Abstract
Objective: The aim of present study was to evaluate whether low-level laser therapy (LLLT) can reverse the impaired wound healing process in diabetic rats. Background data: Impaired wound healing in diabetic patients represents a major health problem. Recent studies have indicated that LLLT may improve wound healing in diabetic rats, but the optimal treatment parameters are still unknown. Materials and methods: Male Sprague–Dawley rats (n=21) were randomly divided into three groups: a healthy control group, a diabetic sham-treated group, and a diabetic LLLT-treated group. Diabetes mellitus was then induced by streptozotocin administration to the two diabetic groups. One 4 cm long full thickness skin incision and one full thickness circular excision (diameter=4 mm) were performed on the back of each rat. An infrared 810 nm laser with an output of 30 mW, a power density of 30 mW/cm2, and a spot size of 1 cm2 was used to irradiate each wound for 30 sec (daily dose of 0.9 J/cm2/wound/day). Results: In diabetic rats, the histology of LLLT-treated excisions revealed a similar healing response to that in nondiabetic controls, with significantly more mature granulation tissue than in the sham-treated diabetic control group. LLLT reduced the loss of tensile strength, and increased the incision wound stiffness significantly compared with sham-irradiated rats, but this did not achieve the same level as in the nondiabetic controls. Conclusions: Our study demonstrates that infrared LLLT can improve wound healing in diabetic rats. Nevertheless, further research needs to be performed to evaluate the exact underlying mechanism and to further optimize LLLT parameters for clinical use.
Introduction
Several factors exist that may negatively affect wound healing. Among these, diabetes mellitus is characterized by glycemia, and micro- and macro-angiopathy, which are risk factors for delayed wound healing and prevalence of chronic wounds.1 The main defects in diabetic wound healing are impaired re-epithelization, and stagnation of granulation tissue (GT) formation as a result of loss of function of diverse growth factors that drive keratinocyte, fibroblast, and endothelial cells functions in tissue repair.2 The importance of the effect of diabetes on wound healing is documented by the fact that >85% of all nontraumatic lower leg amputations are caused by diabetic foot ulcers.3 In addition, a history of diabetic foot ulcer is associated with a significantly increased risk of mortality compared with both nondiabetic and diabetic populations.4
One of many possibilities for improving diabetic healing may be the use of low-level laser therapy (LLLT). LLLT belongs to a group of treatments that induce photochemical interactions within cells of living organisms. Currently, there is no general agreement about the exact way that LLLT may modulate wound healing. However, it has already been shown that LLLT can reduce pain,5–8 and affect all three basic phases (i.e., inflammation, proliferation, and maturation) of wound healing.9–11 In addition, positive LLLT effects have been observed in terms of increased wound tensile strength (TS),12,13 and/or improved microcirculation.14
It has previously been shown that several different wavelengths of laser radiation, such as 633, 670, and 820 nm, can facilitate oxidizing of cytochrome c oxidase.15 From this perspective, it is possible that the mechanisms of LLLT are wavelength independent at the subcellular level, and arise from an increase in the oxidative metabolism in mitochondria. Nevertheless, fibroblasts and endothelial cells responded differently to laser radiation at 665, 675, and 810 nm. Here, the highest proliferation of fibroblasts occurred at 665 and 675 nm, whereas irradiation at 810 nm inhibited cell proliferation. In contrast, endothelial cells showed increased proliferation after exposure to all tested wavelengths.16
The number of persons with diabetes has increased because of population growth, aging, urbanization, and increasing prevalence of risk factors such as obesity and sedentary lifestyle.17 These factors contribute to a poor prognosis of (non-healing) diabetic ulcers, and complications related to diabetes will, therefore, likely continue to increase. In several previously published studies it has been shown that LLLT with selected parameters may improve wound healing in normal and impaired conditions.9–12 As optimal LLLT parameters of infrared lasers are still unknown, we decided to investigate if wound healing could be modulated after laser irradiation with the commonly used wavelength of 810 nm.
We have shown in a number of our previously published articles that there might be a significant difference in the therapeutic approach to an open and a sutured wound.9,10,13 Wound TS measurement and histology have been shown in numerous experimental studies to be appropriate examination techniques for wound healing; therefore, we decided to use these outcomes to evaluate the effects of LLLT on skin repair in diabetic rats using two basic wound healing models (incisional and excisional).18
Materials and Methods
Animal model
The experiment was in compliance with European rules for animal treatment and welfare; therefore, the study was approved by the Ethical Committee of the Faculty of Medicine, Šafárik University in Košice.
Four-month-old male Sprague–Dawley rats (n=21) weighing 300–350 g were included in the experiment, and randomly divided into three groups: control group (C) (n=7), diabetic sham-treated control group (DM-C) (n=7), and diabetic laser-treated group (DM-LLLT) (n=7). One week prior to the wound healing experiment, animals received 50 mg/kg of streptozotocin intraperitoneally (Merck, Darmstadt, Germany). Streptozotocin was prepared in citrate buffer (pH=4.5). Only rats with hyperglycemia in the range of 16–20 mmol/L were included in the experiment. Control animals received analogous volumes of buffer solution.
Wound model
All rats were anesthetized by a combination of ketamine (33 mg/kg; Narkamon a.u.v., Spofa a.s., Czech Republic), xylazine (11 mg/kg; Rometar a. u. v., Spofa a.s., Prague, Czech Republic) and tramadol (5 mg/kg; Tramadol-K, Krka d.d., Slovenia). A circular full-thickness excision (4 mm in diameter) and a full-thickness skin incision (40 mm long) were performed on the back of each rat (Fig. 1). Incisions were immediately sutured by intradermal running suture (Chiraflon 5/0, Chirmax a.s., Prague, Czech Republic). Samples for histological examination (excisions) and wound TS measurement (incisions) were collected at day 14 following surgery.
FIG. 1.
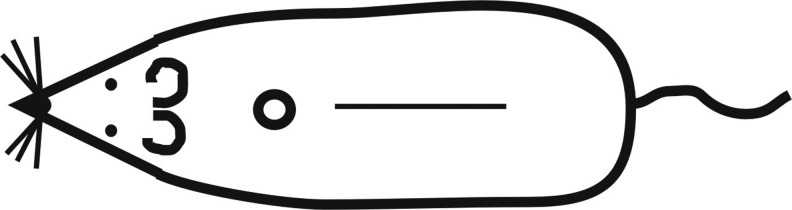
The back of the rats and the positions of the circular excision wound nearest the head, and the longitudinal incision wound nearest the tail.
LLLT
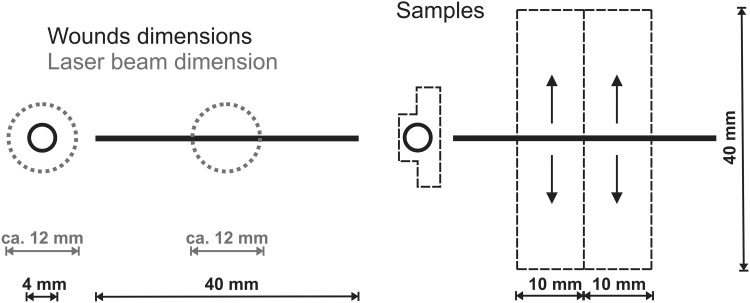
Both wounds (excision and incision) in laser-treated rats were daily (first irradiation immediately after surgery) irradiated in contact mode with a diode laser (Thor Photomedicine Ltd, Chesham, UK) with a continuous output of 30 mW and wavelength of 810 nm (shape of laser beam: round, r=0.6 cm, area=1 cm2). The irradiation area was determined to cover the entire open wound, including an additional 2 mm of the uninjured surrounding area outside the outer margin of the wound (Fig. 2). On the other hand, incisions were irradiated only in the middle of the wound, thus the cranial and caudal wound edges were not directly irradiated (Fig. 2). The power density was 30 mW/cm2 and irradiation time was set to 30 sec/wound/day to achieve the total daily dose of 0.9 J/cm2/wound/day and the energy dose of 0.9 J/wound/day (wounds were irradiated for 7 days; therefore, total therapy lasted 60 sec×7=420 sec/animal; 1.8 J/cm2×7=12.6 J/cm2/animal). Both the nondiabetic and diabetic control groups were sham irradiated to assure similar stress for all animals. During the treatment, all rats were restrained in a Plexiglas cage with a circular opening over the wound.
FIG. 2.
The size of wounds and the diameter of the laser beam used in the present study, as well as the dimensions of the skin samples used for wound tensile strength measurement and the shape of the skin specimen removed for histology.
Wound TS measurement
The device for measuring wound-breaking strength was constructed in our laboratory. Briefly, it is based on a specially shaped arm horizontally pulling one side of a sample whose opposite side is fixed to a measuring tip of a force meter unit (OMEGA Engineering, INC., Stamford, CT). The moving arm is driven by a high-precision stepper motor MDI-17 (Intelligent Motion Systems, Inc., Marlborough, CT) through a linear slider.
This method was described in detail in our previous study.19 Briefly, all animals were killed 14 days after surgery by ether inhalation. Wound samples were then removed and lengthwise placed between the two clamps of the TS testing device (Fig. 2). Pulling (load speed=1 mm/sec) was performed perpendicularly to the original direction of the incision (Fig. 2, arrows).
Maximal breaking strength (MBS) was measured for each sample. TS was calculated by using the following formula: TS=MBS/A [TS=tensile strength (g/mm2), MBS=maximal breaking strength (g), A=wound area (mm2)].
Histological evaluation and morphometry
Excisions were processed routinely for light microscopy [fixation, dehydration, embedding, sectioning, and staining with hematoxylin-eosin (HE) – basic staining and Van Gieson (VG) – nonspecific collagen staining].
The histological structures and processes [polymorphonuclear leukocytes (PMNL), re-epithelization, fibroblasts, and new collagen] were semiquantitatively evaluated in coded slides according to a numerical scale with values: 0, 1, 2, 3, and 4 (Table 1).20
Table 1.
Explanation of Scale Used in the Semiquantitative Evaluation of Histological Sections
| Scale | Epithelization | PMNL | Fibroblasts | Collagen |
|---|---|---|---|---|
| 0 | Thickness of cut edges | Absent | Absent | Absent |
| 1 | Migration of cells (<50%) | Mild ST | Mild ST | Minimal GT |
| 2 | Migration of cells (≥50%) | Mild DL/GT | Mild GT | Mild GT |
| 3 | Bridging the excision | Moderate DL/GT | Moderate GT | Moderate GT |
| 4 | Keratinization | Marked DL/GT | Marked GT | Marked GT |
PMNL, polymorphonuclear cells; ST, surrounding tissue (tissue out of GT); GT, granulation tissue; DL, demarcation line.
To determine the effect of LLLT on GT formation, the area of GT in sections (sections were made from the middle of the wound) made from all animals was measured by means of QuickPHOTO MICRO 2.2 (Promicra, Prague, Czech Republic) software as previously reported.9
Statistical analysis
Data from the wound TS and GT area measurements are presented as mean±SD. ANOVA followed by Tukey–Kramer test was used to compare differences between groups. The semiquantitative data from the histological analysis are presented as medians. Significance was set to p<0.05.
Results
Wound TS
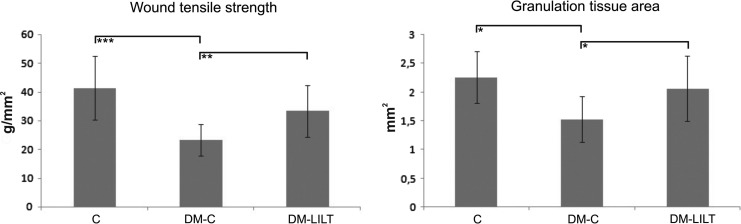
The lowest wound TS results were measured in nontreated diabetic rats (DM-C=23.3±5.4 g/mm2). Higher wound TS results were measured in diabetic LLLT-treated rats (DM-LLLT=33.3±9.1 g/mm2), whereas the highest wound TS results were measured in nondiabetic control animals (C=41.3±11.1 g/mm2) (Fig. 3). The between-group differences were significant between the nondiabetic and diabetic control animals (C vs. DM-C; p<0.001). The difference between the LLLT-irradiated diabetic group, and the nondiabetic control group was not significantly different (C vs. DM-LLLT; p=0.0727), but the TS of the LLLT-irradiated diabetic animals was significantly higher than that for the diabetic sham-irradiated controls (DM-LLLT vs. DM-C; p<0.01) (Fig. 3).
FIG. 3.
Tensile strengths and granulation tissue areas of wounds (all data are presented as mean±SD) removed from control (C), non-laser treated diabetic (DM-C), and laser-treated diabetic animals (DM-LLLT) at day 14 post-surgery (*p<0.05; **p<0.01; ***p<0.001).
Histology
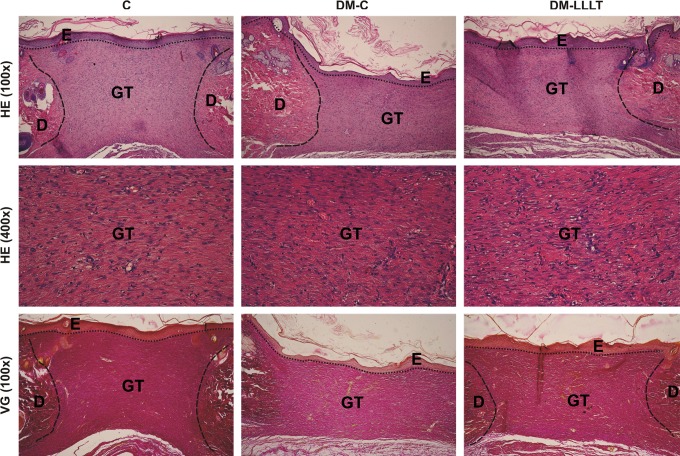
Samples of the wounds are shown in Fig. 4. All wounds demonstrated a normal course of healing, with no infection and/or persistent inflammation (C=0, DM-C=0, DM-LLLT=0). The process of epidermis regeneration was finished, as demonstrated by the presence of a keratin layer in all groups (C=4, DM-C=4, DM-LLLT=4). For this time period, remodeling and reorganization of extracellular matrix (ECM) was characterized, thus the scar was formed (Table 2). In addition, when compared with a wound removed during the proliferation phase (at day 6)20 a mild regress of fibroblasts and luminized vessels in the GT was seen. Here, mild stagnation of GT formation by means of the number of fibroblasts was present in the DM-C group, which was reversed by laser treatment in the DM-LLLT group to the level of the C group (C=3, DM-C=2, DM-LLLT=3). In this context, the GT area measurement revealed significantly reduced GT formation in DM-C rats compared with nondiabetic controls and laser treated diabetic rats (SDM-C=1.532±0.398 mm2 vs. SC=2.263±0.451 mm2 and SDM-LLLT=2.063±0.569 mm2; p<0.05) (C vs. DM-LLLT; p=0.5236). For collagen production, we found the same pattern. The diabetic sham-treated rats exhibited reduced levels of collagen when compared to the other two groups (C=3, DM-C=2, DM-LLLT=3).
FIG. 4.
The histology cuts from one animal in each group – C, DM-C, and DMC-LLLT – shows the amount of granulation tissue (GT) as well as histological structures of skin; dermis (D) and epidermis (E). HE, hematoxylin-eosin; VG, Van Gieson.
Table 2.
Results from the Semiquantitative Evaluation (Data Are Presented as Median) of Histological Sections in Control (C), Non-Laser Treated Diabetic (DM-C), and Laser-Treated Diabetic Animals (DM-LLLT)
| Group | Epithelization | PMNL | Fibroblasts | Collagen |
|---|---|---|---|---|
| C | 4 | 0 | 3 | 3 |
| DM-C | 4 | 0 | 2 | 2 |
| DM-LLLT | 4 | 0 | 3 | 3 |
PMNL, polymorphonuclear leukocytes.
Discussion
Wound healing is a complex process that may be delayed by a number of factors. Imbalance in the production of growth factors may be one of the key factors responsible for delayed wound healing in diabetic patients. It has been shown that LLLT irradiation increased the gene expression and release of certain growth factors by cells, in many cases.21 LLLT with selected parameters was able to significantly reverse the negative impact of diabetes on the healing of skin excisions, and thus increase collagen production and GT formation. Similarly, LLLT with the 980 nm GaAlAs diode laser at 18 J/cm2 has previous been reported to beneficially modulate wound healing in diabetic mice, but LLLT at a higher dose of 36 J/cm2 seemed to decrease the healing process.22 It has also been reported that a better outcome in wound healing was achieved after red LLLT in diabetic rats when compared with infrared LLLT.23 Similarly, by comparing four different wavelengths – 532, 633, 810, and 980 nm – in diabetic rat wound healing, LLLT with a 633 nm wavelength and a dose of 10 J/cm2 was found to give the best result.24 In this context, irradiation with HeNe laser at 10 J/cm2 in both nondiabetic and diabetic rats, significantly promoted wound healing when compared with their controls.25 However, in diabetic patients, to stimulate angiogenesis, target tissues for LLLT are located deeper under the skin. From this perspective, the use of an infrared laser is justified, because of its greater depth of penetration.26,27
The relationships between basic LLLT parameters and their relationships to wound healing are still not fully understood; therefore, the optimal wound type-specific treatment has not yet been determined. In an open wound model, we have previously shown that LLLT at wavelengths 635 and 670 nm was more effective when higher intensity was combined with shorter wavelength and/or lower intensity was combined with higher wavelength.9,10 Hence, it may be suggested that there exists an inverse relationship between wavelength and intensity.28 Furthermore, we also recorded some discrepancy between the effects of irradiation with 670 nm wavelength on the healing of primary sutured and on open wounds.10,13 In this context, open full-thickness wounds were markedly accelerated after LLLT at 810 nm by delivering doses of 5 and 10 J/cm2 (not at 20 and 30 J/cm2).29 This study also reported that the best effect was recorded for the 633 nm red laser. Therefore, in this study, both basic wound models, incision and excision, were used on the same animal. The main difference between primary sutured incisional and open excisional wound healing is in the amount of granulation tissue that is formed during the proliferation phase. Whereas an open wound heals with extensive granulation tissue production, a primary wound forms no or only a negligible amount of granulation tissue.20,30 Therefore, it may be suggested that optimal LLLT probably will be wound-type specific.
LLLT with a pulsed infrared laser at 890 nm demonstrated significantly increased wound stiffness in both healthy and diabetic rats.31 In addition, it has also been shown that LLLT at 830 nm significantly enhanced skin wound TS in a mice diabetic model.12 Laser irradiation at 890 nm displayed dose-dependent diverse effects on skin incisions. LLLT at 0.03 J/cm2 significantly decreased wound stiffness, whereas at 0.2 J/cm2, it significantly increased the maximum load in wounds from both the healthy control and diabetic groups.32 Similarly, LLLT at 808 nm and 10 J/cm2 improved incisional wound healing in diabetic rats; however, the tensile strength of wounds was not evaluated in this investigation.33 Therefore, our study contributes to previously published investigations with novel information regarding wound stiffness in diabetic rats following LLLT at 810 nm. Our study also showed that commonly used parameters of the 810 nm diode laser with 0.9 J/cm2 energy dose promoted open and sutured wound repair slightly differently. Whereas in diabetic rats the open laser-treated wounds achieved a similar tissue quality level as in nondiabetic controls, the wound stiffness of sutured incisions was significantly increased, but did not achieve the same level as in the nondiabetic controls.
From a clinical point of view, this study has some important limitations. We used a mechanical model for inflicting wounds, whereas diabetic wounds typically have an ischemic or neuropathic etiology. Likewise, the rats in our experiment only had had hyperglycemia for 1 week, whereas hyperglycemia may have been present for months in true diabetic patients.
Conclusions
Our study demonstrates that infrared LLLT significantly increased wound TS and stimulated granulation tissue formation in diabetic rats. Although the general molecular mechanisms in wound healing are similar, a direct extrapolation from this experiment to a clinical situation is not possible, because of interspecies variability. Therefore, further studies are needed to optimize LLLT parameters for its use in the clinical practice.
Acknowledgments
We thank Dr. Rodrigo A.B. Lopes-Martins for critically reading this article and for his useful comments. Also we thank Miroslav Chovan and Zuzana Ichniovská for their excellent technical assistance. This study was supported in part by the Slovak Grant Agency (VEGA 1/1095/11), Slovak Research and Development Agency (APVV 0526-11), and internal funds of Bergen University College and the University of Bergen.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Game F.L., Hinchliffe R.J., Apelqvist J., et al. (2012). A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab. Res. Rev. 28Suppl 1, 119–141 [DOI] [PubMed] [Google Scholar]
- 2.Kao H.K., Chen B., Murphy G.F., Li Q., Orgill D.P., and Guo L. (2011). Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann. Surg. 254, 1066–1074 [DOI] [PubMed] [Google Scholar]
- 3.Apelqvist J., and Larsson J. (2000). What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab. Res. Rev. 16Suppl 1, S75–S83 [DOI] [PubMed] [Google Scholar]
- 4.Iversen M.M., Tell G.S., Riise T., et al. (2009). History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care 32, 2193–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iijima K., Shimovama N., Shimovama M., et al. (1989). Effect of repeated irradiation of low-power He-Ne laser in pain relief from postherpetic neuralgia. Clin. J. Pain 5, 271–274 [DOI] [PubMed] [Google Scholar]
- 6.Kreisler M.B., Haj H.A., Noroozi N., et al. (2004). Efficacy of low-level laser therapy in reducing postoperative pain after endodontic surgery—a randomized double-blind clinical study. Int. J. Oral Maxollofac. Surg. 33, 38–41 [DOI] [PubMed] [Google Scholar]
- 7.Nakaji S., Shiroto C., Yodono M., et al. (2005). Retrospective study of adjunctive diode laser therapy for pain attenuation in 662 patients: detailed analysis by questionnaire. Photomed. Laser Surg. 23, 60–65 [DOI] [PubMed] [Google Scholar]
- 8.Toida M., Watanabe F., Goto K., et al. (2003). Usefulness of low-level laser for control of painful stomatitis in patients with handfoot-and-mouth disease. J. Clin. Laser Med. Surg. 21, 363–367 [DOI] [PubMed] [Google Scholar]
- 9.Gál P., Mokrý M., Vidinský B., et al. (2009). Effect of equal daily doses achieved by different power densities of low level laser therapy at 635nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med. Sci. 24, 539–547 [DOI] [PubMed] [Google Scholar]
- 10.Lacjaková K., Bobrov N., Poláková M., et al. (2010). Effects of equal daily doses delivered by different power densities of low-level laser therapy at 670nm on open skin wound healing in normal and corticosteroid-treated rats: a brief report. Lasers Med. Sci. 25, 761–766 [DOI] [PubMed] [Google Scholar]
- 11.Grendel T., Sokolský J., Vaščáková A., et al. (2011). Low-level laser therapy (LLLT) at 830 nm positively modulates healing of tracheal incisions in rats: a preliminary histological investigation. Photomed. Laser Surg. 29, 613–618 [DOI] [PubMed] [Google Scholar]
- 12.Stadler I., Lanzafame R.J., Evans R., et al. (2001). 830-nm irradiation increases the wound tensile strength in a diabetic murine model. Lasers Surg. Med. 28, 220–226 [DOI] [PubMed] [Google Scholar]
- 13.Vasilenko T., Slezák M., Kováč I., et al. (2010). The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670nm on wound tensile strength in rats: a short report. Photomed. Laser Surg. 28, 281–283 [DOI] [PubMed] [Google Scholar]
- 14.Schindl A., Heinze G., Schindl M., et al. (2002). Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc. Res. 64, 240–246 [DOI] [PubMed] [Google Scholar]
- 15.Karu T.I., Afanasyeva N.I., Kolyakov S.F., Pyatibrat L.V., and Welser L. (2001). Changes in absorbance of monolayer of living cells induced by laser radiation at 633, 670 and 820 nm. IEEE J. Quantum Electron. 7, 982–988 [Google Scholar]
- 16.Moore P., Ridgway T.D., Higbee R.G., Howard E.W., and Lucroy M.D. (2005). Effect of wavelength on low-intensity laser irradiationstimulated cell proliferation in vitro. Lasers Surg. Med. 36, 8–12 [DOI] [PubMed] [Google Scholar]
- 17.Wild S., Roglic G., Green A., Sicree R., and King H. (2004). Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053 [DOI] [PubMed] [Google Scholar]
- 18.Dorsett–Martin W.A. (2004). Rat models of skin wound healing: a review. Wound Repair Regen. 12, 591–599 [DOI] [PubMed] [Google Scholar]
- 19.Gál P., Toporcer T., Vidinský B., Hudák R., Živčák J., and Sabo J. (2009). Simple interrupted percutaneous suture versus intradermal running suture for wound tensile strength measurement in rats: a technical note. Eur. Surg. Res. 43, 61–45 [DOI] [PubMed] [Google Scholar]
- 20.Gál P., Kilík R., Mokrý M., et al. (2008). Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: standardization of semi-quantitative and quantitative histological assessments. Vet. Med. (Praha) 53, 652–659 [Google Scholar]
- 21.Peplow P.V., and Baxter G.D. (2012). Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed. Laser Surg. 30, 617–636 [DOI] [PubMed] [Google Scholar]
- 22.Kawalec J.S., Hetherington V.J., Pfennigwerth T.C., Dockery D.S., and Dolce M. (2004). Effect of a diode laser on wound healing by using diabetic and nondiabetic mice. J. Foot Ankle Surg. 43, 214–220 [DOI] [PubMed] [Google Scholar]
- 23.Reddy G.K. (2003). Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg. Med. 33, 344–351 [DOI] [PubMed] [Google Scholar]
- 24.Al-Watban F.A., Zhang X.Y., and Andres B.L. (2007). Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed. Laser Surg. 25, 72–77 [DOI] [PubMed] [Google Scholar]
- 25.Rabelo S.B., Villaverde A.B., Nicolau R., Salgado M.C., Melo Mda S., and Pacheco M.T. (2006). Comparison between wound healing in induced diabetic and nondiabetic rats after low-level laser therapy. Photomed. Laser Surg. 24, 474–479 [DOI] [PubMed] [Google Scholar]
- 26.Langer H., and Lange W. (1992). Comparison of transmission and absorption of HeNe laser and infrared light in human tissue. AKU 20, 19–24 [Google Scholar]
- 27.Joensen J., Ovsthus K., Reed R.K., et al. (2012). Skin penetration time-profiles for continuous 810 nm and Superpulsed 904 nm lasers in a rat model. Photomed. Laser Surg. 30, 688–694 [DOI] [PubMed] [Google Scholar]
- 28.do Nascimento P.M., Pinheiro A.L., Salgado M.A., and Ramalho L.M. (2004). A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats. Photomed. Laser Surg. 22, 513–518 [DOI] [PubMed] [Google Scholar]
- 29.Al-Watban F.A., Zhang X.Y., and Andres B.L. (2007). Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed Laser Surg. 25, 72–77 [DOI] [PubMed] [Google Scholar]
- 30.Vidinský B., Gál P., Toporcer T., et al. (2006). Histological study of the first seven days of skin wound healing in rats. Acta Vet. Brno. 75, 197–202 [Google Scholar]
- 31.Dadpay M., Sharifian Z., Bayat M., Bayat M., and Dabbagh A. (2012). Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J. Photochem. Photobiol. B. 111, 1–8 [DOI] [PubMed] [Google Scholar]
- 32.Dadpay M., Sharifian Z., Bayat M., Bayat M., and Dabbagh A. (2012). Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J. Photochem. Photobiol. B. 111, 1–8 [DOI] [PubMed] [Google Scholar]
- 33.Güngörmüş M., and Akyol U.K. (2009). Effect of biostimulation on wound healing in diabetic rats. Photomed. Laser Surg. 27, 607–610 [DOI] [PubMed] [Google Scholar]