Abstract
Angiotensin II (Ang II) is today considered as one of the essential factors in the pathophysiology of cardiovascular disease, producing acute, hemodynamic and chronic, pleiotropic effects. Although now it is widely accepted that these chronic effects are important, Ang II was initially considered only a short-acting, vasoactive hormone. This view was modified a quarter of a century ago when Dr. Owens and his group published a paper in Circulation Research with initial evidence that Ang II can act as a growth factor that regulates cell hypertrophy. They showed in series of elegant experiments that Ang II promotes hypertrophy and hyperploidy of cultured rat aortic smooth muscle cells. However, Ang II had no effect on hyperplasia. These findings led to a paradigm shift in our understanding of the roles of growth factors and vasoactive substances in cardiovascular pathology and helped to redirect basic and clinical renin-angiotensin system research over the next twenty-five years. Ang II is now known to be a pleiotropic hormone that utilizes multiple signaling pathways to influence most processes that contribute to the development and progression of cardiovascular diseases, ranging from hypertrophy, endothelial dysfunction, cardiac remodeling, fibrosis, and inflammation to oxidative stress.
Keywords: Angiotensin II, Hypertrophy, Growth Factor, ACE inhibitors
The renin-angiotensin system (RAS) is now considered one of the essential factors in the pathophysiology of cardiovascular disease. The main effector of the RAS, angiotensin II (Ang II), contributes to the development of cardiovascular disease as both an endocrine and a local autocrine/paracrine hormone, producing acute (vasoconstriction, water/salt retention) and more importantly chronic (hypertrophy, hyperplasia, oxidative stress, fibrosis, and inflammation) effects. Today it is widely accepted that these chronic effects have a critical role in the development and progression of cardiovascular diseases such as hypertension, atherosclerosis, and heart failure. Initially, Ang II was considered predominantly as a short-acting, vasoactive hormone, and its role in the pathology of cardiovascular disease was thought to result from acute hemodynamic changes. This view was modified a quarter of a century ago when Dr. Owens and his group published a paper in Circulation Research with initial evidence that Ang II can act as a growth factor that regulates cell hypertrophy.1 This article completely changed our perspective on both the pathophysiology of cardiovascular diseases and Ang II’s critical contribution to them, and is the focus of the current commentary.
Research on Ang II as a central component of the RAS began more than a century ago in 1898 with studies conducted by Scandinavian researchers.2 They reported a vasoconstrictor effect of a substance from renal extracts, which they subsequently named renin based on its origin. Interest in the nature of this vasoactive substance released by the kidney was renewed in 1934 when Henry Goldblatt demonstrated that clamping dog renal arteries produced chronic hypertension.3 In the late 1930s, two independent groups in Argentina and the United States utilized the Goldblatt renal ischemia technique to demonstrate secretion of a pressor agent with effects similar to renin.4, 5 This short-acting vasoconstrictor was later identified as an octapeptide product of renin and named Ang.6 Over the next half-century a tremendous amount of research was performed describing in detail the interdependence of the RAS components and the mechanism of action of Ang II, the primary effector molecule of this system.7
Up to this point, research efforts were mainly focused on the role of Ang II as an acute regulator of vasomotor tone. The first FDA-approved ACE inhibitor, captopril, was developed for the treatment of essential hypertension in 1977,8 based on early experimental data on the effect of Ang II on blood pressure in hypertension and chronic heart failure.9 ACE inhibitors proved to be clinically successful in reducing symptoms of hypertension and heart failure,10 despite the fact that there was no experimental evidence indicating that the inhibition of Ang II synthesis is more beneficial than other modalities of antihypertensive therapy. Interestingly, Dr. Owens group was later one of the first to notice a difference in effects between classes of antihypertensive medications.11 However, at that time the RAS was still considered to be an endocrine system in which Ang II had a central role as a potent, short-acting pressor. Furthermore, due to an unfavorable side effect profile of early ACE inhibitors, they were not initially considered as a first choice medication in the treatment of hypertension.12
The dynamics of both basic and clinical RAS research changed in the late 1980’s when evidence first emerged that Ang II can function as a local autocrine and paracrine factor regulating growth of components of the cardiovascular system. Dr. Owens’ seminal paper was the first to show the direct growth effect of Ang II in vascular smooth muscle cells (VSMCs).1 In this article, based on his group’s previous findings of the correlation between hypertension and VSMC hypertrophy13 as well as the differential effectiveness of various antihypertensive drugs on the reversal of hypertrophy11, he postulated that chronic treatment with a contractile agonist such as Ang II can induce hypertrophy of VSMCs. In a series of elegant experiments, the authors proved without doubt that Ang II is an extremely potent inducer of receptor-dependent hypertrophy in VSMCs. This effect was accompanied by the development of hyperploidy, but not hyperplasia, and was fully reversible with a specific Ang II antagonist. Dr. Owens’s group later confirmed these cell culture based results in tissues.14
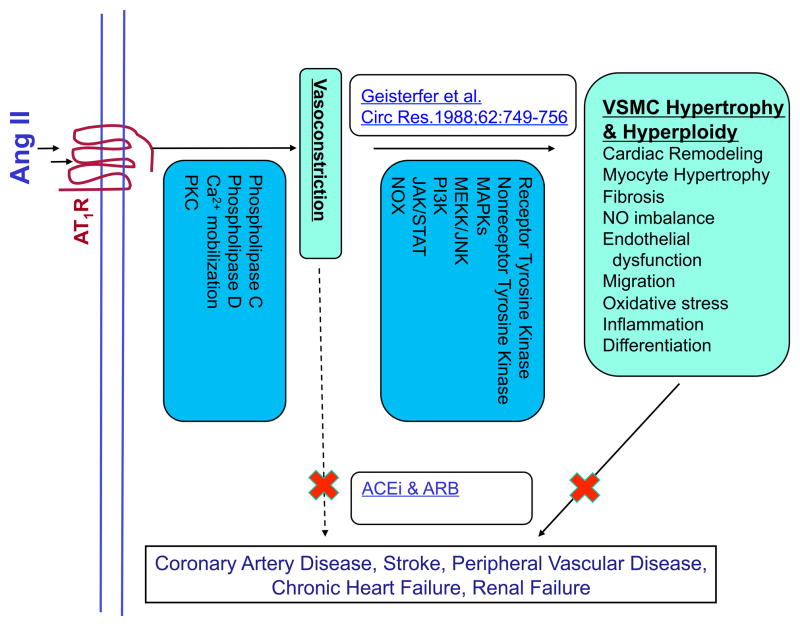
These findings helped to redirect basic and clinical RAS research. Ang II is now known to be a pleiotropic hormone that can influence virtually every process necessary for the development and progression of cardiovascular diseases ranging from hypertension, atherosclerosis, restenosis, and chronic kidney disease to heart failure (Figure 1). Chronic Ang II exposure promotes hypertrophy of VSMCs, phenotype modulation and differentiation, endothelial dysfunction, cardiac remodeling, fibrosis, inflammation, and oxidative stress.1, 15 Dr. Owens’ research efforts in following years showed that Ang II, in addition to hypertrophy, may play an important role in a regulation of differentiation and phenotype switching in VSMCs.16 His group has critically contributed to our understanding of the molecular regulation of expression of VSMCs marker genes. 17–19
Figure 1. Paradigm shift in Angiotensin research: From vasoactive, short acting hormone to pleiotropic growth factor and immunomodulator.
Dr. Owens findings that Ang II is not just an acute vasoactive substance, but also a potent growth factor inspired multiple new studies in different basic research fields from inflammation to tyrosine kinase signaling. These basic research results served as a starting point for clinical research studies that caused a change in therapeutic practices, with ACE inhibitors and ARBs becoming cornerstone of therapy.
Once the vasoactive agonist Ang II was established as growth factor, the converse was also described. Platelet-derived growth factor and epidermal growth factor were found to be vasoconstrictors, suggesting that growth factors and vasoconstrictors shared signaling.20, 21 Similarly, other vasoactive factors, such as thrombin, were shown to have mitogenic effects on VSMCs.22
These novel findings changed the approach to understanding receptor-mediated signaling pathways. As initially Ang II was considered to be a predominantly vasoactive hormone, in the 1980s the majority of signaling research was focused on the stimulation of phospholipase C and Ca2+ mobilization via G protein coupled receptors, as well as activation of phospholipase D and protein kinase C and their effect on smooth muscle contraction.15 However, when Dr. Owens’ paper described the pro-hypertrophic effects of Ang II, basic research in the 1990s shifted to studying the activation of tyrosine kinases, transactivation of receptor tyrosine kinases and activation of NADPH oxidases (Figure 1). In one of the earliest reports, Tsuda et al.23 demonstrated that vasoconstrictors such as Ang II can specifically stimulate the tyrosine phosphorylation of multiple proteins in vascular smooth muscle cells. This early success sparked research efforts that identified numerous additional tyrosine phosphorylated proteins. Of special significance was the discovery of Ang II induced transactivation of receptor tyrosine kinases (epidermal growth factor, platelet-derived growth factor, insulin receptor) and nonreceptor tyrosine kinases (c-Src family kinases, focal adhesion kinase and Janus kinases) leading to phosphorylation and modulation of activity of multiple downstream targets such as mitogen-activated protein kinases (summarized in ref 15). Another important line of research that followed was the signaling mediated by activation of the NADPH oxidases and reactive oxygen species (ROS) synthesis.24 Ang II was initially considered a nonspecific, potent mediator of oxidative stress, but was later shown to utilize ROS as specific second messengers that mediate signaling in different pathways that can contribute to the development of cardiovascular diseases. Ang II was shown to mediate VSMC growth, differentiation, migration, fibrosis and remodeling as a basis of its physiological and pathological roles (Figure 1).
These findings led to a shift in clinical research and ultimately therapeutic approaches. In the 1980s, the focus of clinical research was on the hemodynamic effects of ACE inhibitor therapy25 and its correlation with symptomatic improvement in congestive heart failure and hypertension.26 In the 1990s, after publication of Dr. Owens’ study and based on new basic science findings of the pleiotropic effects of chronic Ang II stimulation beyond its acute hemodynamic effects, the focus of clinical studies changed to the effect of ACE inhibitors on mortality. This is an example of translational research at best. Dr. Owens’ paper created a paradigm shift in our understanding of Ang II’s role in cardiovascular pathology and inspired multiple new basic research studies that served as a starting point for further clinical research. Subsequently ACE inhibitors were shown to promote survival of patients with congestive heart failure, myocardial infarction, coronary artery disease, and hypertension.27–29 It also became clear that they have a renoprotective effect independent of their effects on blood pressure.30 These results confirmed hypothesis originating from basic research that ACE inhibitors, in comparison to other classes of antihypertensive medications, have pleiotropic, disease-modifying effects beyond decreasing blood pressure. This triggered a complete change in therapeutic approaches. Consequently, ACE inhibitors and Ang II receptor blockers have become an indispensable and primary component of cardiovascular disease treatment.31–33
Supplementary Material
Acknowledgments
Sources of funding
The authors’ work is supported by National Institutes of Health grants HL38206, HL092120 and HL058863.
Non-standard Abbreviations and Acronyms
- Ang II
Angiotensin II
- RAS
Renin-angiotensin system
- VSMCs
Vascular smooth muscle cells
- ROS
Reactive oxygen species
Footnotes
Disclosures: None
References
- 1.Geisterfer A, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circulation Research. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 2.Tigerstedt R, Bergman PQ. Niere und kreislauf1. Skandinavisches Archiv Für Physiologie. 1898;8:223–271. [Google Scholar]
- 3.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension : I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasciolo JC, Leloir LF, Munoz JM, Braun-Menendez E. On the specificity of renin. Science. 1940;92:554–555. doi: 10.1126/science.92.2398.554. [DOI] [PubMed] [Google Scholar]
- 5.Page IH, Helmer OM, Kohlstaedt KG, Fouts PJ, Kempf GF. Reduction of arterial blood pressure of hypertensive patients and animals with extracts of kidneys. J Exp Med. 1941;73:7–41. doi: 10.1084/jem.73.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun-Menendez E, Page IH. Suggested revision of nomenclature--angiotensin. Science. 1958;127:242. doi: 10.1126/science.127.3292.242-a. [DOI] [PubMed] [Google Scholar]
- 7.Atlas SA. The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 9.Gavras H, Flessas A, Ryan TJ, Brunner HR, Faxon DP, Gavras I. Angiotensin II inhibition: Treatment of congestive cardiac failure in a high-renin hypertension. JAMA. 1977;238:880–882. doi: 10.1001/jama.238.8.880. [DOI] [PubMed] [Google Scholar]
- 10.Corea L, Bentivoglio M, Verdecchia P. Low-dose captopril therapy in mild and moderate hypertension. Randomized comparison of twice daily vs three times daily doses. Hypertension. 1983;5:III157–159. doi: 10.1161/01.hyp.5.5_pt_2.iii157. [DOI] [PubMed] [Google Scholar]
- 11.Owens GK. Differential effects of antihypertensive drug therapy on vascular smooth muscle cell hypertrophy, hyperploidy, and hyperplasia in the spontaneously hypertensive rat. Circulation research. 1985;56:525–536. doi: 10.1161/01.res.56.4.525. [DOI] [PubMed] [Google Scholar]
- 12.Borer JS. Angiotensin-converting enzyme inhibition: A landmark advance in treatment for cardiovascular diseases. European Heart Journal Supplements. 2007;9:E2–E9. [Google Scholar]
- 13.Owens GK. Influence of blood pressure on development of aortic medial smooth muscle hypertrophy in spontaneously hypertensive rats. Hypertension. 1987;9:178–187. doi: 10.1161/01.hyp.9.2.178. [DOI] [PubMed] [Google Scholar]
- 14.Holycross BJ, Peach MJ, Owens GK. Angiotensin II stimulates increased protein synthesis, not increased DNA synthesis, in intact rat aortic segments, in vitro. Journal of Vascular Research. 1993;30:80–86. doi: 10.1159/000158979. [DOI] [PubMed] [Google Scholar]
- 15.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. American Journal of Physiology Cell Physiology. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 16.Turla MB, Thompson MM, Corjay MH, Owens GK. Mechanisms of angiotensin II- and arginine vasopressin-induced increases in protein synthesis and content in cultured rat aortic smooth muscle cells. Evidence for selective increases in smooth muscle isoactin expression. Circulation Research. 1991;68:288–299. doi: 10.1161/01.res.68.1.288. [DOI] [PubMed] [Google Scholar]
- 17.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of Srf binding to CArG box chromatin regulates smooth muscle gene expression in vivo. The Journal of Clinical Investigation. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T, Hoofnagle MH, Owens GK. Myocardin and prx1 contribute to angiotensin II-induced expression of smooth muscle alpha-actin. Circulation research. 2004;94:1075–1082. doi: 10.1161/01.RES.0000125622.46280.95. [DOI] [PubMed] [Google Scholar]
- 19.Hoofnagle MH, Neppl RL, Berzin EL, Teg Pipes GC, Olson EN, Wamhoff BW, Somlyo AV, Owens GK. Myocardin is differentially required for the development of smooth muscle cells and cardiomyocytes. American Journal of Physiology. Heart and Circulatory Physiology. 2011;300:H1707–1721. doi: 10.1152/ajpheart.01192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merkel L, Rivera L, Colussi D, Perrone M. Inhibition of EGF-induced vasoconstriction in isolated rabbit aortic rings with the tyrosine kinase inhibitor RG50864. Biochemical and Biophysical Research Communications. 1993;192:1319–1326. doi: 10.1006/bbrc.1993.1560. [DOI] [PubMed] [Google Scholar]
- 21.Berk BC, Alexander RW, Brock Ta, Gimbrone MA, Webb RC. Vasoconstriction: A new activity for platelet-derived growth factor. Science. 1986;232:87–90. doi: 10.1126/science.3485309. [DOI] [PubMed] [Google Scholar]
- 22.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, 2nd, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. The Journal of Clinical Investigation. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda T, Kawahara Y, Shii K, Koide M, Ishida Y, Yokoyama M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS letters. 1991;285:44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- 24.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 25.Lejemtel TH, Keung E, Frishman WH, Ribner HS, Sonnenblick EH. Hemodynamic effects of captopril in patients with severe chronic heart failure. The American Journal of Cardiology. 1982;49:1484–1488. doi: 10.1016/0002-9149(82)90365-4. [DOI] [PubMed] [Google Scholar]
- 26.Ader R, Chatterjee K, Ports T, Brundage B, Hiramatsu B, Parmley W. Immediate and sustained hemodynamic and clinical improvement in chronic heart failure by an oral angiotensin-converting enzyme inhibitor. Circulation. 1980;61:931–937. doi: 10.1161/01.cir.61.5.931. [DOI] [PubMed] [Google Scholar]
- 27.Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: Results of the cooperative north scandinavian enalapril survival study (consensus) The American Journal of Cardiology. 1988;62:60A–66A. doi: 10.1016/s0002-9149(88)80087-0. [DOI] [PubMed] [Google Scholar]
- 28.Fox KM Investigators EUtOrocewPiscAd. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 29.van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, Boersma E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: A meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non--insulin-dependent diabetes mellitus: A 7-year follow-up study. Archives of Internal Medicine. 1996;156:286. [PubMed] [Google Scholar]
- 31.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Fagard R, Narkiewicz K, Redan J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F List of authorsTask Force. 2013 practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2013;31:1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 33.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA World Heart Foundation, the Preventive Cardiovascular Nurses Assoc. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.