Abstract
Objective: The present study was conducted to investigate the effects of helium-neon (He-Ne) laser irradiation on the proliferation, migration, and differentiation of cultured human epidermal stem cells (ESCs). Background data: A He-Ne laser with a wavelength of 632.8 nm is known to have photobiological effects, and is widely used for accelerating wound healing; however, the cellular mechanisms involved have not been completely understood. Methods: The ESCs were prepared from human foreskin, and irradiated by using He-Ne laser at 632.8 nm with 2 J/cm2. The ESC proliferation, migration, and differentiation were examined by using XTT assay, scratch assay, and flow cytometry technology, respectively. The phosphorylation of extracellular signal-regulated kinases (ERK) was analyzed by using Western blotting. Results: He-Ne laser irradiation markedly promoted cell proliferation and migration accompanied by an increase in the phosphorylation of ERK, but did not significantly influence cell differentiation. Conclusion: Our data indicated that photostimulation with a He-Ne laser resulted in a significant increase in human ESC proliferation and migration in vitro, which might contribute, at least partially, to accelerated wound re-epithelialization by low-level laser therapy.
Introduction
The process of tissue healing is very complex and involves several biological effects, such as vascular and cell changes, epithelial proliferation, fibroblast proliferation, synthesis and deposition of collagen, production of elastin and proteoglycans, revascularization, and wound contraction.1 The incorporation of the laser as a therapeutic tool in the biomedical field has been investigated since 1960.2 It has been observed that laser photostimulation influences macrophage production of growth factors, which increases cell proliferation.3–5 The effect of the laser on living tissue, known as laser photobiomodulation, has been increasingly used with the purpose of improving the quality of wound healing.6 The therapeutic effects of the laser on the different biological types are broad, and include trophic-regenerative, anti-inflammatory, and analgesic effects,7 It has also been demonstrated that tissue regeneration becomes more effective when treated with a low-level laser.8,9 Additionally, it was reported that there are reports that laser irradiation stimulates the release of fibroblast growth factor (FGF) and the replication of these cells.10 Irradiation with a Helium-Neon (He-Ne) laser accelerated the healing process, with a better weave of collagen fibers and greater collagen deposition, combined with faster re-epithelialization and neovascularization.11–14
The re-epithelialization of the wound surface is a critical process in wound healing. The migration and proliferation of keratinocytes contribute to the re-epithelialization of the wound surface. With the current progress in stem cell research, epidermal stem cells (ESCs) have been found to reside in the basal layer of the epidermis, the bulge region of the hair follicle, and the germinal hair follicle matrix, and play an important role in not only self-renewal of the epidermis but also in wound closure.15,16 However, little is known about the effects of laser irradiation on the biological function of ESCs.
Therefore, in the present study, we employed the cultured ESCs prepared from human foreskin, and focused mainly on the effect of He-Ne laser irradiation at 632.8 nm on the proliferation, migration, and differentiation of ESCs. Our results demonstrated that He-Ne laser irradiation could promote ESC proliferation and migration accompanied by an increase in the phosphorylation of ERK, with no change in ESC differentiation, suggesting that the increase in ESC proliferation and migration might contribute to the accelerated the re-epithelialization of wound surface by He-Ne laser therapy.
Materials and Methods
Source of specimens
Foreskin tissue samples were obtained from plastic surgery performed for the circumcision of normal and healthy children. Informed consent was obtained from each individual and their parents, and the study was approved by the Medical and Ethical Committees of the First Affiliated Hospital of Jinan University.
Isolation and culture of human ESCs
Human ESCs were isolated from foreskins as described previously.17,18 Briefly, the epidermal layer of was obtained from foreskin tissue that had been disinfected, after an overnight incubation in 100 mg/L dispase (Roche, Germany) at 4°C. The epidermi were placed for 4 min in a sterile tube containing 0.25% trypsin and ethylenediaminetetraacetic acid (EDTA) with rapid stirring using a pipette, and dissociated into individual cells. The trypsin was inactivated by addition of 10% fetal bovine serum (Invitrogen) in Dulbecco's modified Eagle medium (DMEM), and the cells were centrifuged at 1000 rpm and resuspended in keratinocyte-serum-free medium (K-SFM, 17005-024, Gibco), which was supplemented with 0.25 ng/mL human recombinant epidermal growth factor (rEGF) and 25 μg/mL bovine pituitary extract (BPE). The cells obtained were allowed to adhere for 20 min at 37°C to culture dishes coated with the collagen IV substrates (Sigma). Spent media was exchanged with fresh complete K-SFM medium every 2 or 3 days. The monolayers were dissociated using 0.25% trypsin and EDTA in phosphate-buffered saline (PBS). Second-passage ESCs were washed twice in PBS, resuspended in K-SFM, and seeded at 5×104 cells per well into 3.5 cm Petri dishes. The cells were used immediately for laser irradiation (described subsequently). After laser irradiation, the K-SFM in the culture plates was removed and replaced with fresh culture medium.
Immunofluorescence staining
Cultured cells at the second passage were fixed in 4% paraformaldehyde for immunofluorescent staining using the method described previously.17 Slides were incubated with primary antibodies (mouse monoclonal anti-keratin 19 (K19) antibody (sc-51584) and mouse monoclonal anti-β1-integrin (sc-71388) (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight, washed in PBS, followed by the addition of the secondary antibodies fluorescein (FITC)-conjugated goat anti-mouse immunoglobulins (Jackson Immunoresearch, USA) for detecting K19, and Texas red-conjugated rabbit anti-mouse immunoglobulin for detecting β1-integrin (Jackson Immunoresearch, USA). The sections were extensively washed with 0.01 M PBS between incubations. The stained sections were mounted using a glycerol-based mounting medium (Permafluor; Shandon, Pittsburgh, PA) and stored in the refrigerator. As negative controls, the primary antibodies were omitted, whereas the secondary antibodies were included for the sections. Immunostaining was examined under an Olympus FV1000 confocal laser scanning microscope (Japan).
He-Ne laser irradiation
A He-Ne laser was used at a wavelength of 632.8 nm and a terminal power output of 25 mW for irradiation. The terminal power output was measured using a laser power meter. The laser beam was delivered using an optical fiber, and irradiated a circular area of 1 cm2. Irradiation of the human ESCs was for 80 sec, and the total energy was 2 J/cm2.
Cell proliferation assay
Second passage ESCs were seeded in 200 μL of medium/well in 96 well plates with 5000 cells/well at 37°C under 5% CO2. After randomization, the cells were treated with or without laser irradiation. Cell culture was continued under the same conditions. To detect the activity of ESC proliferation at 1, 3, 5, and 7 days, the cell enzyme-linked immunosorbent XTT Viability assay kit (Genmed, USA) was used according to the manufacturer's instructions. Finally, optical density was determined at 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader.
In vitro wound healing assay
To investigate the effect of He-Ne laser irradiation on the ESC migration, the scratch assay was performed. Cells were seeded in six-well plates at a density of 5×106 cells/mL. After 24 h, a scratch was made through each well using a sterile pipette tip as described previously.19 Then, the cells were treated with or without laser irradiation. The scratches were investigated under the microscope (magnification×100) immediately after irradiation and following cultivation in an incubator (37°C, 5% CO2) for 15 h. Pictures were taken at each time point using a NikonDS-L2 camera (Nikon Instruments Inc. Japan). For data evaluation, wound closure rate was calculated using image analyzing software (NIH image) at the indicated time points. Experiments were performed in triplicate and repeated at least five times.
Flow cytometric analysis of the keratin-10 (K10) expression
Cultured cells at the second passage were processed for K10 staining together with the appropriate negative controls and single color positive controls to establish a compensation setting on for fluorescence-activated cell sorting. Cells were fixed and permeabilized simultaneously in 4% paraformldehyde and 0.3%TritonX-100 in PBS for 10 min at room temperature. Cells were incubated with primary antibody (mouse polyclonal anti-K10 antibody, Abcam ab9025) at 4°C overnight after blocking in 3 mL blocking buffer (10% donkey serum in PBS) for 30 min. Cells were washed twice with 1M PBS and incubated with isotype-specific secondary antibodies (donkey anti-mouse antibody, Invitrogen) for 1 h at room temperature. Finally, the cells were fixed and resuspended at 1×106 cells/L for flow cytometry analysis of expression.20
Western blot analysis
Total proteins were prepared from the cultured human ESCs, and Western blot was performed as previously described.21 Immunoblotting was done using anti-extracellular signal-regulated kinase (ERK), anti-phospho-ERK (Santa Cruz Biotechnology, Santa Cruz, CA).
Data analysis
Values are expressed as mean±SEM in the text and figures. The data were analyzed using ANOVA. If a statistically significant effect was found, post-hoc analysis was performed to detect the difference between the groups. Values of p<0.05 were considered to be statistically significant.
Results
Identification of the cultured ESCs derived from human skin
As shown in Fig. 1A, the isolated cells formed large clones at 7 days after the inoculation, and displayed the typical ESC morphology of small-sized cells with a high nuclear/cytoplasmic ratio. To confirm the undifferentiated state of the cultured human ESCs, we examined K19/β1-integrin expression in the cultured cells from each holoclone. The results from immunofluorescent double labeling showed that the cells were strongly stained for β1-integrin and K19 (Fig. 1B and C), as the putative surface markers for ESCs, indicating that these cells could be ESCs.
FIG. 1.
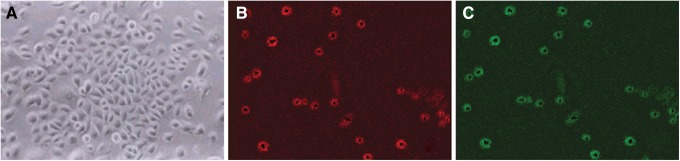
Characterization of cultured human epidermal stem cells (ESCs). (A) Holoclone formation of rapidly adherent cells cultured up to 1 week (inverted phase contrast microscope×200). (B) and (C) Representative double-labeled immunostaining of the holoclone, using the antibodies directed against the mouse β1-integrin and K19 (original magnification×400). Red indicates positive staining for β1-integrin. Blue indicates positive staining for K19.
Effect of He-Ne laser irradiation on the proliferation of human ESCs in vitro
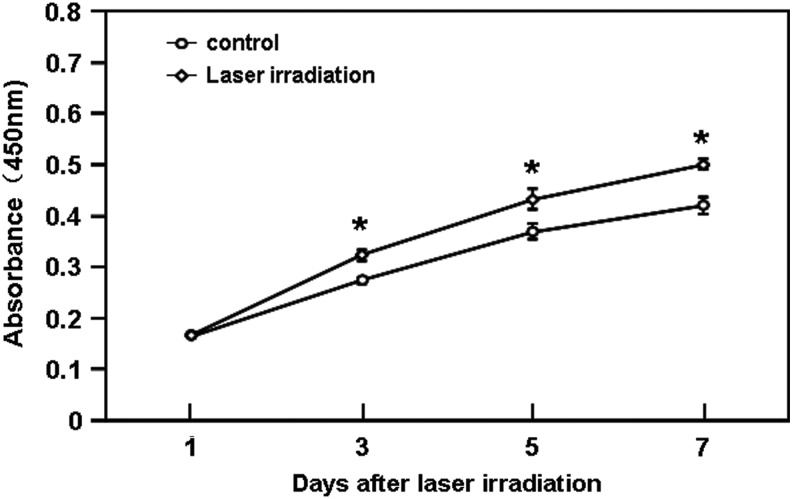
ESC proliferation is essential for achieving cutaneous wound re-epithelialization. To explore the effect of He-Ne laser irradiation on ESC proliferation, XTT assays were performed. As shown in Fig. 2, treatment with He-Ne laser irradiation at 2 J/cm2 markedly promoted the ESC proliferation from day 3 to day 7 after irradiation, when compared with the unirradiated group (p<0.05).
FIG. 2.
Effects of He-Ne laser irradiation on the proliferation of cultured human epidermal stem cells (ESCs). The cells (5000 cells/well) were treated with or without a single exposure to 2 J/cm2 of 632.8 nm laser, and cell proliferation was assessed using the XTT assay as described in the Materials and Methods section. The quantitative data are presented as means±SEM of three independent experiments. Control versus treatment groups, *p<0.05.
Effect of He-Ne laser irradiation on the migration of human ESCs in vitro
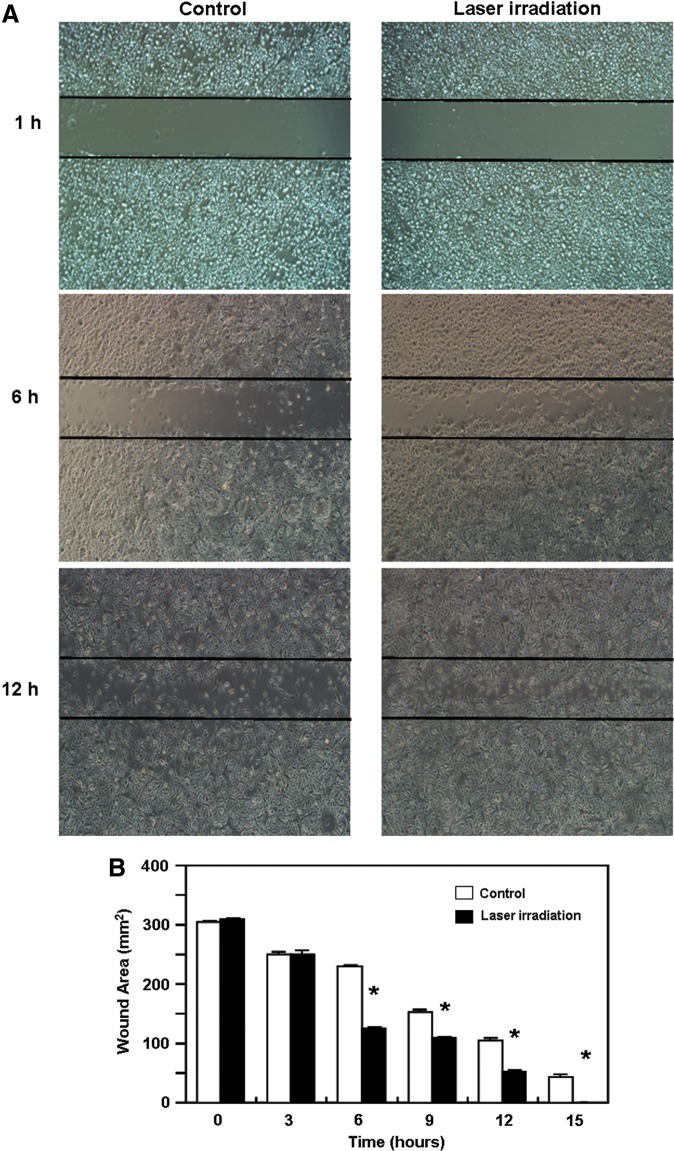
ESC migration plays an important role in epithelial regeneration during wound healing. Therefore, we investigated the effects of He-Ne irradiation on ESC migration and its ability to accelerate the closure of a “wound” that had been created by scratching through a cell monolayer. As shown in Fig. 3A and B, ESC migration was markedly accelerated in the He-Ne laser-treated group at the indicated time points when compared with the unirradiated group (p<0.05), which led to faster “wound closure.”
FIG. 3.
Effect of He-Ne laser irradiation on the migration of cultured human epidermal stem cells (ESCs). The cells were seeded in six-well plates at a density of 5×106 cells/mL, and cell migration assays were performed using a scratch wound assay method as described in the Materials and Methods section. The scratch wounds and the migration cells in the scratch were photographed at the indicated time points after scratching. (A) Represent photographs of the scratch wounds and migration cells in the scratch (original magnification×400). (B) The quantitative data are presented as means±SEM of three independent experiments. Control versus treatment groups, *p<0.05.
Effect of He-Ne laser irradiation on the differentiation of human ESCs in vitro
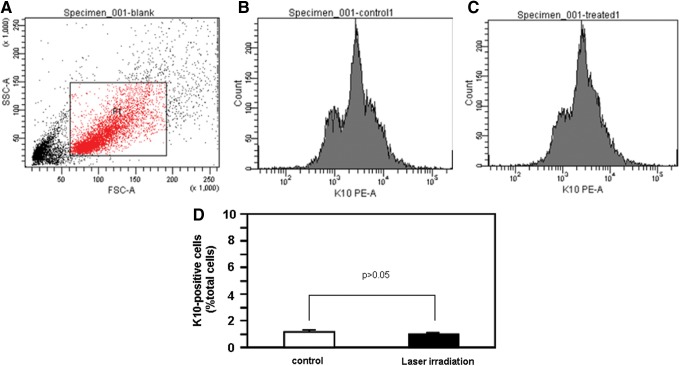
ESCs, which give rise to differentiated keratinocytes, play an important role in wound healing and the maintenance of homeostasis in the epidermis. Therefore, we investigated the effect of He-Ne irradiation on ESC differentiation. The cells were cultured for 5 days after He-Ne laser irradiation, and the number of K10-positive cells was examined as described in the Materials and Methods section, as K10 expression was presumed to identify the differentiation of ESCs into keratinocytes. As demonstrated in Fig. 4, He-Ne laser irradiation did not significantly affect the number of K10-positive cells, suggesting that He-Ne irradiation did not influence ESC differentiation into keratinocytes.
FIG. 4.
Effect of He-Ne laser irradiation on the number of K10-positive cells in cultured human epidermal stem cells (ESCs). The cells were cultured for 5 days after He-Ne laser irradiation, and the number of K10-positive cells was determined as described in the Materials and Methods section. (A) Representative flow cytometric analysis showing background fluorescence intensity. (B) Representative flow cytometric analysis showing K10 staining in the control group. (C) Representative flow cytometric analysis showing K10 staining in the laser-treated group. (D) The quantitative data are presented as means±SEM of three independent experiments.
Effect of He-Ne laser irradiation on the phosphorylation of ERK in cultured human ESCs
To further examine the possible signaling mechanism by which He-Ne laser irradiation effectively increased cell proliferation and migration in cultured human ESCs, we focused on ERK activity, because the ERK pathway is critical for cell growth and migration during wound repair.22 As shown in Fig. 5, treatment with He-Ne laser irradiation at 2 J/cm2 markedly increased the phosphorylation of ERK in cultured human ESCs when compared with the unirradiated group (p<0.05).
FIG. 5.
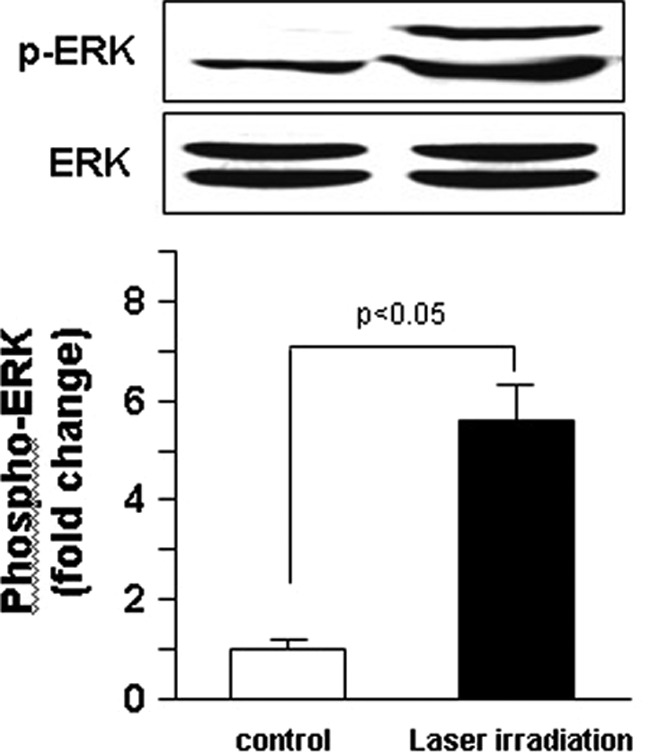
Effect of He-Ne laser irradiation on extracellular signal-regulated kinases (ERK) phosphorylation in cultured human epidermal stem cells (ESCs). Western blotting showing ERK phosphorylation in cultured human ESCs with or without a single exposure to 2 J/cm2 of 632.8 nm He-Ne laser. Results shown are representative data of three separate experiments. Values are fold changes of basal phospho-ERK levels and are expressed as mean±SEM of three independent experiments.
Discussion
Non-healing ulcers represent a significant dermatological problem. Recently, conventional therapy-resistant chronic ulcers have been treated with low-energy lasers, which are recognized as an effective therapeutic method by the United States Food and Drug Administration (FDA), particularly to improve tissue healing.23 This form of treatment has had great appeal because of its novelty, relative ease, and low morbidity profile. Despite its documented therapeutic effects, the regulatory mechanisms imparted by low-energy lasers have not been fully elucidated. In the present study, we demonstrated, for the first time, that He-Ne laser irradiation promoted ESC proliferation and migration. The enhancement of the capacity of ESCs for proliferation and migration by He-Ne laser irradiation might contribute at least partially to the explanation of the promotion of wound closure. Our data further support the beneficial role of low-energy laser therapy in the acceleration of the wound repair processes in skin.
Laser irradiation is regarded to have an important effect in triggering cellular proliferation, differentiation, and apoptosis in various cell types.24 The restoration of cutaneous barrier function by re-epithelialization following wounding is one of the essential events during the wound healing process. Cells participating in the re-epithelialization of wounds include basal and suprabasal keratinocytes lining the wound edge. Among the cells, ESCs are thought to be a main functional cell population, and contribute to wound closure through increased proliferation, migration, and differentiation. Growing evidence has indicated that He-Ne laser irradiation promotes epidermal regeneration possibly by influencing keratinocyte function.25,26 When subconfluent keratinocyte cultures were irradiated three times within 24 h with a He-Ne laser at 0, 0.8, 3, or 7.2 J/cm2, the capacity of keratinocyte migration was enhanced without any change in its differentiation state.27 Yu et al. have reported that low-energy He-Ne laser irradiation stimulated interleukin-1α and interleukin-8 release from cultured human keratinocytes in a concentration-dependent manner, and induced an increase in the rate of keratinocyte migration and proliferation.4 Furthermore, a recent study on the effect of He-Ne laser irradiation on the hair follicle growth cycle of testosterone-treated and untreated Swiss albino mice has showed that laser irradiation led to a significant increase in the percentage of anagen and enhanced the growth cycle of hair follicles, which harbor ESCs, indicating the possible role of He-Ne laser in ESC biological function.28 Consistent with the findings in the cultured keratinocytes, our experimental data demonstrated the enhanced capacity of ESC proliferation and migration by He-Ne laser irradiation, which further supports the notion that He-Ne laser irradiation increases the capacity for epidermal regeneration during wound healing.
The He-Ne laser is a low-energy laser emitting radiation at the visible light spectrum. As the thermal effects associated with low-energy laser irradiation are extremely minute, the physiologic effects imparted by low-energy lasers were attributed to their direct biostimulation of exposed cells.29–31 The cellular mechanisms associated with the stimulatory effects of the He-Ne laser have not been fully elucidated. One possibility may be that the laser energy is absorbed by intracellular chromophores and converted to metabolic energy.31–33 It was shown that cellular adenosine triphosphate (ATP) levels increased almost twofold after He-Ne laser irradiation in cultured HeLa cells.34 Examinations of the mechanisms of ATP production have led to the discovery of a correlation with reactive oxygen species (ROS) production and the currently predicted model of elevated chemiosmotic gradient as the primary effect of light stimulation.35 Furthermore, the effects of the light-induced electrical field on molecular and cell structure provide a general explantation for the observed cellular light effect as reviewed by Amat et al.36 In addition, the He-Ne laser was found to impart significant effects on cellular signal transduction pathways.37 We observed that the increase of ESC proliferation and migration by laser irradiation was accompanied by the enhancement of ERK phosphorylation, suggesting that phototherapy induces ERK phosphorylation in ESCs. The further dissection of the molecular mechanisms involved in the biostimulatory effects of the He-Ne laser on ESCs might assist in initiating a new rational concept for the clinical treatment of non-healing wounds.
Conclusions
The present study has shown that He-Ne laser irradiation increased the capacity for ESC proliferation and migration accompanied by the enhancement of ERK phosphorylation, which might lead to the accelerated re-epithelialization of wounds during healing. Our results provide new evidence for beneficial role of He-Ne laser in wound repair.
Acknowledgments
This work was supported by grant from the National Basic Science and Development Programme, P.R. China (973 Programme, 2012CB518105), the National Nature and Science Foundation, P.R. China (No.30973127, 81272100, 81372065), and the Guangzhou City Science and Technology Project, P.R. China (No.201300000091).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Busnardo V.L., and Biondo-Simões M.L. (2010). Effects of low-level helium-neon laser on induced wound healing in rats. Rev. Bras. Fisioter. 14, 45–51 [PubMed] [Google Scholar]
- 2.England S. (1988). Introduction to mid laser therapy. Physiotherapy. 74, 100–103 [Google Scholar]
- 3.Young S., Bolton P., Dyson M., Harvey W., and Diamantopoulos C. (1989). Macrophage responsiveness to light therapy. Lasers Surg. Med. 9, 497–505 [DOI] [PubMed] [Google Scholar]
- 4.Yu H.S., Chang K.L., Yu C.L., Chen J.W., and Chen G.S. (1996). Low-energy helium neon laser irradiation stimulate interleukin-1 alpha and interleukin-8 release from cultured human keratinocytes. J. Invest. Dermatol. 107, 593–596 [DOI] [PubMed] [Google Scholar]
- 5.Skinner S.M., Gage J.P., Wilce P.A., and Shaw R.M. (1996). A preliminary study of the effects of laser radiation on collagen metabolism in cell culture. Aust. Dent. J. 41, 188–192 [DOI] [PubMed] [Google Scholar]
- 6.David R., Nissan M., Cohen I., and Soudry M. (1996). Effect of low-power He-Ne laser on fracture healing in rats. Lasers Surg. Med. 19, 458–464 [DOI] [PubMed] [Google Scholar]
- 7.Woodruff L.D., Bounkeo J.M., Brannon W.M., et al. (2004). The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed. Laser Surg. 22, 241–247 [DOI] [PubMed] [Google Scholar]
- 8.Hawkins D., Houreld N., and Abrahamse H. (2005). Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann. N.Y. Acad. Sci. 1056, 486–493 [DOI] [PubMed] [Google Scholar]
- 9.Silveira P.C., Streck E.L., and Pinho R.A. (2007). Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J. Photochem. Photobiol. B. 86, 279–282 [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro M.S., Da Silva D., de F, De Araujo C.E., et al. (2004). Effects of low –intensity polarized visible laser radiation on skin burns: a light microscopy study. J. Clin. Laser Med. Surg. 22, 59–66 [DOI] [PubMed] [Google Scholar]
- 11.de Araujo C.E., Ribeiro M.S., Favaro R., Zezell D.M., and Zorn T.M. (2007). Ultrastructural and autoradiographical analysis show a faster skin repair in He-Ne laser-treated wounds. J. Photochem. Photobiol. B. 86, 87–96 [DOI] [PubMed] [Google Scholar]
- 12.Medrado A.R., Pugliese L.S., Reis S.R., and Andrade Z.A. (2003). Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg. Med. 32, 239–244 [DOI] [PubMed] [Google Scholar]
- 13.Carvalho P.T., Mazzer N., dos Reis F.A., Belchior A.C., and Silva I.S. (2006). Analysis of the influence of low-power HeNe laser on the healing of skin wounds in diabetic and non-diabetic rats. Acta Cir. Bras. 21, 177–183 [DOI] [PubMed] [Google Scholar]
- 14.Corazza A.V., Jorge J., Kurachi C., and Bagnato V.S. (2007). Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed. Laser Surg. 25, 102–106 [DOI] [PubMed] [Google Scholar]
- 15.Bickenbach J.R., Stern M.M., Grinnell K.L., Manuel A., and Chinnathambi S. (2006). Epidermal stem cells have the potential to assist in healing damaged tissues. J. Invest. Dermatol. 11, 118–123 [DOI] [PubMed] [Google Scholar]
- 16.Watt F.M., Celso C.L., and Vargas V.S. (2006). Epidermal stem cells: an update. Curr. Opin. Genet. Dev. 16, 518–524 [DOI] [PubMed] [Google Scholar]
- 17.Li A., Simmons P.J., and Kaur P. (1998). Indentification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. U.S.A. 95, 3902–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackie R. and Bickenbach E. C. (1998). Selection and extended growth of murine epidermal stem cells in culture. Exp. Cell Res. 244, 184–195 [DOI] [PubMed] [Google Scholar]
- 19.Chen C.Y., Cheng K.C., Chang A.Y., Lin Y.T., Hseu Y.C., and Wang H.M. (2012). 10-Shogaol, an antioxidant from Zingiber officinale for skin cell proliferation and migration enhancer. Int. J. Mol. Sci. 13, 1762–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franssen M.E., Boezeman J.B., Van De Kerkhof P.C., and Van Erp P.E. (2004). Monitoring hyperproliferative disorders in human skin: flow cytometry of changing cytokeratin expression. Cytometry B. Clin. Cytom. 57, 32–39 [DOI] [PubMed] [Google Scholar]
- 21.Liu H.W., Cheng B., Yu W.L., Sun R.X., Zeng D., Wang J., Liao Y.X., and Fu X.B. (2006). Angiotensin II regulates phosphoinositide 3 kinase/Akt cascade via a negative crosstalk between AT1 and AT2 receptors in skin fibroblasts of human hypertrophic scars. Life Sci. 79, 475–483 [DOI] [PubMed] [Google Scholar]
- 22.Heldin C.H., Ostman A., and Ronnstrand L. (1998). Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1378, F79–F113 [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves W.L., Souza F.M., Conti C.L., et al. (2007). Influence of He-Ne laser therapy on the dynamics of wound healing in mice treated with anti-inflammatory drugs. Braz. J. Med. Biol. Res. 40, 877–884 [DOI] [PubMed] [Google Scholar]
- 24.Bibikova A., and Oron U. (1993). Promotion of muscle regeneration in the toad (Bufo viridis) gastrocnemius muscle by low-energy laser irradiation. Anat. Rec. 235, 374–380 [DOI] [PubMed] [Google Scholar]
- 25.Santuzzi C.H., Buss H.F., Pedrosa D.F., Freire M.O., Nogueira B.V., Gonçalves W.L. (2011). Combined use of low level laser therapy and cyclooxygenase-2 selective inhibition on skin incisional wound reepithelialization in mice: a preclinical study. An. Bras. Dermatol. 86, 278–283 [DOI] [PubMed] [Google Scholar]
- 26.Dixit S., Maiya A., Rao L., Rao M.A., Shastry B.A., and Ramachandra L. (2012). Photobiomodulation by helium neon and diode lasers in an excisional wound model: a single blinded trial. Adv. Biomed. Res. 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rood P.A., Haas A.F., Graves P.J., Wheeland R.G., and Isseroff R.R. (1992). Low-energy helium neon laser irradiation does not alter human keratinocyte differentiation. J. Invest. Dermatol. 99, 445–448 [DOI] [PubMed] [Google Scholar]
- 28.Shukla S., Sahu K., Verma Y., Rao K.D., Dube A., and Gupta P.K. (2010). Effect of helium-neon laser irradiation on hair follicle growth cycle of Swiss albino mice. Skin Pharmacol. Physiol. 23, 79–85 [DOI] [PubMed] [Google Scholar]
- 29.Basford J.R. (1989) Low-energy laser therapy: controversies and new research findings. Lasers Surg. Med. 9, 1–5 [DOI] [PubMed] [Google Scholar]
- 30.Babapour R., Glassberg E., and Lask G.P. (1995). Low-energy laser systems. Clin. Dermatol. 13, 87–90 [DOI] [PubMed] [Google Scholar]
- 31.Belkin M., and Schwartz M. (1989). New biological phenomena associated with laser radiation. Health Phys. 56, 687–690 [DOI] [PubMed] [Google Scholar]
- 32.Conlan M.J., Rapley J.W., and Cobb C.M. (1996). Biostimulation of wound healing by low-energy laser irradiation. A review. J. Clin. Periodontol. 23, 492–496 [DOI] [PubMed] [Google Scholar]
- 33.Karu T. (1999). Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B. 49, 1–17 [DOI] [PubMed] [Google Scholar]
- 34.Karu T., Pyatibrat L., and Kalendo G. (1995). Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J. Photochem. Photobiol. B. 27, 219–223 [DOI] [PubMed] [Google Scholar]
- 35.Prindeze N.J., Moffatt L.T., and Shupp J.W. (2012). Mechanisms of action for light therapy: a review of molecular interactions. Exp. Biol. Med. (Maywood). 237, 1241–1248 [DOI] [PubMed] [Google Scholar]
- 36.Amat A., Rigau J., Waynant R.W., Ilev I.K., and Anders J.J. (2006). The electric field induced by light can explain cellular responses to electromagnetic energy: a hypothesis of mechanism. J. Photochem. Photobiol. B. 82, 152–160 [DOI] [PubMed] [Google Scholar]
- 37.Shefer G, Oron U., Irintchev A., Wernig A., and Halevy O. (2001). Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J. Cell Physiol. 187, 73–80 [DOI] [PubMed] [Google Scholar]