Abstract
OBJECTIVES
Intravenous catheter placement is one of the most common sources of pain for children in inpatient settings. We sought to compare the efficacy of two cryotherapeutic treatments for this procedure: vapocoolant spray versus topical ice-pack.
METHODS
We prospectively enrolled 95 patients, age 9–18 years, in a pediatric emergency department who required IV catheters as part of their treatment. Subjects were randomly assigned to receive vapocoolant spray, or topical ice-pack for three minutes, prior to IV catheter placement. Subjects completed visual analog scale (VAS) scores for three time points: baseline, pre-treatment with ice or spray, and IV insertion. The principal investigator, and two physicians viewing video recordings of the procedure, also completed VAS scores for observed pain levels. VAS scores were compared using the Wilcoxon Rank Sum test.
RESULTS
Although median VAS scores were similar, the change in VAS from baseline was of greater magnitude in the Painease® group, indicating that it may be more effective. More subjects in the Painease® group (76%) felt their treatment worked well, compared to 49% in the ice group. Physician-assigned VAS scores were lower and less variable than those of subjects. Most IV insertions were successful (83%).
CONCLUSIONS
Vapocoolant spray may be more effective than ice as an analgesic for IV insertion. Subjects were more satisfied with vapocoolant spray. Neither agent caused a decrease in successful IV insertion rates.
Keywords: ice, vapocoolant, Painease®, intravenous catheter, pain
INTRODUCTION
Intravenous (IV) catheter placement is one of the most common sources of pain for pediatric patients (1), as well as a significant source of distress for the child and their family. (2) Topical creams, intradermal injections, and specialized anesthetic delivery systems have all been utilized to alleviate pain, (3) however no single method has achieved universal acceptance in pediatric emergency departments. Each method possesses different advantages and disadvantages for its use and convenience in the emergency setting.
Ice has been used for centuries as a topical pain reliever. It works by several mechanisms, including slowing conduction of peripheral nerve fibers, promoting sensory competition, and decreasing release of inflammatory and nociceptive mediators. (4) It is cheap, readily available, and achieves skin anesthesia relatively quickly. Although not widely used for procedural pain, ice has been utilized as an analgesic for subcutaneous and intramuscular injections with favorable results. (5, 6) It has been evaluated for IV catheter placement in adult volunteers (7), but has not yet been studied in a pediatric population. Similarly, vapocoolant sprays (such as ethyl chloride and newer halogenated compounds) are cryotherapeutic agents used for short, painful procedures. Although proven effective for intramuscular injections (8), literature has been conflicting on whether vapocoolant sprays are also effective for IV catheter placement. (9–16)
In light of the controversy surrounding vapocoolant sprays for IV catheter placement, as well as the safety and other potential benefits of ice, we sought to compare the efficacy of these two methods. Because the two agents share a similar mechanism of action, we hypothesized that the median change in VAS from baseline to IV placement would be similar between the two groups.
MATERIALS AND METHODS
Patients aged 9 to 18 years were prospectively enrolled in a pediatric emergency department if their care required IV placement. The study was designed as a convenience sample. Patients were excluded if they were in moderate to severe pain prior to IV placement. Moderate pain was defined as a score >2 (“hurts a little more”) on a Wong-Baker Faces scale, as assessed by the treating physician and/or principal investigator. (17) Patients were also excluded if they had significant developmental, cognitive, or motor delays preventing them from completing a VAS score. Additional exclusion criteria were the presence of sickle cell disease (due to risk of vaso-occlusive crisis with ice), prior adverse skin reaction to vapocoolant spray, parenteral narcotic injection within six hours of enrollment, or need for emergent IV access due to patient illness.
Data were collected from the patient’s family and the medical record regarding presence of chronic medical conditions, reason for ED visit and IV placement, use of narcotic pain medicine in the preceding 6 months, treatment with acetaminophen or ibuprofen within the past 6 hours, and the number of prior IV catheters the patient had received in their lifetime.
Patients were randomized to receive either vapocoolant spray (Painease® medium stream spray, Gebauer Company, Cleveland, Ohio) or topical ice-pack prior to IV catheter insertion. Painease® is a topical anesthetic refrigerant composed of 1,1,1,3,3-pentafluoropropane and 1,1,1,2-tetrafluoroethane liquid. It was administered according to the manufacturer’s instructions, spraying from a distance of 8 to 18 centimeters for 4 to 10 seconds or until the skin turned white, whichever was sooner. (18) Ice treatment consisted of approximately 250 mL of crushed ice and water slurry within a plastic re-sealable bag applied to the skin for 3 minutes prior to IV insertion. Pediatric emergency medicine nurses with extensive experience in pediatric venous access placed all IV catheters. The PI consented, enrolled, and explained the VAS to each subject. Patients filled out a VAS score for three time points: pain at baseline, treatment with Painease® or ice, and actual IV placement (the moment the needle pierced the skin). Baseline pain was assessed by asking “How much pain are you in as you sit here now?” This was assessed 15–30 minutes prior to IV catheter placement, and was intended to assess pain from the subject’s acute medical condition, from chronic pain, or from any other source besides the IV. Based on observation of the patient at the three time points, the PI also assigned VAS scores for each subject. Subjects were videotaped, and two independent physician observers (DL and VW) reviewed all video recordings. Each of these physicians assigned a VAS score for the moment of IV placement.
If the subject’s first IV attempt failed, they received the opposite treatment for their second IV attempt. The subject completed a VAS score for the second IV attempt and was asked which treatment he/she preferred. The PI and physician observers also assigned VAS scores for these events. After the IV was placed, nurses were asked to complete a form noting the catheter size and location, the degree of difficulty for IV placement, and whether they thought that ice or Painease® pre-treatment affected their ability to visualize the vein or successfully achieve IV catheter placement.
Statistical Analysis
Sample size calculation was based on prior studies (6, 7, 13, 14), and was estimated to be 90 patients (45 in each group) to detect a 15 millimeter difference in change in VAS score from baseline with 80% power and significance level of 5%. Estimates of the minimum clinically significant difference in VAS score range from 13 mm to 17 mm, and thus a midpoint of 15 mm was chosen. (19, 20) All data analyses were performed using SAS® software Version 9.2 (Copyright 2008, SAS Institute Inc.). Patient baseline characteristics were compared using Chi-square and Fisher’s exact test for independent categorical variables. Median VAS scores were compared using the Wilcoxon Rank Sum test. Because neither ice nor vapocoolant spray is a universally accepted gold standard of therapy, we did not design this study as an equivalence trial. Instead, we compared the median change in VAS (ΔVAS) from baseline to IV insertion with each method.
RESULTS
Ninety-five patients were enrolled in the study between August 2010 and February 2011. Fifty subjects were randomly assigned to receive ice, 45 received Painease® spray. Baseline characteristics for the two groups are listed in Table 1. The two groups were similar in age, gender, ethnicity, and frequency of chronic medical conditions. The mean age of all patients was 13.7 years, and the majority of patients were Hispanic. The most common reason for ED visit was abdominal pain (37%). A significant number of patients had a chronic medical condition (43%), for which many of them had received more than 25 IVs in their lifetime (14%). However a large percentage of subjects (37%) had never had an IV before their enrollment in this study. None had received oral or IV narcotics on the day of the study. One patient had received intranasal fentanyl approximately one hour before the study. Since the intranasal route of this medication was not listed as an exclusion criterion (its use in our emergency department began after study enrollment had begun), this patient was included in the study.
Table 1.
Baseline Characteristics
| Painease® n=50 |
Ice n=45 |
Total n=95 |
|
|---|---|---|---|
| Mean age (yrs) | 13.7 | 13.7 | 13.7 |
| Gender | |||
| Male | 20 (40%) | 20 (44%) | 40 (42%) |
| Female | 30 (60%) | 25 (56%) | 55 (58%) |
| Ethnicity | |||
| Hispanic | 36 (72%) | 33 (73%) | 69 (73%) |
| Caucasian | 5 (10%) | 6 (13%) | 11 (12%) |
| African-American | 4 (8%) | 3 (7%) | 7 (7%) |
| Other | 5 (10%) | 3 (7%) | 8 (8%) |
| Chronic Medical Condition | 23 (46%) | 18 (40%) | 41 (43%) |
| Reason for Visit | |||
| Abdominal pain | 20 (40%) | 15 (33%) | 35 (37%) |
| Fracture | 4 (8%) | 3 (7%) | 7 (7%) |
| Fever | 3 (6%) | 1 (2%) | 4 (4%) |
| Other | 23 (46%) | 26 (58%) | 49 (52%) |
| Reason for IV Placement | |||
| Lab draw | 32 (64%) | 29 (64%) | 61 (64%) |
| IV fluid | 7 (14%) | 8 (18%) | 15 (16%) |
| IV antibiotic | 4 (8%) | 4 (9%) | 8 (9%) |
| Procedural sedation | 3 (6%) | 1 (2%) | 4 (4%) |
| Other | 4 (8%) | 3 (7%) | 7 (7%) |
| Number of IVs lifetime | |||
| 0 | 19 (38%) | 16 (35%) | 35 (37%) |
| 1–3 | 13 (26%) | 7 (16%) | 20 (21%) |
| 4–10 | 7 (14%) | 11 (24%) | 18 (19%) |
| 11–25 | 2 (4%) | 7 (16%) | 9 (9%) |
| >25 | 9 (18%) | 4 (9%) | 13 (14%) |
| Acetaminophen or Ibuprofen in last 6 hrs | 19 (38%) | 16 (35%) | 35 (37%) |
| Narcotics in last 6 months | 5 (10%) | 4 (9%) | 9 (10%) |
Median VAS scores (and 25%–75% interquartile ranges) are listed in Table 2. Of note, one subject was not able to complete a VAS score due to subtle learning delays and difficulty with spatial-motor tasks that became apparent only after enrollment. This patient was not included in the VAS score data, but was included in the nursing assessment regarding IV placement and satisfaction.
Table 2.
Comparison of Median VAS (25,75 IQR) between Subject, Investigator, and Video Reviewers
| Painease® n=49 |
Ice n=45 |
p value | |
|---|---|---|---|
| Subject VAS-S | |||
| Baseline | 35 (2, 69) | 22 (0, 62) | p=0.18 |
| Treatment | 4 (1, 19) | 15 (2, 32) | p=0.13 |
| IV Placement | 9 (2, 24) | 13 (3, 42) | p=0.08 |
| Investigator VAS-I | |||
| Baseline | 4 (2, 11) | 3 (0, 5) | p=0.0398 |
| Treatment | 1 (0, 3) | 3 (0, 11) | p=0.0477 |
| IV Placement | 2 (0, 7) | 4 (2, 16) | p=0.0086 |
| Video Reviewers * | |||
| IV Placement | 0.5 (0, 7) | 2 (0, 6.5) | p=0.25 |
| Subject Δ VAS-S | |||
| Δ Treatment - Baseline | −20 (−60, 1) | 0 (−28, 10) | p=0.0356 |
| Δ IV - Baseline | −20 (−56, 0) | 0 (−38, 19) | p=0.0127 |
| Δ IV - Baseline | 0 (−8, 9) | 2 (−11, 12) | p=0.77 |
For video reviewers Painease® n=34, Ice n=32
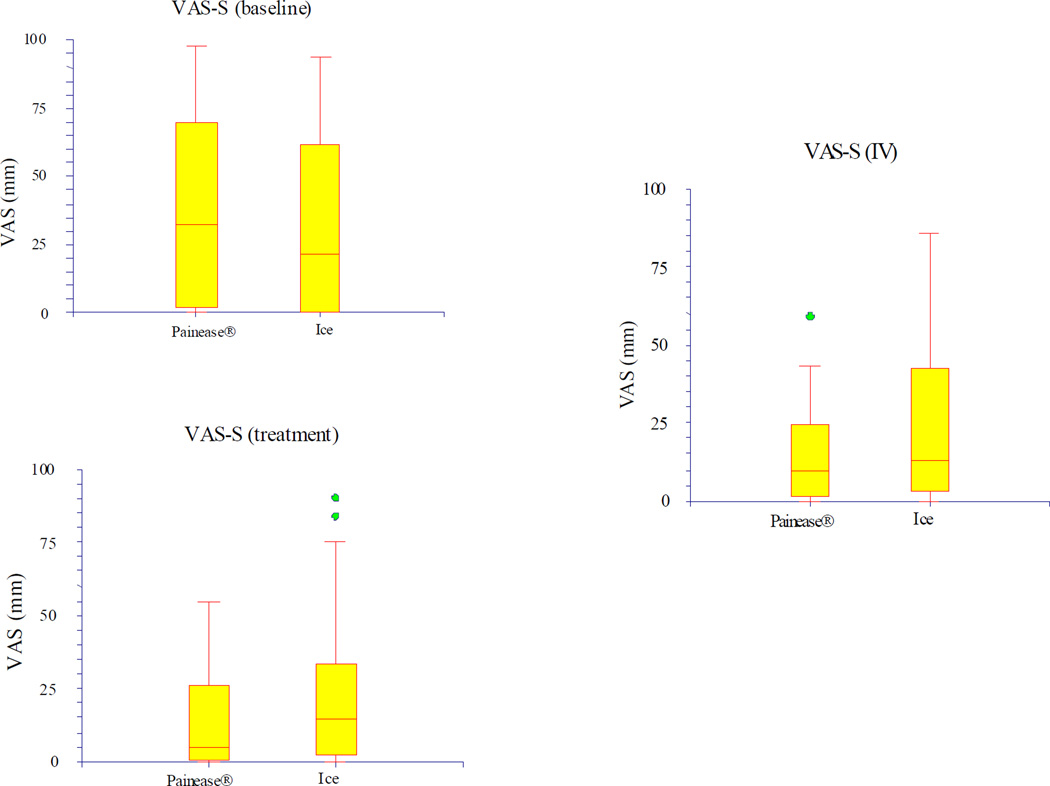
The median VAS score assigned by subjects (VAS-S) at baseline, treatment, and with IV placement did not differ by a statistically significant amount between the two groups. For Painease®, the VAS-S was 35 mm (IQR 2, 69) at baseline, and 9 mm (IQR 2, 24) with IV placement. For ice, the VAS-S was 22 mm (IQR 0, 62) at baseline, and 13 mm (IQR 3, 42) with IV placement. Of note, the interquartile ranges reflect the wide variability of pain rankings, as well as the large number of low rankings (25% of subjects were less than 3 mm). Figure 1 illustrates the median VAS-S and IQR for both groups at the three time points.
Figure 1.
VAS-subject (VAS-S)
Differences in subjects' VAS scores between two different time points (ΔVAS-S) are also listed in Table 2. Although the individual VAS scores did not differ between groups, the ΔVAS-S did achieve statistical and clinical significance in some cases. This was most notable in the ΔVAS-S for treatment minus baseline and IV minus baseline, which were −20 for Painease®, and 0 for ice, with p< 0.05 for this difference between groups.
The median VAS scores assigned by the PI (VAS-I) were significantly lower than those rated by subjects. The median VAS-I for the Painease® group was 4 mm (IQR 2, 11) at baseline, and 2 mm (IQR 0, 7) for IV placement. For ice, the median VAS-I was 3 mm (IQR 0, 5) at baseline, and 4 mm (IQR 2, 16) for IV placement. These were statistically significance differences, but none of the differences reached the 15 mm level of clinical significance.
Sixty-six video recordings were reviewed and assigned a VAS score per protocol (29 were excluded due to poor video quality). Fifty-three of these videos were successfully blinded for treatment group. Thirteen were non-blinded (due to inadvertent factors which may have indicated to the viewer which treatment the patient had received). The median VAS scores for independent video reviewers are listed in Table 2. As with the VAS-I scores, the medians are significantly lower and less variable than the VAS-S scores assigned by subjects. There was not a statistically significant difference between the two groups’ VAS scores at IV placement.
Patient satisfaction data is listed in Table 3. Overall, 76% of subjects who had received Painease® thought that it worked “well”, versus 49% of subjects who had received ice. This difference was statistically significant (p=0.0167). In addition, a greater number of patients who had received Painease® (90%) would want that treatment again, versus 69% of patients who had received ice. Parents’ approval tended to correlate with subjects; however, there were no statistically significant differences detected. Nine subjects had a failed first attempt at an IV, and crossed over into the opposite treatment group for their second IV. Of these, six patients preferred Painease®, and three preferred ice.
Table 3.
Patient Satisfaction
| Painease® n=50 |
Ice n=45 |
p value | |
|---|---|---|---|
| Worked well | 38 (76%) | 22 (49%) | p=0.0167 |
| Worked a little bit | 10 (20%) | 16 (36%) | |
| Did not work at all | 2 (4%) | 7 (16%) | |
| Subject would want again | 45 (90%) | 31 (69%) | p=0.0192 |
| Parent would want again | 45 (90%) | 34 (76%) | p=0.09 |
For video reviewers Painease® n=34, Ice n=32
Information collected from nurses is listed in Table 4. Ninety-three RNs successfully filled out data sheets. Overall, 83% of IV catheters were successfully placed on the first attempt, and 70% of all IVs were classified as “easy” to start. The two groups were similar with regards to the size and location of IV catheters placed, the proportion of successful placements on first attempt, and the difficulty in placing the IV. Despite this, some RNs (31% in the Painease® group, 23% in the ice group) felt that IV placement was more difficult due to cryotherapeutic analgesia, and 26% overall expressed a negative concern, citing potential vasoconstriction and effects on visibility of veins.
Table 4.
Nursing Data and Satisfaction
| Painease® n=49 |
Ice n=45 |
p value | |
|---|---|---|---|
| IV location | |||
| Hand | 29 (59%) | 24 (55%) | p=0.66 |
| Antecubital Fossa | 17 (35%) | 15 (34%) | |
| Other | 3 (6%) | 15 (34%) | |
| IV Size | |||
| #24 G | 4 (8%) | 2 (5%) | p=0.65 |
| #22 G | 38 (78%) | 36 (82%) | |
| #20 G | 6 (12%) | 5 (11%) | |
| #18 G | 0 (0%) | 1 (2%) | |
| IV Success on first attempt | 41 (84%) | 36 (82%) | p=1.0 |
| Difficulty | |||
| Easy | 36 (74%) | 29 (66%) | p=0.82 |
| Slightly Difficult | 7 (14%) | 7 (16%) | |
| Moderately Difficult | 1 (2%) | 2 (4%) | |
| Unsuccessful | 5 (10%) | 6 (14%) | |
| Was the IV more difficult than if nothing had been used? (% Yes) | 15 (31%) | 10 (23%) | p=0.48 |
DISCUSSION
We compared ice and vapocoolant spray as cryotherapeutic analgesics for IV catheter placement in children. Although pain scores at the moment of IV placement (as rated by subjects, investigators, and independent reviewers) were similar between the two groups, Painease® was associated with a larger magnitude and more negative change in pain level from baseline. This does not imply that Painease® was able to reduce a subject’s baseline level of pain. Rather, our interpretation of these results is that a greater number of patients in the ice group assigned a higher VAS to ice pre-treatment, or receiving an IV, than was assigned at baseline. This shifted the ΔVAS (IV minus baseline, and treatment minus baseline) towards a positive number, and indicates that in this measure, Painease® was a more effective analgesic for this procedure. A greater proportion of subjects in the Painease® group felt that their treatment worked “well” and that they would prefer it for future IV placement. Most IV starts were successful and easily placed by participating nurses, however a significant number of nurses expressed concern regarding the use of cryotherapeutic agents.
Numerous analgesic agents are available for IV placement. Topical formulations, such as EMLA® or LMX®, are effective but require long application times. (21–23) Intradermal injections of an anesthetic (such as lidocaine) have been proven effective in alleviating pain (15, 24), however the need for multiple needle injections has limited their use in younger patients. Newer delivery systems, such as J-Tip® or ultrasound-assisted anesthetic delivery, are likely to be fast and effective (25, 26), but are more costly and not universally available.
The use of cold to produce anesthesia dates back thousands of years. (4) Both ice and vapocoolant sprays achieve anesthesia by lowering the temperature of the surrounding skin. This drop in temperature decreases nerve conduction velocity of C and A-delta fibers, thus dampening the transmittance of pain signals. (18) Kuwuhara, et al. (5), compared EMLA, ice, and no anesthesia in a group of 20 adult volunteers prior to an intradermal injection of lidocaine. The authors found that while EMLA was superior to ice, ice was superior to no anesthetic (p<0.01). Sixty percent of subjects felt there was no difference in performance between EMLA and ice. In another study, Yoon, et al. compared vapocoolant spray to ice for an intradermal antibiotic skin test. (6) They found that 84% of subjects felt ice to be more effective, versus only 2% preferring vapocoolant spray.
In one of the only studies to look at ice for IV catheter placement, Richman et al., compared ice to no treatment in 28 healthy adult volunteers receiving both techniques. (7). The authors found a lower median VAS score with ice (27 mm) than with no pre-treatment (37.5 mm). This difference was not statistically significant. In their study, 39% of subjects felt that ice was more painful than IV catheter placement. Because of this finding, our study design included a significantly shorter application time for ice: 3 minutes versus 10 minutes (in their study). To date, our study is the first to evaluate ice as a topical analgesic for IV catheter placement in a pediatric population.
Our results have several interesting implications. First, although VAS scores for the moment of IV insertion were similar between the two groups, subjects in the Painease® group had a larger and more negative change in their VAS score compared to baseline. A greater number in the Painease® group also felt that their treatment worked “well” and would prefer it for future IV placement. This could be due to actual effect, or could be due to a potential bias in our study. Subjects may have perceived vapocoolant spray as a more “novel” therapy, and because it is not available at home, more “effective” than ice. A second interesting outcome in our study regards nursing satisfaction with the two treatments. Drop in skin temperature, whether by ice or vapocoolant spray, is well known to cause peripheral vasoconstriction. In prior studies, actual success rates at IV placement do not seem to be affected by pre-treatment with a skin coolant. (11, 15, 27) In our study, nurses were able to exceed the average success rate for pediatric IV catheter insertion, which is 53–76% on first attempt (28, 29), even in a population with many chronic illnesses and multiple past IVs. However, a significant proportion of nurses also felt that the IV was harder to start than if nothing was used, and many of them had concerns about visualization of the vein.
Limitations
Our study has several limitations. First, the rating of physical pain is inherently difficult for children who have difficulty separating anxiety and fear from physical pain. We attempted to lessen this difficulty by choosing older children (age 9–18 years) who might be less anxious and more able to assess physical pain independently, and we used a pain scoring system which has been well validated in this age group. Despite all these efforts, fear and anxiety were clearly present, especially in the younger subjects in this study. Including a Child Life specialist or another means of distraction might have had a significant effect on our results, but would have added other confounding variables.
Although we attempted to estimate a sample size large enough to detect a clinical difference based on previous studies, we found that subjects’ pain ratings in our study were not normally distributed and were highly variable. This made the confidence intervals in our study large, and speaks to the inherent difficulties in pain research in children. Although we attempted to lessen the subjects’ variability by having the PI explain all VAS scores in depth, and in the same way, to each patient, our subjects’ pain scores remained variable. The difference in the two groups’ pain at baseline (35 mm versus 22 mm) did not exceed our 15 mm level of clinical significance, but it did approach it. We decided a priori to look at differences in VAS scores (ΔVAS) between time points in order to account for this possibility. It is possible that with much higher numbers, or a smaller clinical outcome measure, a clinical or statistical difference may have been found.
Lastly, there are logistical and other practitioner-dependent variables which limited this study. Such factors include speed in catheter placement, the need to re-adjust or “fish” for the vein, and practitioner-dependent comforting of the patient during IV catheter placement. This study included older patients, many with chronic diseases and who had difficult IV access. Due to inherent variability between centers, our results may not be generalizable to younger patients or different patient populations.
CONCLUSIONS
In this study, we compared ice and vapocoolant spray as topical analgesic agents for IV catheter placement in children 9–18 years of age. Although median pain scores at IV insertion were similar between the two groups, Painease® resulted in a larger magnitude and more negative change from baseline, and may be a more effective analgesic. More patients in the Painease® group were satisfied with their therapy and would prefer it for future IVs. Despite nurses’ concerns regarding vasoconstriction, IV insertion success rates were above average, and most IVs were easily placed.
Acknowledgments
This work was supported in part by Grant Number 1UL1RR031986, Children’s Hospital Los Angeles Clinical Translational Science Institute, with funds provided by the National Center for Research Resources (NCRR), NIH.
Abbreviations
- IV
intravenous
- ED
emergency department
- VAS
Visual Analog Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest or corporate sponsors.
REFERENCES
- 1.Cummings EA, Graham JR, Finley GA, et al. Prevalence and source of pain in pediatric inpatients. Pain. 1996;68:25–31. doi: 10.1016/S0304-3959(96)03163-6. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey GB, Boon CM, Chiquit van Linden van den Huevell GF, et al. The Occurrence of High Levels of Acute Behavioral Distress in Children and Adolescents Undergoing Routine Venipunctures. Pediatrics. 1992;90(1):87–91. [PubMed] [Google Scholar]
- 3.Zempsky WT. Pharmacologic Approaches for Reducing Venous Access Pain in Children. Pediatrics. 2008;122:S140–S153. doi: 10.1542/peds.2008-1055g. [DOI] [PubMed] [Google Scholar]
- 4.Ernst E, Fialka V. Ice Freezes Pain? A Review of the Clinical Effectiveness of Analgesic Cold Therapy. Journal of Pain and Symptom Management. 1994;9:56–59. doi: 10.1016/0885-3924(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 5.Kuwuhara R, Skinner R. EMLA Versus Ice as Topical Anesthetic. Dermatologic Surgery. 2001;27:495–496. doi: 10.1046/j.1524-4725.2001.00343.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoon WY, et al. Analgesic pretreatment for antibiotic skin test: vapocoolant spray vs. ice cube. The American Journal of Emergency Medicine. 2008;26:59–61. doi: 10.1016/j.ajem.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Richman PB, Singer AJ, Flanagan M, et al. The Effectiveness of Ice as a Topical Anesthetic for Insertion of Intravenous Catheters. The American Journal of Emergency Medicine. 1999;17(3):255–257. doi: 10.1016/s0735-6757(99)90119-5. [DOI] [PubMed] [Google Scholar]
- 8.Reis EC, Holubkov R. Vapocoolant Spray is Equally Effective as EMLA Cream in Reducing Immunization Pain in School-aged Children. Pediatrics. 1997;100:e5. doi: 10.1542/peds.100.6.e5. [DOI] [PubMed] [Google Scholar]
- 9.Selby IR, Bowles BJ. Analgesia for venous cannulation: a comparison of EMLA (5 minutes application), lignocaine, ethyl chloride, and nothing. Journal of the Royal Society of Medicine. 1995;88:264–267. [PMC free article] [PubMed] [Google Scholar]
- 10.Davies EH, Molloy A. Comparison of ethyl chloride spray with topical anesthetic in children experiencing venipuncture. Paediatric Nursing. 2006;18:39–43. doi: 10.7748/paed2006.04.18.3.39.c8136. [DOI] [PubMed] [Google Scholar]
- 11.Farion KJ, Splinter KL, Newhook K, et al. Effect of Vapocoolant Spray on Pain Due to IV Cannulation in Children: a Randomised Controlled Trial. Canadian Medical Association Journal. 2008;179(1):31–36. doi: 10.1503/cmaj.070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijazi R, Taylor D, Richardson J. Effect of topical alkane vapocoolant spray on pain with IV cannulation in patients in emergency departments: randomized double blind placebo controlled trial. British Medical Journal. 2009;338:b215–b220. doi: 10.1136/bmj.b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsook C, Kozinetz CA, Moro-Sutherland D. Efficacy of ethyl chloride as a local anesthetic for venipuncture and intravenous cannula insertion in a pediatric emergency department. Pediatric Emergency Care. 2001;17(5):341–343. doi: 10.1097/00006565-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Costello M, Ramundo M, Christopher NC, et al. Ethyl Chloride Vapocoolant Spray Fails to Decrease Pain Associated with Intravenous Cannulation in Children. Clinical Pediatrics. 2006;45:628–632. doi: 10.1177/0009922806291013. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong P, Young C, McKeown D. Ethyl Chloride and venipuncture pain: a comparison with intradermal lidocaine. Canadian Journal of Anesthesia. 1990;37:656–658. doi: 10.1007/BF03006485. [DOI] [PubMed] [Google Scholar]
- 16.Hartstein BH, Barry JD. Mitigation of pain during intravenous catheter placement using a topical skin coolant in the emergency department. Emergency Medical Journal. 2008;25:257–261. doi: 10.1136/emj.2006.044776. [DOI] [PubMed] [Google Scholar]
- 17.Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in Pediatric Emergency Department Patients. Academic Emergency Medicine. 2010;17(1):50–54. doi: 10.1111/j.1553-2712.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 18.Gebauer Company. Painease® Technical Data Sheet. [Accessed on June 7, 2011]; Available at: http://gebauer.com/getmedia/6ddc613b-142f-44cb-ac1f-b399cfb48854/PETS_ENG.aspx. [Google Scholar]
- 19.Todd KH, Funk KG, Funk JP, et al. Clinical Significance of Reported Changes in Pain Severity”. Annals of Emergency Medicine. 1996;27(4):485–489. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 20.Mark MSM, Au TTS, et al. The minimum clinically significant difference in visual analogue scale pain score in a local emergency setting. Hong Kong Journal of Emergency Medicine. 2009;16(4):233–236. [Google Scholar]
- 21.Eichenfield LF, Funk A, Fallon-Friedlander S, et al. A clinical study to evaluate the efficacy of ELA-Max (4% liposomal lidocaine) as compared with eutectic mixture of local anesthetics cream for pain reduction in venipuncture in children. Pediatrics. 2002;109(6):1093–1099. doi: 10.1542/peds.109.6.1093. [DOI] [PubMed] [Google Scholar]
- 22.Friedman PM, Mafong EA, Friedman ES. Topical Anesthetics Update: EMLA and Beyond. Dermatologic Surgery. 2001;27:1019–1026. doi: 10.1046/j.1524-4725.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- 23.Koh JH, Harrison D, Myers R, et al. A randomized, double-blind comparison study of EMLA and ELA-Max for topical anesthesia in children undergoing intravenous insertion. Paediatric Anaesthesia. 2004;14(12):977–982. doi: 10.1111/j.1460-9592.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 24.Luhmann J, Hurt S, Shootman M, Kennedy R. A comparison of buffered lidocaine versus ELA-Max before peripheral intravenous catheter insertions in children. Pediatrics. 2004;113(3):e217–e220. doi: 10.1542/peds.113.3.e217. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez N, Bradford H, Seidel KD, et al. A comparison of a needle free injection system for local anesthesia versus EMLA for intravenous catheter insertion in the pediatric patient. Anesthesia and Analgesia. 2006;102(2):411–414. doi: 10.1213/01.ane.0000194293.10549.62. [DOI] [PubMed] [Google Scholar]
- 26.Zempsky WT, Robbins B, McKay K. Reduction of Topical Anesthetic Onset Time Using Ultrasound: A Randomized Controlled Trial Prior to Venipuncture in Young Children. Pain Medicine. 2008;9(7):795–802. doi: 10.1111/j.1526-4637.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 27.Page DE, Taylor DM. Vapocoolant spray vs. subcutaneous lidocaine injection for reducing the pain of intravenous cannulation: a randomized, controlled, clinical trial. British Journal of Anesthesia. 2010;105(4):519–525. doi: 10.1093/bja/aeq198. [DOI] [PubMed] [Google Scholar]
- 28.Rauch D, Dowd D, Eldridge D, et al. Peripheral Difficult Venous Access in Children. Clinical Pediatrics. 2009;48(9):895–901. doi: 10.1177/0009922809335737. [DOI] [PubMed] [Google Scholar]
- 29.Lininger RA. Pediatric peripheral I.V. insertion success rates. Pediatric Nursing. 2003;29(5):351–354. [PubMed] [Google Scholar]