Abstract
The product of the von Hippel-Lindau (VHL) tumor suppressor gene, the gene inactivated in VHL disease and in sporadic clear-cell renal carcinomas, has recently been shown to have as a functional target the transcription elongation complex, elongin (also called SIII). Here it is shown that there is a tightly regulated, cell-density-dependent transport of VHL into and/or out of the nucleus. In densely grown cells, the VHL protein is predominantly in the cytoplasm, whereas in sparse cultures, most of the protein can be detected in the nucleus. We have identified a putative nuclear localization signal in the first 60 and first 28 amino acids of the human and rat VHL protein, respectively. Sequences in the C-terminal region of the VHL protein may also be required for localization to the cytosol. These findings provide the initial indication of a novel cell density-dependent pathway that is responsible for the regulation of VHL cellular localization.
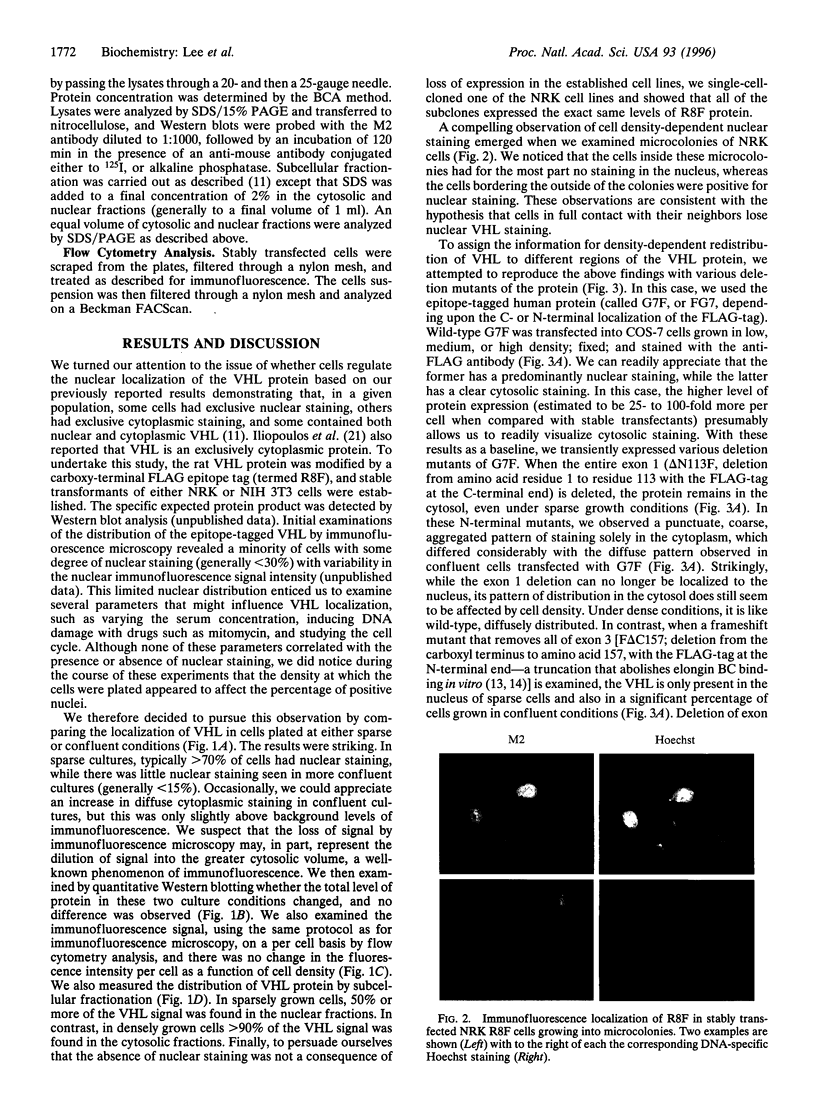
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso T., Lane W. S., Conaway J. W., Conaway R. C. Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science. 1995 Sep 8;269(5229):1439–1443. doi: 10.1126/science.7660129. [DOI] [PubMed] [Google Scholar]
- Boulikas T. Nuclear localization signals (NLS). Crit Rev Eukaryot Gene Expr. 1993;3(3):193–227. [PubMed] [Google Scholar]
- Bradsher J. N., Jackson K. W., Conaway R. C., Conaway J. W. RNA polymerase II transcription factor SIII. I. Identification, purification, and properties. J Biol Chem. 1993 Dec 5;268(34):25587–25593. [PubMed] [Google Scholar]
- Bradsher J. N., Tan S., McLaury H. J., Conaway J. W., Conaway R. C. RNA polymerase II transcription factor SIII. II. Functional properties and role in RNA chain elongation. J Biol Chem. 1993 Dec 5;268(34):25594–25603. [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Kishida T., Yao M., Hustad T., Glavac D., Dean M., Gnarra J. R., Orcutt M. L., Duh F. M., Glenn G. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat. 1995;5(1):66–75. doi: 10.1002/humu.1380050109. [DOI] [PubMed] [Google Scholar]
- Crossey P. A., Richards F. M., Foster K., Green J. S., Prowse A., Latif F., Lerman M. I., Zbar B., Affara N. A., Ferguson-Smith M. A. Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet. 1994 Aug;3(8):1303–1308. doi: 10.1093/hmg/3.8.1303. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Duan D. R., Humphrey J. S., Chen D. Y., Weng Y., Sukegawa J., Lee S., Gnarra J. R., Linehan W. M., Klausner R. D. Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6459–6463. doi: 10.1073/pnas.92.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D. R., Pause A., Burgess W. H., Aso T., Chen D. Y., Garrett K. P., Conaway R. C., Conaway J. W., Linehan W. M., Klausner R. D. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995 Sep 8;269(5229):1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- Foster K., Prowse A., van den Berg A., Fleming S., Hulsbeek M. M., Crossey P. A., Richards F. M., Cairns P., Affara N. A., Ferguson-Smith M. A. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994 Dec;3(12):2169–2173. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- Gao J., Naglich J. G., Laidlaw J., Whaley J. M., Seizinger B. R., Kley N. Cloning and characterization of a mouse gene with homology to the human von Hippel-Lindau disease tumor suppressor gene: implications for the potential organization of the human von Hippel-Lindau disease gene. Cancer Res. 1995 Feb 15;55(4):743–747. [PubMed] [Google Scholar]
- Garrett K. P., Aso T., Bradsher J. N., Foundling S. I., Lane W. S., Conaway R. C., Conaway J. W. Positive regulation of general transcription factor SIII by a tailed ubiquitin homolog. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7172–7176. doi: 10.1073/pnas.92.16.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett K. P., Tan S., Bradsher J. N., Lane W. S., Conaway J. W., Conaway R. C. Molecular cloning of an essential subunit of RNA polymerase II elongation factor SIII. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5237–5241. doi: 10.1073/pnas.91.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra J. R., Tory K., Weng Y., Schmidt L., Wei M. H., Li H., Latif F., Liu S., Chen F., Duh F. M. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994 May;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Herman J. G., Latif F., Weng Y., Lerman M. I., Zbar B., Liu S., Samid D., Duan D. S., Gnarra J. R., Linehan W. M. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Weinberg R. A. Tumor suppressor genes. Curr Opin Genet Dev. 1994 Feb;4(1):135–141. doi: 10.1016/0959-437x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O., Kibel A., Gray S., Kaelin W. G., Jr Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995 Aug;1(8):822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Karin M., Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995 Jul 1;5(7):747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- Kibel A., Iliopoulos O., DeCaprio J. A., Kaelin W. G., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995 Sep 8;269(5229):1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- Knudson A. G. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Linehan W. M., Lerman M. I., Zbar B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer. JAMA. 1995 Feb 15;273(7):564–570. [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J., Riordan J. F. Nuclear translocation of angiogenic proteins in endothelial cells: an essential step in angiogenesis. Biochemistry. 1994 Oct 25;33(42):12535–12539. doi: 10.1021/bi00208a001. [DOI] [PubMed] [Google Scholar]
- Odenwald W. F., Taylor C. F., Palmer-Hill F. J., Friedrich V., Jr, Tani M., Lazzarini R. A. Expression of a homeo domain protein in noncontact-inhibited cultured cells and postmitotic neurons. Genes Dev. 1987 Jul;1(5):482–496. doi: 10.1101/gad.1.5.482. [DOI] [PubMed] [Google Scholar]
- Rihs H. P., Jans D. A., Fan H., Peters R. The rate of nuclear cytoplasmic protein transport is determined by the casein kinase II site flanking the nuclear localization sequence of the SV40 T-antigen. EMBO J. 1991 Mar;10(3):633–639. doi: 10.1002/j.1460-2075.1991.tb07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs H. P., Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989 May;8(5):1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuin T., Kondo K., Torigoe S., Kishida T., Kubota Y., Hosaka M., Nagashima Y., Kitamura H., Latif F., Zbar B. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994 Jun 1;54(11):2852–2855. [PubMed] [Google Scholar]
- Tan S., Aso T., Conaway R. C., Conaway J. W. Roles for both the RAP30 and RAP74 subunits of transcription factor IIF in transcription initiation and elongation by RNA polymerase II. J Biol Chem. 1994 Oct 14;269(41):25684–25691. [PubMed] [Google Scholar]







