuous exposure to water usually causes significant skin damage, including maceration, disruption of the well-organized structure, and injury to the epidermis. However, this does not occur during fetal development. Vernix caseosa plays a role.
Vernix caseosa and skin development
In utero
Vernix consists of sebaceous gland secretions, lanugo and epithelial cells that cover the fetal and newborn skin.25 It is a complex mixture of 80% water, 10% protein, and 10% lipids consisting of densely packed, flattened corneocytes that are coated with an amorphous lipid matrix.26 The cell envelopes are∼1–2 μm thick, lack distinct nuclei, and have lower keratin levels than fully mature SC cells.26 There is no evidence of corneodesmosomes or distinct cellular organization, and individual cells vary in the stage of keratinization.27 The high water content is associated with the cells.
Presumably, fetal epidermis has a high water flux potential that is driven by osmotic gradients, as cornification is incomplete. Epidermal cornification occurs about the same time as the production of vernix.7 Vernix coats the fetus from head to toe and back to front, presumably under adrenal hormonal control during the last trimester, and facilitates SC formation in the wet uterine environment.28 Vernix lipids include types produced by the sebaceous glands.29 The cornified cells in vernix originate from the hair follicles. In this scenario, vernix is extruded out through the hair shaft and onto the interfollicular epidermis, eventually spreading over the entire surface throughout gestation (Fig. 6).8 The vernix coating protects the epidermis from water exposure and creates a drier condition under which the SC can develop. Vernix may impose a semi-regulated barrier and/or physiological gradient for transepidermal water and nutrients in utero, facilitating cornification via increased DNA and lipid synthesis. Neonates <28 weeks of gestation lack significant vernix caseosa.
Figure 6.
Vernix caseosa formation. Vernix lipids include types produced by the sebaceous glands.29 Vernix is extruded out through the hair shaft and onto the interfollicular epidermis, eventually spreading over the entire surface throughout gestation.8 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
After birth
Vernix has multiple roles after birth. It contains antimicrobial peptides such as lysozyme and lactoferrin that are bioactive against common fungal and bacterial pathogens.30,31 Vernix retention on the skin at birth yields increased hydration 24 h later28 compared with vernix removal. Skin acidity is lower with vernix retention, suggesting that it assists in acid mantle development.28 Treatment with native vernix facilitates skin barrier repair in superficial wounds relative to controls in human, murine, and mini-pig models.32,33
Full-term neonatal skin adaptation
At birth, the skin barrier is highly effective, as indicated by very low TEWL, equal to or lower than adults.34 The skin acidity is relatively neutral, decreases significantly during the first 1–4 days, and continues to drop during the first 3 months.35,36 An acidic surface (acid mantle) is necessary for enzyme function during SC formation, lipid metabolism, lipid bilayer structure formation, ceramide synthesis, and desquamation.37
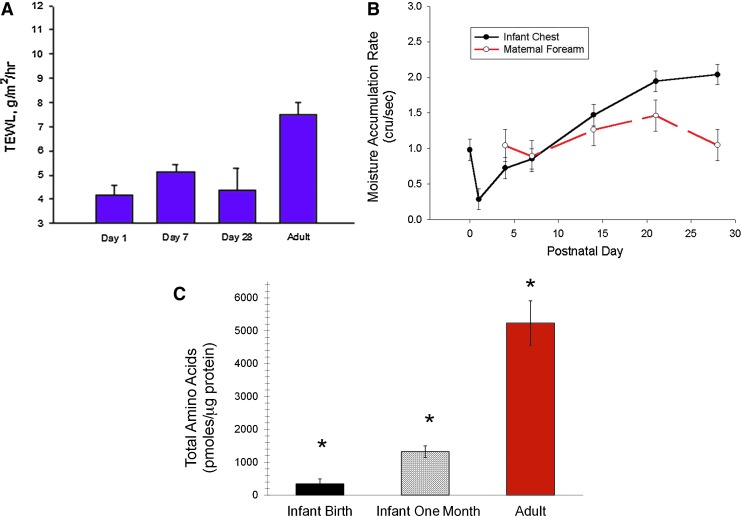
The presence of water (hydration) at an optimum level is required for proper SC function and plasticity.38 Too much hydration causes maceration, lipid disruption, desmosomal degradation, corneocyte swelling, increased permeability, inflammation, irritation, and urticaria.39,40 Too little hydration produces dryness/scaling, aberrant desquamation, cracking, and itching. Skin hydration is measured non-invasively as capacitive reactance. Transepidermal water moves through the upper epidermis and accumulates under a sensor. The rate of this moisture accumulation is a measure of skin hydration. Within minutes to hours after birth, full-term skin hydration varies with body site, environmental temperature, and the presence of vernix.28 Hydration decreases rapidly during the first day and then increases during the first 2 weeks, in contrast to constant hydration in maternal skin (Fig. 7). Water-binding ability increases during the first 2 weeks, indicating adaptation to the dry environment.36 The levels of water-binding free amino acids (FAA, constitutes 40% of the NMF) are extremely low at birth, increase over the first month in parallel with increased hydration but remain markedly lower than adult levels.41 FAAs are lower across 10 layers of neonatal foreskin (1–2 days old), suggesting that filaggrin proteolysis may not take place in utero (100% humidity) but occurs once a water gradient is established after birth.42
Figure 7.
Full-term infant skin adaptation. (A) TEWL is very low at birth, equal to or lower than adults, indicating a highly effective skin barrier. (B) Hydration decreases rapidly during the 1st day and then increases during the 1st 2 weeks, in contrast to constant hydration in maternal skin.36 (C) The water-binding free amino acids (constitutes 40% of the NMF) are extremely low at birth, increase over the 1st month in parallel with increased hydration, but remain markedly lower than adult levels.41 *Indicates significant differences among all three groups. TEWL, transepidermal water loss. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Premature neonatal skin
Status
The premature epidermis is thinner, not fully formed, and has only a few cornified layers.43 Superficial wounds such as abrasions and tape stripping typically remove some or all of the cornified layers. Premature infant skin is essentially “wounded” skin. As a result, the premature infant is at risk for increased permeability to exogenous materials, infection, additional skin compromise, and delayed skin barrier maturation.44 The dermis is deficient in structural proteins. The mechanical properties are poor, and the skin is easily damaged.45 Consequently, it is more easily wounded in response to environmental effects and common procedures, for example, tape removal. Acceleration of skin maturation, that is, wound repair, is critical for this population.
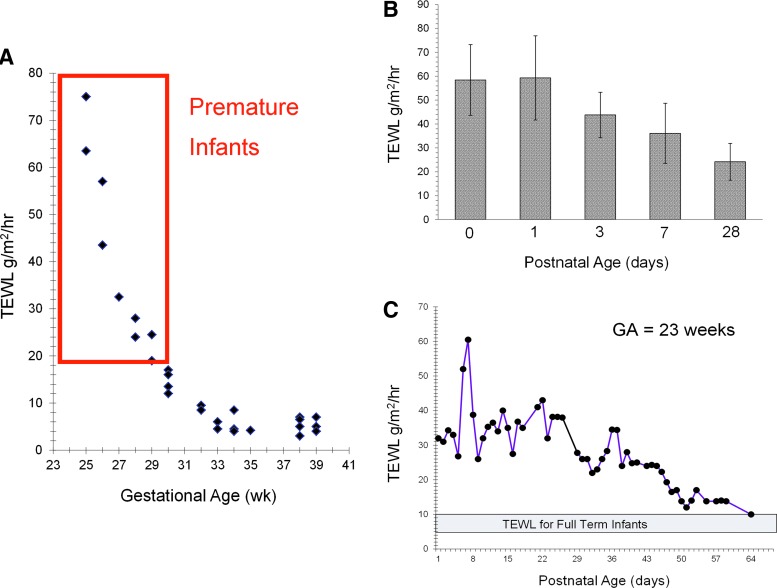
The extent of epidermal barrier maturation depends on gestational age (GA) at birth. Very low-birth-weight infants are at greatest risk for skin damage. At 23 weeks, the SC is nearly absent with TEWL of 75 g/m2/h, similar to what they would be in an open wound (Fig. 8).46 By week 26, a few cornified layers have formed (TEWL of ∼45 g/m2/h). At 29 weeks, TEWL is 17 g/m2/h and markedly higher than values of 5–6 g/m2/h observed in the full-term infant. Around weeks 34–35, the barrier is relatively well formed, but the protective functionality is currently unknown.
Figure 8.
Premature infant skin and adaptation. (A) The extent of epidermal barrier maturation depends on GA at birth. At 23 weeks, the SC is nearly absent with TEWL values that are similar to what they would be in an open wound.46 (B) The TEWL remains significantly higher for premature infants versus normal full-term infants even 1 month after birth.48 (C) The immature SC of premature infants is functionally compromised for several weeks after birth with estimates of full maturation time varying from 2 to 9 weeks of postnatal age.50 GA, gestational age. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Ontogeny of barrier maturation
SC formation is accelerated with exposure to a dry environment.47 However, even after 1 month, TEWL remains significantly higher than normal full-term infants.48 Skin hydration is significantly higher for infants <30 weeks than infants >30 weeks due to the high TEWL.47 By day 5, hydration is significantly lower for infants <27 weeks GA, indicating rapid barrier development postnatally. NMF levels in premature skin are quite low under the conditions of rapid skin development.42 The immature SC of premature infants is functionally compromised for several weeks with estimates of full maturation time varying from 2 to 9 weeks postnatal age49,50 and even longer (months) for complete acid mantle formation.51 The effect of epidermal maturation in the absence of vernix, as seen in infants <28 weeks, is unknown. Evidence suggests that epidermal barrier formation in premature infants is a wound repair process.
Biomarkers: structural proteins
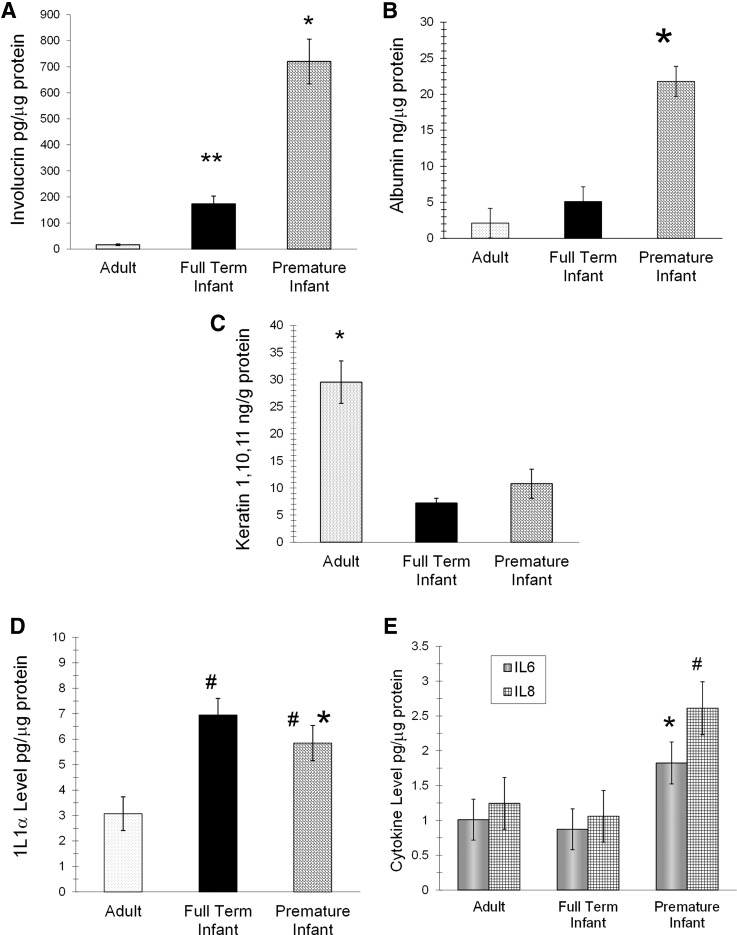
Specific biomarkers are present on the skin surface of premature infants, full-term infants, and adults.52 Neonates ≤32 weeks GA have significantly higher levels of involucrin and albumin than full-term infants and adults (Fig. 9). Involucrin and albumin levels are inversely related to GA, most likely indicative of age effects on barrier maturation. Early involucrin expression is linked with barrier disruption and inflammation.53,54 Albumin is higher in lesional and uninvolved atopic skin than non-atopic controls, positively correlated with TEWL and negatively correlated to skin hydration.55
Figure 9.
Biomarkers of innate immunity in premature infants, full-term infants, and adults. (A, B) Neonates ≤32 weeks of GA had higher levels of involucrin and albumin than full-term infants and adults (p<0.05).52 * indicates significant difference from all, and ** from adults. (C) Keratin 1,10,11 was lower in both infant groups than adults. *Indicates significant difference from all. (D) The proinflammatory cytokine IL1α was higher in both infant groups than adults and higher in premature than full-term infants when adjusted for GA. #Indicates significant difference from adults, and * from full-term infants. (E) The premature infants had higher levels of the proinflammatory cytokines interleukin 6, interleukin 8, interleukin 1 beta, and monocyte chemotactic protein-1 than full-term infants and adults. #Indicates significant difference from full-term infants, and * from all.
Keratin 1,10,11 is significantly lower in both infant groups than adults. Decreased keratin 1,10,11 has been associated with higher skin dryness56 and chronic hyperproliferation.57 Full-term infants have low SC hydration at birth.36 Premature infants frequently exhibit abnormal desquamation, indicating SC hyperproliferation.47
Biomarkers: cytokines
The proinflammatory cytokine interleukin 1-alpha (IL1α) is higher in both infant groups than adults and higher in premature than full-term infants when adjusted for GA. All infants transition rapidly from high to low humidity at birth. IL1α is higher in the upper SC of neonatal animals that are kept at low humidity (<10%), increases in neonatal rat skin at birth, and increases the rate of barrier formation when topically applied to fetal explants.58,59 The higher IL1α level in infants versus adults suggests a similar function. Epidermal keratinocytes secrete cytokines, such as IL1, in response to SC damage and coordinate the recovery processes.60 Premature infants also have higher levels of the proinflammatory cytokines interleukin 6, interleukin 8 (IL8), interleukin 1 beta (IL1β), and monocyte chemotactic protein-1 than full-term infants (Fig. 9) and higher IL1β and IL8 than adults. However, secondary to a higher incidence of chorioamnionitis in premature infants, the significance of the increased cytokine levels is currently unknown. Levels of IL8 and IL6 are higher in the amniotic fluid with premature labor or preterm premature rupture of membranes.61 Specific cytokines may indicate conditions that are associated with local or systemic inflammation and/or genetic factors.62,63
Future Perspective
A worldwide initiative is underway to substantially reduce the morbidity and mortality associated with premature birth. Research on application of emollients to enhance newborn barrier integrity is in progress. The application of sunflower seed oil to the skin of premature infants <33 weeks GA reduced the incidence of nosocomial blood infections by 41% in developing countries.64 Topically applied lipids penetrate the SC to the granular layer to alter the structure of the lamellar bodies, that is, become a part of the lamellar granule membrane and co-extrude to form SC lamellae.65 The processes of granule formation and lipid secretion are not fully developed in the premature infant.66–68 Investigation of preterm epidermal response to topically applied lipids is warranted. Efforts to address poor skin integrity and iatrogenic skin compromise are underway as a strategy to decrease portals of entry for infectious agents.
The basic science research on premature skin barrier maturation is limited. Clinical data describe the general skin condition and response to topical agents, environmental effects, and routine care procedures, for example, tape stripping from adhesives. Clinical evaluations rely on visual inspection of the irritant response, that is, erythema and scaling. Instrumental measures of SC integrity and function, including TEWL, skin hydration, and acidity, have been published, but there are no reports of the physiology and biological processes underlying these measures. Investigations to determine SC structure, composition, integrity, permeability, cohesiveness as a function of GA at birth, that is, ontogeny, and the skin microflora versus GA, barrier maturation, and environmental effects are warranted. Relevant questions include: When does the SC become fully competent, that is, comparable to full-term healthy newborn at 1–3 months of life? How would premature skin variability due to GA influence treatment strategies? How can SC barrier maturation be effectively accelerated to minimize the risk of infection? Clinicians need to know when premature skin becomes fully competent and comparable to healthy newborn skin. This information will guide treatment selection and the development of innovations for optimizing skin integrity.
Take-Home Messages.
• During gestation, fetal skin progresses from a single layer derived from the ectoderm to a complex, multi-layer tissue with the SC as the outermost layer in contact with the environment. Specific proteins are responsible for the structural integrity.
• The fully developed epidermis is designed to continually replenish itself, provide a protective barrier against environmental insults, and repair tissue injuries. The skin is a highly competent “would healing agent” that is equipped to repair itself from damage from environmental assaults.
• Vernix caseosa is a mixture of water, protein, and lipids consisting of flattened cells coated with a lipid matrix. Produced by the hair follicles and sebaceous glands, vernix spreads over the fetus, protects the epidermis from water exposure, and enhances SC formation. Vernix has antimicrobial, hydrating, and wound-healing properties.
• The skin provides innate immunity via a complex process involving a balance of pro- and anti-inflammatory cytokines, structural proteins, specific antigen-presenting cells, and a direct physical SC barrier.
• Full-term neonatal skin is well developed with excellent integrity (low transepidermal water loss) despite being exposed to water and amniotic fluid for 9 months. However, full-term skin is very dry at birth. It undergoes marked changes, including increasing hydration, increasing levels of water-binding NMFs, and decreases in acidity (acid mantle), for 6–12 months after birth as it adapts to the dry environment.
• Premature skin has a markedly thinner epidermis, a poorly formed SC, and a deficiency of dermal structural proteins versus full-term skin. Premature infant skin is essentially “wounded” skin. Poor SC integrity puts the premature infant at risk for increased exposure to infectious agents and irritants, increased permeability, and high water loss. Acceleration of skin maturation, that is, wound repair, is critical for this population.
• Premature skin is significantly compromised for weeks to months after birth. Time to full maturation may be as long as 9 weeks of postnatal age and longer for complete formation of the protective acid mantle.
• The key unanswered question is: When does premature infant skin become functionally full-term skin? The scientific information on premature skin maturation is sparse and lacks a sufficiently comprehensive understanding of the underlying physiological processes. Once this evidence is obtained, the development of effective interventions will likely occur.
Abbreviations and Acronyms
- BMP
bone morphogenic protein
- FAA
free amino acid
- FGF
fibroblast growth factor
- GA
gestational age
- HBD
human beta defensins
- hCAP18
catelicidin (hCAP18)
- IL1α
interleukin 1-alpha
- IL1β
interleukin 1 beta
- IL8
interleukin 8
- LC
Langerhans cell
- LL-37
human catelicidin
- NICU
neonatal intensive care unit
- NMF
natural moisturizing factor
- SC
stratum corneum
- TEWL
transepidermal water loss
Acknowledgments and Funding Sources
The authors have not received funding for this work.
Author Disclosure and Ghostwriting
No competing financial interests exist. This article was written entirely by the authors. Ghostwriters were not used to write this article.
About the Authors
Marty O. Visscher, PhD, is the Director of the Skin Sciences Program in the Division of Plastic Surgery at the Children's Hospital Medical Center and an Associate Professor in the Department of Surgery in the College of Medicine at the University of Cincinnati. Her research areas include the ontogeny of epidermal barrier maturation in infants, vernix biology, neonatal skin adaptation, and environmental interactions; the effects of stress on skin maturation and innate immunity; and application of quantitative multimodal imaging methods for quantitation of skin condition. Dr. Visscher has conducted numerous major research projects in the NICU setting. Vivek Narendran, MD, MRCP, MBA, is a Neonatologist in the Perinatal Institute of the Cincinnati Children's Hospital Medical Center and a Professor in the Department of Pediatrics in the College of Medicine at the University of Cincinnati. He is the Medical Director of the NICU and Newborn Nurseries of the University Hospital and Christ Hospital. His research interests are innate immunity of the skin with a particular focus on epidermal biomarkers and antimicrobial peptides on the skin surface. His clinical interests are in non-invasive ventilation of the premature infant and business cases for quality improvement.
References
- 1.Kaplan HC, Lannon C, Walsh MC, et al. : Ohio statewide quality-improvement collaborative to reduce late-onset sepsis in preterm infants. Pediatrics 2011; 127:427. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs E: Scratching the surface of skin development. Nature 2007; 445:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane AT: Human fetal skin development. Pediatr Dermatol 1986; 3:487. [DOI] [PubMed] [Google Scholar]
- 4.Holbrook KA. and Odland GF: The fine structure of developing human epidermis: light, scanning, and transmission electron microscopy of the periderm. J Invest Dermatol 1975; 65:16. [DOI] [PubMed] [Google Scholar]
- 5.Koster MI. and Roop DR: Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol 2007; 23:93. [DOI] [PubMed] [Google Scholar]
- 6.Blanpain C. and Fuchs E: Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009; 10:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardman MJ, Moore L, Ferguson MW, et al. : Barrier formation in the human fetus is patterned. J Invest Dermatol 1999; 113:1106. [DOI] [PubMed] [Google Scholar]
- 8.Hardman MJ, Sisi P, Banbury DN, et al. : Patterned acquisition of skin barrier function during development. Development 1998; 125:1541. [DOI] [PubMed] [Google Scholar]
- 9.Chorro L, Sarde A, Li M, et al. : Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med 2009; 206:3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster CA, Holbrook KA, and Farr AG: Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J Invest Dermatol 1986; 86:240. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan DH, Li MO, Jenison MC, et al. : Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med 2007; 204:2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster C, Vaculik C, Fiala C, et al. : HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J Exp Med 2009; 206:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster C, Vaculik C, Prior M, et al. : Phenotypic characterization of leukocytes in prenatal human dermis. J Invest Dermatol 2012; 132:2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candi E, Schmidt R, and Melino G: The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005; 6:328. [DOI] [PubMed] [Google Scholar]
- 15.Kalinin A, Marekov LN, and Steinert PM: Assembly of the epidermal cornified cell envelope. J Cell Sci 2001; 114:3069. [DOI] [PubMed] [Google Scholar]
- 16.Williams ML, Hincenbergs M, and Holbrook KA: Skin lipid content during early fetal development. J Invest Dermatol 1988; 91:263. [DOI] [PubMed] [Google Scholar]
- 17.Tachi M. and Iwamori M: Mass spectrometric characterization of cholesterol esters and wax esters in epidermis of fetal, adult and keloidal human skin. Exp Dermatol 2008; 17:318. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Liu Y, Yang Z, et al. : Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005; 11:1351. [DOI] [PubMed] [Google Scholar]
- 19.Drake DR, Brogden KA, Dawson DV, et al. : Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res 2008; 49:4. [DOI] [PubMed] [Google Scholar]
- 20.Rieg S, Seeber S, Steffen H, et al. : Generation of multiple stable dermcidin-derived antimicrobial peptides in sweat of different body sites. J Invest Dermatol 2006; 126:354. [DOI] [PubMed] [Google Scholar]
- 21.Niyonsaba F, Suzuki A, Ushio H, et al. : The human antimicrobial peptide dermcidin activates normal human keratinocytes. Br J Dermatol 2009; 160:243. [DOI] [PubMed] [Google Scholar]
- 22.Aberg KM, Man MQ, Gallo RL, et al. : Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol 2008; 128:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker VP, Akinbi HT, Meinzen-Derr J, et al. : Host defense proteins on the surface of neonatal skin: implications for innate immunity. J Pediatr 2008; 152:777. [DOI] [PubMed] [Google Scholar]
- 24.Fluhr JW, Kao J, Jain M, et al. : Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol 2001; 117:44. [DOI] [PubMed] [Google Scholar]
- 25.Mosby's Medical Dictionary (8th edn.). St Louis, MO: Elsevier, 2009 [Google Scholar]
- 26.Pickens WL, Warner RR, Boissy YL, et al. : Characterization of vernix caseosa: water content, morphology, and elemental analysis. J Invest Dermatol 2000; 115:875. [DOI] [PubMed] [Google Scholar]
- 27.Agorastos T, Hollweg G, Grussendorf EI, et al. : Features of vernix caseosa cells. Am J Perinatol 1988; 5:253. [DOI] [PubMed] [Google Scholar]
- 28.Visscher MO, Narendran V, Pickens WL, et al. : Vernix caseosa in neonatal adaptation. J Perinatol 2005; 25:440. [DOI] [PubMed] [Google Scholar]
- 29.Rissmann R, Groenink HW, Weerheim AM, et al. : New insights into ultrastructure, lipid composition and organization of vernix caseosa. J Invest Dermatol 2006; 126:1823. [DOI] [PubMed] [Google Scholar]
- 30.Akinbi HT, Narendran V, Pass AK, et al. : Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol 2004; 191:2090. [DOI] [PubMed] [Google Scholar]
- 31.Tollin M, Bergsson G, Kai-Larsen Y, et al. : Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci 2005; 62:2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oudshoorn MH, Rissmann R, van der Coelen D, et al. : Development of a murine model to evaluate the effect of vernix caseosa on skin barrier recovery. Exp Dermatol 2009; 18:178. [DOI] [PubMed] [Google Scholar]
- 33.Visscher MO, Barai N, LaRuffa AA, et al. : Epidermal barrier treatments based on vernix caseosa. Skin Pharmacol Physiol 2011; 24:322. [DOI] [PubMed] [Google Scholar]
- 34.Yosipovitch G, Maayan-Metzger A, Merlob P, et al. : Skin barrier properties in different body areas in neonates. Pediatrics 2000; 106:105. [DOI] [PubMed] [Google Scholar]
- 35.Hoeger PH. and Enzmann CC: Skin physiology of the neonate and young infant: a prospective study of functional skin parameters during early infancy. Pediatr Dermatol 2002; 19:256. [DOI] [PubMed] [Google Scholar]
- 36.Visscher MO, Chatterjee R, Munson KA, et al. : Changes in diapered and nondiapered infant skin over the first month of life. Pediatr Dermatol 2000; 17:45. [DOI] [PubMed] [Google Scholar]
- 37.Schmid-Wendtner MH. and Korting HC: The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol 2006; 19:296. [DOI] [PubMed] [Google Scholar]
- 38.Gloor M, Bettinger J, and Gehring W: [Modification of stratum corneum quality by glycerin-containing external ointments]. Hautarzt 1998; 49:6.[In German]. [DOI] [PubMed] [Google Scholar]
- 39.Kligman AM: Hydration injury to human skin. In: The Irritant Contant Dermatitis Syndrome, edited by van der Valk PMH. Boca Raton, FL: CRC Press, 1996, p. 187 [Google Scholar]
- 40.Warner RR, Stone KJ, and Boissy YL: Hydration disrupts human stratum corneum ultrastructure. J Invest Dermatol 2003; 120:275. [DOI] [PubMed] [Google Scholar]
- 41.Visscher MO, Utturkar R, Pickens WL, et al. : Neonatal skin maturation—vernix caseosa and free amino acids. Pediatr Dermatol 2011; 28:122. [DOI] [PubMed] [Google Scholar]
- 42.Scott IR. and Harding CR: Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol 1986; 115:84. [DOI] [PubMed] [Google Scholar]
- 43.Cartlidge P: The epidermal barrier. Semin Neonatol 2000; 5:273. [DOI] [PubMed] [Google Scholar]
- 44.Rutter N: Clinical consequences of an immature barrier. Semin Neonatol 2000; 5:281. [DOI] [PubMed] [Google Scholar]
- 45.Eichenfield LF. and Hardaway CA: Neonatal dermatology. Curr Opin Pediatr 1999; 11:471. [DOI] [PubMed] [Google Scholar]
- 46.Sedin G, Hammarlund K, and Stromberg B: Transepidermal water loss in full-term and pre-term infants. Acta Paediatr Scand Suppl 1983; 305:27. [DOI] [PubMed] [Google Scholar]
- 47.Okah FA, Wickett RR, Pickens WL, et al. : Surface electrical capacitance as a noninvasive bedside measure of epidermal barrier maturation in the newborn infant. Pediatrics 1995; 96:688. [PubMed] [Google Scholar]
- 48.Agren J, Sjors G, and Sedin G: Transepidermal water loss in infants born at 24 and 25 weeks of gestation. Acta Paediatr 1998; 87:1185. [DOI] [PubMed] [Google Scholar]
- 49.Agren J, Sjors G, and Sedin G: Ambient humidity influences the rate of skin barrier maturation in extremely preterm infants. J Pediatr 2006; 148:613. [DOI] [PubMed] [Google Scholar]
- 50.Kalia YN, Nonato LB, Lund CH, et al. : Development of skin barrier function in premature infants. J Invest Dermatol 1998; 111:320. [DOI] [PubMed] [Google Scholar]
- 51.Visscher M, Odio M, Taylor T, et al. : Skin care in the NICU patient: effects of wipes versus cloth and water on stratum corneum integrity. Neonatology 2009; 96:226. [DOI] [PubMed] [Google Scholar]
- 52.Narendran V, Visscher MO, Abril I, et al. : Biomarkers of epidermal innate immunity in premature and full-term infants. Pediatr Res 2010; 67:382. [DOI] [PubMed] [Google Scholar]
- 53.Hirao T, Terui T, Takeuchi I, et al. : Ratio of immature cornified envelopes does not correlate with parakeratosis in inflammatory skin disorders. Exp Dermatol 2003; 12:591. [DOI] [PubMed] [Google Scholar]
- 54.Ekanayake-Mudiyanselage S, Aschauer H, Schmook FP, et al. : Expression of epidermal keratins and the cornified envelope protein involucrin is influenced by permeability barrier disruption. J Invest Dermatol 1998; 111:517. [DOI] [PubMed] [Google Scholar]
- 55.Yamane Y, Moriyama K, Yasuda C, et al. : New horny layer marker proteins for evaluating skin condition in atopic dermatitis. Int Arch Allergy Immunol 2009; 150:89. [DOI] [PubMed] [Google Scholar]
- 56.Engelke M, Jensen JM, Ekanayake-Mudiyanselage S, et al. : Effects of xerosis and ageing on epidermal proliferation and differentiation. Br J Dermatol 1997; 137:219. [DOI] [PubMed] [Google Scholar]
- 57.HogenEsch H, Boggess D, and Sundberg JP: Changes in keratin and filaggrin expression in the skin of chronic proliferative dermatitis (cpdm) mutant mice. Pathobiology 1999; 67:45. [DOI] [PubMed] [Google Scholar]
- 58.Ashida Y, Ogo M, and Denda M: Epidermal interleukin-1 alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol 2001; 144:238. [DOI] [PubMed] [Google Scholar]
- 59.Jiang YJ, Lu B, Crumrine D, et al. : IL-1alpha accelerates stratum corneum formation and improves permeability barrier homeostasis during murine fetal development. J Dermatol Sci 2009; 54:88. [DOI] [PubMed] [Google Scholar]
- 60.Nickoloff BJ. and Naidu Y: Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol 1994; 30:535. [DOI] [PubMed] [Google Scholar]
- 61.Hillier SL, Witkin SS, Krohn MA, et al. : The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993; 81:941. [PubMed] [Google Scholar]
- 62.Ambalavanan N, Carlo WA, D'Angio CT, et al. : Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 2009; 123:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moonen RM, Paulussen AD, Souren NY, et al. : Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res 2007; 62:188. [DOI] [PubMed] [Google Scholar]
- 64.Darmstadt GL, Saha SK, Ahmed AS, et al. : Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet 2005; 365:1039. [DOI] [PubMed] [Google Scholar]
- 65.Mao-Qiang M, Brown BE, Wu-Pong S, et al. : Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol 1995; 131:809. [DOI] [PubMed] [Google Scholar]
- 66.Madison KC: Barrier function of the skin: “la raison d'etre” of the epidermis. J Invest Dermatol 2003; 121:231. [DOI] [PubMed] [Google Scholar]
- 67.Feingold KR: Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res 2007; 48:2531. [DOI] [PubMed] [Google Scholar]
- 68.Proksch E, Brandner JM, and Jensen JM: The skin: an indispensable barrier. Exp Dermatol 2008; 17:1063. [DOI] [PubMed] [Google Scholar]