Abstract
Sex differences in ultradian activity rhythms (URs) and circadian rhythms (CRs) were assessed in Siberian hamsters kept in long day (LD) or short day (SD) photoperiods for 40 weeks. For both sexes URs of locomotor activity were more prevalent, greater in amplitude and more robust in SDs. The UR period was longer in females than males in both day lengths. The reproductive system underwent regression and body mass declined during the initial 10 weeks of SD treatment, and in both sexes these traits spontaneously reverted to the LD phenotype at or before 40 weeks in SD, reflecting the development of neuroendocrine refractoriness to SD patterns of melatonin secretion. Hamsters of both sexes, however, continued to display SD-like URs at the 40 weeks time point. CRs were less prevalent and the waveform less robust and lower in amplitude in SDs than LDs; the SD circadian waveform also did not revert to the long-day phenotype after 40 weeks of SD treatment. Short day lengths enhanced ultradian and diminished circadian rhythms in both sexes. Day length controls several UR characteristics via gonadal steroid and melatonin-independent mechanisms. Sex differences in ultradian timing may contribute to sex diphenisms in rhythms of sleep, food intake and exercise.
Keywords: Photoperiod, Seasonality, Circadian, Photorefractoriness
1. Introduction
Despite well-established ultradian rhythms (URs) in sleep [1], food consumption [2,3], water intake [4], locomotor activity [5], heart rate [6] and body temperature [7], the substrates that mediate behavioral URs have rarely been investigated. A Web of Science search for the years 1990–2012, coupling the search terms “hormones” and “behavior” with either “ultradian” or “circadian”, yielded 52 and 5364 citations, respectively, evidence of the relative neglect of this class of biological rhythms. The neuroendocrine mechanisms that subserve URs are poorly understood and the existence and nature of sex differences in behavioral URs, if any, remain unspecified.
Robustness, amplitude, and presence of locomotor activity URs vary as a function of the stages of the Syrian hamster estrous cycle [8]. The period (τ′), amplitude and complexity of male Siberian hamster locomotor URs increases when day lengths fall below 13 h light/day, which is associated with testicular regression [9] and marked reductions in blood androgen concentrations [10]. These observations raised the possibility that gonadal steroids influence ultradian behavior rhythms. However, gonadectomy alone did not alter the UR waveform of male hamsters housed in long days; testosterone and estradiol were likewise without effect on male locomotor URs [11], suggesting that the marked seasonal changes in the UR waveform occur largely independently of concurrent changes in gonadal hormones. It remains unknown, however, whether this conjecture applies to females; thus, a principal aim of this investigation was to evaluate URs in locomotor activity in intact female Siberian hamsters housed in long and short day lengths and to determine whether URs are similar or differ in females and males.
In Siberian hamsters, melatonin-dependent responses to short days (e.g., changes in body mass, pelage molt, reproductive and immune function) become refractory to extended (>20 weeks) short-day patterns of melatonin secretion, and exhibit ‘spontaneous’ reversion to the long-day phenotype. Despite the prominent role of melatonin in mediating mammalian physiological responses to changes in photoperiod [12], several aspects of the UR waveform response to short days are unaffected by extirpation of the pineal gland. UR τ′, for instance, increases from 2 h in long days to 4 h in short days, independent of pineal status [11]. In contrast, short-day induced decreases in UR mesor activity levels are counteracted by pinealectomy; short day modulation of the amplitude of the UR waveform is governed by both pineal-dependent and -independent mechanisms. These relations were established after 12 weeks of short day treatment of male Siberian hamsters [11]. It remains unknown whether the UR waveform eventually becomes refractory to short days. Short-day changes in UR τ′ could spontaneously revert to the long day phenotype after 40 weeks of treatment, suggesting melatonin-dependent control of seasonal timing. Alternatively, failure of prolonged short-day exposure to trigger the long-day phenotype would establish that URs do not become refractory to short days, and imply that the prevailing photoperiod, rather than melatonin, controls seasonal changes in the UR waveform. The present study evaluated these hypotheses.
2. Materials and methods
2.1. Animals and housing
Siberian hamsters (Phodopus sungorus) from a breeding colony maintained at the University of Chicago on a light:dark cycle of 15L:9D (15L; lights off at 17:00 CST) were housed in polypropylene cages (28×17×12 cm) on wood shaving bedding (Harlan Sani-Chips, Harlan Inc., Indianapolis, IN, USA). Ambient temperature was 20±0.5 °C, and relative humidity 53±2%. Food (Teklad Rodent Diet 8604, Harlan Inc.), filtered tap water and cotton nesting material were continuously available in the cages. All procedures conformed to the USDA Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Chicago (Protocols 71383 and 71443). The experiments were conducted at the University of Chicago.
2.2. Photoperiods
Adult male and female hamsters, 60–90 days of age, from the 15L:9D breeding colony (n = 106) were housed singly. On week 0, hamsters were transferred to a short day (SD) photoperiod (9L:15D; lights off at 18:00 h CST), where they remained for the next 40 weeks (SD40 group); other groups remained in the 15L photoperiod for 40 weeks (LD group) or for 30 weeks, at which time they were transferred to SDs for the final 10 weeks of the experiment (SD10 group). Final sample sizes were: LD: n = 11 females, 13 males; SD40: n = 25 females, and 24 males; SD10: n = 12 females, and 16 males.
2.3. Somatic and reproductive measures
On weeks 10, 20, 28 and 40, hamsters were weighed (±0.1 g) and reproductive status assessed under methoxyflurane anesthesia. These time points were selected so as to capture the temporal dynamics of somatic and behavioral changes during the development of refractoriness, and to allow sufficient time for refractoriness/recrudescence to manifest. The length and width of the left testis were measured (±0.1 mm). The product of testis width squared times testis length provides a measure of estimated testis volume (ETV) highly correlated (R>0.9) with testis weight [13]. Vaginal patency was determined using a blunt 2 mm stainless steel probe. Stage in the pelage color cycle was assessed using an integer scale of 1–4 (1 = dark ‘summer’ fur, 4 = white ‘winter’ fur; [14]) without knowledge of the animal's treatment condition.
Males that failed to exhibit gonadal regression (ETV>400) and females with patent vaginas after 10 weeks of SD treatment (i.e., at week 10 for SD40 hamsters; at week 40 for SD10 hamsters), that also did not exhibit a winter pelage (fur score = 1), were identified as photoperiod nonresponders (6 females and 6 males) and omitted from all subsequent analyses.
2.4. Activity measurements
We measured URs and CRs of spontaneous general locomotor activity – a non-food-specific behavior that correlates highly with daily rhythms of sleep-wakefulness, body temperature, and drinking behavior [15]. URs of locomotor activity correlate positively with feeding rhythms [5] and permit detailed analysis of underlying ultradian timing systems. Locomotor activity was monitored in the home cage between weeks 9–11 (“week 10”), weeks 23–27 (“week 25”), and weeks 38–40 (“week 40”) for a minimum of 10 consecutive days with passive infrared motion detectors (Coral Plus, Visonic, Bloomfield, CT) positioned 22 cm above the cage floor that registered activity when 3 of 27 zones were crossed. Activity triggered closure of an electronic relay, recorded by a computer running ClockLab software (Actimetrics, Evanston, IL). Cumulative activity counts were collected at 6 min intervals.
2.5. Activity analyses
2.5.1. Ultradian rhythms (URs)
Activity data collected at 6 min intervals were parsed into light-phase only activity (150 or 90 data points/24 h) and dark-phase only activity (90 or 150 data points/24 h) files. The number of photophases and scotophases sampled was adjusted to provide 900 data points for each hamster to equalize statistical power in periodogram analyses from different photoperiods. For LD hamsters, 10 consecutive nights and 6 consecutive days generated dark-phase and light-phase activity files, each with 900 data points. For SD hamsters, 6 nights and 10 days were collated. Successive days of scotophase- and photophase-specific activity data were concatenated into separate files subjected to Lomb–Scargle periodogram (LSP) and cosinor periodogram analyses, as described in detail elsewhere [8].
2.5.2. Circadian rhythms (CRs)
Unparsed files (240 data points/24 h) 10 days in length were subjected to LSP and cosinor periodogram analyses.
2.6. Statistical analyses
Quantitative parameters of UR and CR waveforms were determined using LSP and cosinor analyses, as described previously [8]. LSP analyses [16] identified the statistical presence/absence of URs and CRs, and the number of significant peaks in the UR spectrum (range: 0.3–7.9 h). The level of statistical significance was set to 0.01. Cosinor analyses determined several quantitative measures of locomotor URs (range: 0.3–7.9 h) and CRs (range: 22–26 h): robustness (or ‘prominence’, the percent of variance accounted for by the best-fit cosine model, which corresponds to the coefficient of determination R2 in regression analyses; [17]); mesor (rhythm-adjusted mean value around which the waveform oscillates); amplitude (the difference between the peak or trough value and the mesor), expressed as absolute values (activity counts) and relative values referenced to the photophase-specific mesor value to incorporate baseline activity levels during each photophase in determining rhythm amplitude. Acrophase was computed as the average time relative to the onset or offset of light at which the waveform peaks. The level of statistical significance was set to 0.05.
The LSP optimizes detection of URs by not displaying peaks at multiples of all rhythms detected [18,19]. Supplemental analyses after completion of LSP analysis [20] were adopted as recommended by Refinetti et al. [17], hence the cosinor periodogram [21], a preferred curve-fitting tool to quantify rhythm parameters [17], was used.
Analyses of variance (ANOVAs) and post-hoc pairwise comparisons were performed with Statview 5.0 (SAS Institute, Cary, NC, USA) and LSP and cosinor analyses with software written by R. Refinetti (available at http://www.circadian.org/softwar.html; [17]). Effects of day length on reproductive measures and body mass were assessed by ANOVA and effects of day length on fur color with Kruskal–Wallis tests (H scores), followed by Mann–Whitney U tests. The proportion of hamsters displaying URs and CRs was evaluated with chi-square and Fisher's exact tests (FET). Quantitative aspects of URs and CRs were examined with ANOVA. Pairwise comparisons were performed with Fisher's PLSD tests or unpaired, two-tailed t tests. Differences were considered significant if P≤0.05.
3. Results
3.1. Reproductive and somatic responses to photoperiod
3.1.1. Males
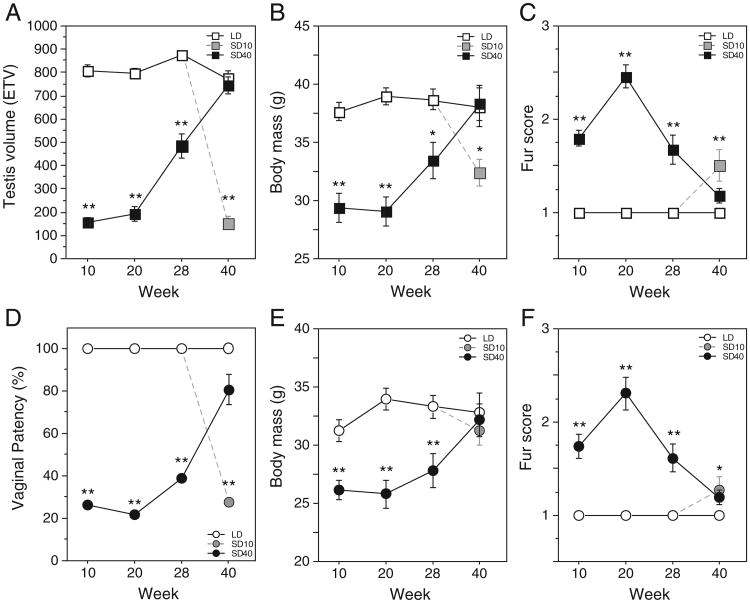
Photoperiod treatments affected testis volume (F>41.8, P<0.001). Males housed in SDs for 40 weeks (SD40 group) exhibited gonadal regression by week 10 (Fig. 1A), and recrudescence beginning between weeks 20 and 28. ETV values of SD40 hamsters were lower than those of LD controls on weeks 10, 20 and 28 (P<0.001, all comparisons) but by week 40 were comparable to those of LD hamsters (P>0.50; Fig. 1A), indicative of complete testicular recrudescence. Males transferred from LDs to SDs on week 30 exhibited a significant decrease in ETV by week 40 (SD10, P<0.001 vs. LD group, Fig. 1A). The pattern of body mass (F>3.3, P<0.05, Fig. 1B) and fur color changes (weeks 10–28: U>14.5, P<0.001; week 40: H = 8.9, P<0.05; Fig. 1C) mirrored that for the testes, with attainment of the short-day phenotype by week 10–20, followed by recovery of the LD phenotype by week 40.
Fig. 1.
Responses to long and short day lengths. Mean±SEM estimated testis volume (A) male body mass (B), male fur color (C) vaginal patency (D), female body mass (E) and female fur color (F) of hamsters maintained in LDs for 40 wks (LD), SDs for 40 wks (SD40) or transferred to SDs for 10 weeks beginning at wk 30 (SD10). *P<0.05 vs LD and **P<0.005 vs. LD.
3.1.2. Females
Photoperiod affected vaginal patency (F> 12.3, P<0.001). SD treatments reduced the proportion of SD40 hamsters with patent vaginas on weeks 10, 20, and 28(P<0.001, all comparisons; Fig. 1D). Vaginal patency was restored in >80% of these females at week 40 (P>0.10 vs LD females), indicative of restoration of the LD vaginal phenotype. Transfer from LDs to SDs on week 30 resulted in non-patent vaginas in >75% of females 10 weeks later (SD10, P<0.001 vs. LD, Fig. 1D).
Similar SD patterns were evident for body mass (F> 10.1, P<0.005; Fig. 1E) and fur color (U> 52, P<0.001, all time points; Fig. 1F) through week 28. On week 40, the body mass of SD40 hamsters did not differ from that of LD females (P>0.50), completing the reversion to the LD phenotype; fur scores of SD40 females on week 40 declined from peak values at week 20 but remained significantly higher than those of LD females. (U = 48, P = 0.05). The SD10 females had initiated the transition to the winter pelage after 10 weeks of treatment.
3.2. Ultradian responses to photoperiod
3.2.1. Males
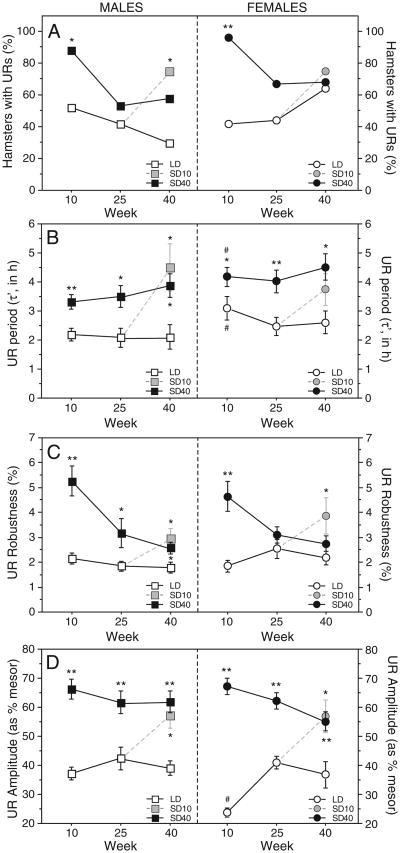
URs were evident in a greater proportion of SD than LD hamsters (Fig. 2A). On week 10, 52% (15/29) of LD and 88% (21/24) of SD40 hamsters exhibited dark-phase URs (χ2 = 7.7, P<0.01); this difference was no longer significant on week 25 (χ2 = 0.4, P>0.5). On week 40, URs were evident in 30% (4/13) of LD compared to 75% (12/16) of males transferred to SD on week 30 (χ2 = 5.7, P<0.05 vs. LD), and 58% (14/24) of SD40 hamsters (χ2 = 2.6, P<0.10 vs. LD).
Fig. 2.
Sex differences in UR prevalence, period, robustness and amplitude. Percent hamsters of each sex exhibiting URs in SDs (filled black symbols) and LDs (open white symbols) (A). Mean±SEM period (τ′) (B), robustness (C) and amplitude (D) of the dark-phase general locomotor activity waveform. The shaded symbols at week 40 represent values of hamsters transferred from LDs to SDs at wk 30 (SD10). *P≤0.05 vs LD; **P≤0.005 vs. LD; # P≤0.05 vs. male value within same photoperiod.
The period (τ′) of dark-phase URs was longer in SD than LD males on weeks 10 (F = 11.0, P<0.005), 25 (F = 7.7, P<0.01) and 40 (F = 4.9; P<0.05; Fig. 2B). Transfer from LDs to SDs on week 30 significantly lengthened τ′ over the next 10 weeks (P<0.01, SD10 vs. LD).
UR robustness was significantly greater in SD40 relative to LD hamsters on weeks 10 (F = 26.3, P<0.001), 25 (F = 4.4, P<0.05) and 40 (F = 3.8, P<0.05, Fig. 2C); significant increases in UR robustness also were evident in hamsters 10 weeks after transfer to SD on week 30 (SD10 vs LD, P<0.05).
UR amplitude was markedly enhanced in SD40 compared to LD males on week 10 (F = 51.8, P<0.001), week 25 (F = 11.7, P<0.001) and week 40 (F = 9.2, P<0.001; Fig. 2D); this SD-enhancement was also evident in males transferred from LDs to SDs on week 30 (SD10 vs LD; P<0.01; Fig. 2D).
3.2.2. Females
On week 10, 42% of LD and 96% of SD40 hamsters exhibited dark-phase URs (χ2 = 17.7, P<0.001; Fig. 2A). This effect of SD was not significant on week 25 (χ2 = 2.5, P>0.1). On week 40, URs were evident in 64% LD and 75% of hamsters transferred to SD on week 30 (χ2 = 0.4, P>0.50 vs. LD), and 68% (17/25) of SD40 hamsters (χ2 = 0.1, P>0.70 vs. LD).
Dark-phase τ′ was significantly longer in SD40 than LD females on week 10 (F = 4.1, P<0.05) week 25 (F = 9.6, P<0.005) and week 40 (P = 0.01; Fig. 2B). Transfer from LDs to SDs on week 30 resulted in an increase in τ′ that was not statistically significant (P = 0.10; Fig. 2B).
UR robustness was significantly greater on week 10 in SD40 relative to LD females (F = 21.4, P<0.001; Fig. 2C) but this enhancement no longer was evident after 25 (F = 1.7, P>0.20) and 40 weeks (F = 2.9, P>0.05)of SD treatment; LD to SD transfer on week 30 increased UR robustness 10 weeks later (SD10 vs. LD, P<0.05; Fig. 2C).
UR amplitude was greater in SD40 than LD hamsters at all 3 time points (week 10: F = 189, P<0.001; week 25: F = 35.8, P<0.001; week 40: F = 5.7, P<0.01; Fig. 2D). Persistence of increased UR amplitude in SD40 females on week 40 indicates failure to reestablish the LD phenotype after 40 weeks in SD. Transfer from LD to SD on week 30 acutely increased UR amplitude (SD10 vs. LD females, P<0.01; Fig. 2D).
3.3. Ultradian sex differences
There was a main effect of sex on τ′ on week 10 (F = 8.3, P<0.005): τ′s were longer in females than males in both LD and SD40 groups on week 10 (P<0.05, both comparisons, Fig. 2B). Also on week 10, sex and photoperiod interacted to affect UR amplitude (F = 8.0, P<0.01): amplitude was comparable in males and females in SDs (P>0.70), but significantly lower in LD females than LD males (P<0.001; Fig. 2D).
3.4. Circadian responses to photoperiod
3.4.1. Males
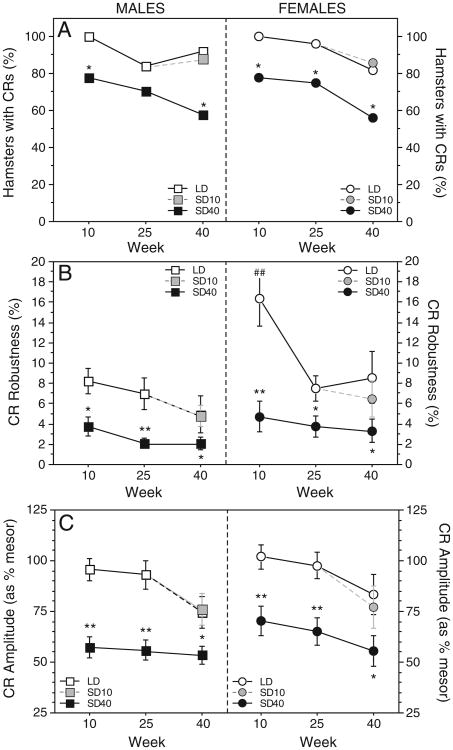
After 10 weeks of treatment, 78% of SD40 hamsters compared to 100% of LD males displayed CRs (FET: P<0.05; Fig. 3A). This photoperiod effect also was significant on week 25, when 84% of LD and 53% of SD hamsters exhibited CRs (χ2 = 4.26, P<0.02). On week 40, significant CRs were evident in 58% of SD40 hamsters, 92% of LD males and 88% of hamsters transferred to SDs on week 30 (χ2 > 3.9, P<0.05, SD40 vs. each of the other groups).
Fig. 3.
Effects of long and short day lengths on prevalence, robustness and amplitude of circadian rhythms in male and female hamsters. Prevalence (A), mean±SEM robustness (B), and amplitude (C) of circadian general locomotor activity waveforms of hamsters in SDs (filled black symbols) and LDs (open white symbols). The shaded symbols at week 40 represent values of hamsters transferred from LD to SD at wk 30 (SD10). *P≤0.05 vs LD and **P≤0.005 vs. LD value. ## P≤0.005 vs. male value within same photoperiod.
CRs were more robust in LD than SD40 hamsters on week 10 (F = 8.3, P<0.01) and week 25 (F = 9.6, P<0.005; Fig. 3B). On week 40 robustness was greater in LD than SD40 hamsters (P<0.05; Fig. 3B) but did not differ between LD hamsters and hamsters transferred to SD on week 30 (LD vs. SD10, P>0.90; Fig. 3B).
Photoperiod treatments did not affect CR mesor values on week 10 (F = 3.6, P>0.05) or week 25 (F = 0.2, P>0.6), or differentiate among LD, SD, and SD40 groups on week 40 (F = 3.2, P>0.05, all comparisons).
CR amplitude was greater in LD than SD40 hamsters on week 10 (F = 25.2, P<0.001), week 25 (F = 18.5, P<0.001) and week 40 (F = 4.7, P = 0.01; Fig. 3C) but did not differ between LD and SD10 hamsters on week 40 (P>0.80).
3.4.2. Females
After 10 weeks of treatment, 78% of SD40 compared to 100% of LD females displayed CRs (FET: P<0.05; Fig. 3A). A similar effect was evident on week 25 (χ2 = 4.4, P<0.05). On week 40, CRs were present in 82% of LD and 86% of SD10 hamsters; 56% of SD40 hamsters exhibited CRs (P<0.05, both comparisons vs. LD and SD10 females).
CR robustness was much greater in LD than SD40 females on week 10 (F = 12.3, P<0.005), and was still elevated in LDs on week 25 (F = 5.2, P<0.05). On week 40, CR robustness of LD hamsters continued to exceed that of SD40 hamsters (P<0.05; Fig. 3B), but transfer to SD on week 30 did not result in a significant decrease in CR robustness 10 weeks later (P>0.40, LD vs. SD10; Fig. 3B).
Photoperiod treatments did not affect mesor values on week 10 (F = 1.9, P>0.10), week 25 (F = 0.3, P>0.50), or week 40 (F = 0.8, P>0.40, all comparisons).
CR amplitude was greater in LD than SD40 females on week 10 (F = 11.6, P = 0.001) and week 25 (F = 12.2, P = 0.001; Fig. 3C). On week 40, CR amplitude of LD hamsters was greater than that of SD40 hamsters (P<0.05; Fig. 3C), but transfer of LD hamsters to SDs on week 30 did not reduce CR amplitude on week 40 (LD vs. SD10, P>0.60; Fig. 3C).
3.5. Circadian sex differences
Photoperiod and sex interacted to affect CR robustness on week 10 (F = 3.9, P<0.05). Robustness was similar in males and females housed in SDs (Fig. 3B),but in LD was markedly greater in females than males at week 10 (P = 0.001; Fig. 3B), but not thereafter.
4. Discussion
4.1. Ultradian rhythms
This study established robust sex differences in the period (τ′) of the ultradian locomotor rhythm (UR). The longer female τ′ was stable across both long and short day lengths. Previously we documented τ′-lengthening effects of short day lengths on male URs [9]; this relation evidently also obtains in females. The contribution of activational and organizational effects of gonadal steroids, or hormone-independent genetic factors (reviewed in [22]) to UR sex differences is unknown; neither orchidectomy of male Siberian hamsters housed in long day lengths, nor replacement treatment of males with testosterone or estradiol in adulthood affected UR characteristics [11]. In male LEW rats [23], locomotor activity URs also were unaffected by gonadectomy; URs, which were not detected in intact female LEW rats, emerged after ovariectomy and were suppressed by estradiol treatment [23].
The present report replicated the observation of acute (≤12 weeks) SD treatments on URs, and extended this effect to older hamsters transferred to SDs after 30 weeks of LD treatment. Photoperiodic engagement of the UR system was not compromised in older hamsters, but CR responses to SDs either were attenuated or absent in the older hamsters. The latter observation is consistent with reduced responsiveness of aged males to short day lengths [24].
Marked effects of day length on UR locomotor activity also were evident in the activity records of male Siberian hamsters ([25]; Fig. 2), but were nearly completely absent in rats [26], which unlike hamsters, are minimally responsive to seasonal variations in day length [27].
Seasonal changes in gonadal and somatic traits induced by short day lengths (reviewed in [12,28]) wane after 15–20 weeks and eventually revert to the long-day phenotype in Siberian hamsters (e.g., [29,30]), even though the increased duration of nocturnal melatonin secretion is maintained throughout housing in short days [31]. The increased prevalence of URs after 10 weeks of SD treatment, in common with SD changes in body mass and reproductive physiology, reverted to the long-day pattern 15 and 30 weeks later in both sexes, as did UR robustness in females (Fig. 2C). Spontaneous recovery of the LD phenotype reflects development of neuroendocrine refractoriness to SDs and long-duration melatonin signals (reviewed in [32,33]). In contrast, increases in UR period length, robustness, and amplitude, evident after 10 weeks of SD treatment, were sustained throughout 40 weeks of testing in males, suggesting that day length controls several UR characteristics via gonadal steroid-and melatonin-independent mechanisms (cf. [11]). Because hamsters manifest photoperiodic responses when transferred on week 30 to SDs, we infer that UR phenotypic plasticity is not compromised by advanced age.
Changes in food consumption are unlikely to account for photoperiodic changes in Siberian hamster URs. Food intake does not change in the first 12 weeks after the transition from long to short day lengths [25,34] and increased meal frequency, which is higher in the dark than light phase in LDs and during the first 4 weeks in SDs, no longer is evident after 8 and 16 weeks in SDs [25].
4.2. Circadian rhythms
CRs were detected in a higher percentage of LD than SD males after 10 and 40 weeks and in females after 10, 25 and 40 weeks of treatment. Robustness and amplitude of CRs were greater in LDs than SDs for both sexes. The nocturnal locomotor activity rhythm of male Siberian hamsters is diminished after 8 and 16 weeks of SD treatment, with accompanying deceases in nocturnal oxygen consumption and carbon dioxide production [25]. In contrast, the incidence, robustness and amplitude of CRs of males and females retained SD characteristics throughout 40 weeks of treatment, despite the concurrent recovery of the long-day body mass and reproductive phenotypes. The prevailing day length rather than gonadal steroid and pineal melatonin secretion appears to control all CR and several UR characteristics. Other than a transient marked increase in CR robustness in LD females compared to LD males, there were no sex differences in the circadian responses to photoperiod manipulations.
The longer period of the ultradian locomotor activity rhythm in females in both summer and winter day lengths may reflect overall decreased mobility of females than males ([35] cited in [36]). The significance of this sex difference remains to be established; hormone manipulations will be required to assess contributions of the perinatal and adult hormonal milieu to the establishment and maintenance of this sex difference.
Sex differences in locomotor activity were more pronounced for ultradian than circadian rhythms. Robustness of circadian locomotion was substantially lower in both sexes in short than long day lengths but the opposite relation obtained for ultradian activity, with more robust URs in winter-like day lengths. This extends earlier observations [8,9,37], in which URs were more prominent when circadian rhythms were reduced in amplitude [8,9] or eliminated as a result of functional disruption [37] or surgical damage [38] to the suprachiasmatic nucleus, or long-term maintenance in constant light [39].
Many traits, including body mass and reproduction in Siberian hamsters that vary seasonally in photoperiodic rodents, spontaneously revert from the winter to the summer phenotype during prolonged maintenance in short day lengths. Most ultradian and circadian rhythm components, however, retained short-day characteristics for 40 weeks. Because it requires 4–6 weeks to reverse winter reproductive quiescence, it is advantageous to initiate recovery long before the advent of long day lengths; the development of reproductive refractoriness beginning in mid-winter ensures that photoperiodic rodents will be capable of reproduction at the very outset of favorable spring conditions [40]. No such temporal constraints apply to the re-entrainment of locomotor activity rhythms, which can be achieved in a matter of days [41] with the advent of spring day lengths; this may account for the failure of locomotor activity to develop refractoriness to short day lengths.
In summary, we confirmed robust effects of photoperiod on the temporal structure of URs [9], and extended the earlier observation to describe sex differences in the ultradian timing system. SD-induced lengthening of τ′ (Fig. 2) has been proposed to conserve energy during winter by increasing the interval between energetically-expensive foraging bouts [9] and may also contribute to the increase in non-REM sleep in SD [42]. Sex differences in ultradian rhythms of hamsters were more prominent under long than short day lengths: female hamsters exhibited behavioral URs lower in amplitude and with longer periods than those expressed by males. The functional significance of these sex differences remains unknown. Although sex diphenisms in sleep, a rhythmic behavior with strong ultradian components, are pervasive (humans: [43]; mice: [44,45]; rats: [46]), no studies have examined sex differences in the temporal aspects of sleep in Siberian hamsters. Food intake, which also expresses strong ultradian characteristics, does not differ between males and females in either LD or SD [34,47]; in LD, however, male hamsters hoard significantly more food than do females [48]. To the extent that the longer ultradian home-cage activity periods in females reflect decreases in foraging tendencies, they may contribute to decreased food hoarding.
Highlights.
Sex and day length affect Siberian hamster ultradian (UR) and circadian (CR) rhythms.
UR period was longer in females than in males in both long (LD) and short (SD) days.
CRs were less prevalent, less robust and lower in amplitude in SDs than LDs.
Neuroendocrine refractoriness to SD was compatible with persistent SD-like URs.
Acknowledgments
We thank Kenneth Onishi, Janine Kirin, and Dr. Betty Theriault for their expert assistance.
References
- 1.Sei H, Azekawa T, Morita Y. Ultradian rhythm of 100 min in the dark phase EEG of the rat. Physiol Behav. 1991;49:207–10. doi: 10.1016/0031-9384(91)90255-m. [DOI] [PubMed] [Google Scholar]
- 2.Gattermann R. Zur biorhthmik des goldhamsters (Mesocricetus auratus Waterhouse 1839) Zool Jb. 1985;89:107–13. [Google Scholar]
- 3.Gerkema MP, Groos GA, Daan S. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J Biol Rhythms. 1990;5:81–95. doi: 10.1177/074873049000500201. [DOI] [PubMed] [Google Scholar]
- 4.Fitzsimons TJ, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol. 1969;67:273–83. doi: 10.1037/h0026772. [DOI] [PubMed] [Google Scholar]
- 5.Gerkema MP, Daan S, Wilbrink M, Hop MW, van der Leest F. Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J Biol Rhythms. 1993;8:151–71. doi: 10.1177/074873049300800205. [DOI] [PubMed] [Google Scholar]
- 6.Tornatzky W, Cole JC, Miczek KA. Recurrent aggressive episodes entrain ultradian heart rate and core temperature rhythms. Physiol Behav. 1998;63:845–53. doi: 10.1016/s0031-9384(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 7.Heldmaier G, Steinlechner S, Ruf T, Wiesenger H, Klingenspor M. Photoperiod and thermoregulation in vertebrates: body temperature rhythms and thermogenic acclimation. J Biol Rhythms. 1989;4:251–65. [PubMed] [Google Scholar]
- 8.Prendergast BJ, Beery AK, Paul MJ, Zucker I. Enhancement and suppression of ultradian and circadian rhythms across the female hamster reproductive cycle. J Biol Rhythms. 2012;27:246–56. doi: 10.1177/0748730412441315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prendergast BJ, Zucker I. Photoperiodic influences on ultradian rhythms of male Siberian hamsters. PLoS One. 2012;7:e41723. doi: 10.1371/journal.pone.0041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–10. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast BJ, Cable EJ, Cisse YM, Stevenson TJ, Zucker I. Pineal and gonadal influences on ultradian locomotor rhythms of male Siberian hamsters. Horm Behav. 2013;63:54–64. doi: 10.1016/j.yhbeh.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- 13.Gorman MR, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in day length controls annual testicular and body weight rhythms. Biol Reprod. 1995;53:116–25. doi: 10.1095/biolreprod53.1.116. [DOI] [PubMed] [Google Scholar]
- 14.Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. I. Role of the gonads and pituitary. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- 15.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- 16.Lomb N. Least-squares frequency analysis of unequally spaced data. Astrophys Space Sci. 1976;39:447–62. [Google Scholar]
- 17.Refinetti R, Cornélissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruf T. The Lomb–Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res. 1999;30:178–201. [Google Scholar]
- 19.van Dongen HPA, Olofsen E, Van Hartevelt JH, Kruyt EW. A procedure of multiple period searching in unequally spaced time series with the Lomb–Scargle method. Biol Rhythm Res. 1999;30:149–77. doi: 10.1076/brhm.30.2.149.1424. [DOI] [PubMed] [Google Scholar]
- 20.van Dongen HPA, Ruf T, Olofsen E, van Hartevelt JH, Kruyt EW. Analysis of problematic time series with the Lomb–Scargle method, a reply to ‘Emphasizing difficulties in the detection of rhythms with Lomb–Scargle periodograms’. Biol Rhythm Res. 2001;32:347–54. doi: 10.1076/brhm.32.3.347.1348. [DOI] [PubMed] [Google Scholar]
- 21.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 22.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;4:677–83. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wollnik F, Döhler KD. Effects of adult or perinatal hormonal environment on ultradian rhythms in locomotor activity of laboratory LEW/Ztm rats. Physiol Behav. 1986;38:229–40. doi: 10.1016/0031-9384(86)90158-7. [DOI] [PubMed] [Google Scholar]
- 24.Horton TH, Yellon SM. Aging, reproduction, and the melatonin rhythm in the Siberian hamster. J Biol Rhythms. 2001;16:243–53. doi: 10.1177/074873040101600307. [DOI] [PubMed] [Google Scholar]
- 25.Warner A, Jethwa PH, Wyse CA, I'anson H, Brameld JM, Ebling FJ. Effects of photo-period on daily locomotor activity, energy expenditure, and feeding behavior in a seasonal mammal. Am J Physiol. 2010;298:R1409–16. doi: 10.1152/ajpregu.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siebert U, Wollnik F. Wheel-running activity rhythms in two inbred strains of laboratory rats under different photoperiods. Physiol Behav. 1991;50:1137–43. doi: 10.1016/0031-9384(91)90574-8. [DOI] [PubMed] [Google Scholar]
- 27.Nelson RJ, Zucker I. Photoperiodic control of reproduction in olfactory bulbectomized rats. Neuroendocrinology. 1981;32:266–71. doi: 10.1159/000123171. [DOI] [PubMed] [Google Scholar]
- 28.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, brain, and behavior. Vol. 2. San Diego: Elsevier Science Press; 2002. pp. 93–156. [Google Scholar]
- 29.Schlatt S, Niklowitz P, Hoffmann K, Nieschlag E. Influence of short photoperiods on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus. Biol Reprod. 1993;49:243–50. doi: 10.1095/biolreprod49.2.243. [DOI] [PubMed] [Google Scholar]
- 30.Kauffman AS, Freeman DA, Zucker I. Termination of neuroendocrine refractoriness to melatonin in Siberian hamsters (Phodopus sungorus) J Neuroendocrinol. 2003;15:191–6. doi: 10.1046/j.1365-2826.2003.00966.x. [DOI] [PubMed] [Google Scholar]
- 31.Rollag MD, Panke ES, Reiter RJ. Pineal melatonin content in male hamsters throughout the seasonal reproductive cycle. Proc Soc Exp Biol Med. 1980;165:330–4. doi: 10.3181/00379727-165-40981. [DOI] [PubMed] [Google Scholar]
- 32.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–90. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 33.Prendergast BJ, Nelson RJ, Zucker I. Seasonal rhythms of mammalian behavioral neuroendocrinology. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, brain, and behavior. 2nd. Vol. 1. San Diego: Academic Press; 2009. pp. 507–38. [Google Scholar]
- 34.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight, and body composition in Siberian hamsters. Am J Physiol. 1984;246:R26–30. doi: 10.1152/ajpregu.1984.246.1.R26. [DOI] [PubMed] [Google Scholar]
- 35.Meyer MN. Peculiarities of the reproduction and development of Phodopus sungorus Pallas of different geographical populations. Zool Zh. 1967;46:604–14. [Google Scholar]
- 36.Ross PD. Phodopus sungorus. Mamm Species. 1998;595:1–9. [Google Scholar]
- 37.Prendergast BJ, Cisse YM, Cable EJ, Zucker I. Dissociation of sultradian and circadian phenotypes in female and male Siberian hamsters. J Biol Rhythms. 2012;27:287–98. doi: 10.1177/0748730412448618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav. 1984;32:357–68. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- 39.Honma KI, Hiroshige T. Endogenous ultradian rhythms in rats exposed to prolonged continuous light. Am J Physiol. 1978;235:R250–6. doi: 10.1152/ajpregu.1978.235.5.R250. [DOI] [PubMed] [Google Scholar]
- 40.Piekarski DJ, Jarjisian SG, Zucker I. Winter day lengths counteract stimulatory effects of apomorphine and yohimbine on sexual behavior of male Syrian hamsters. Chronobiol Int. 2012;29:850–6. doi: 10.3109/07420528.2012.699125. [DOI] [PubMed] [Google Scholar]
- 41.Prendergast BJ, Freeman DA. Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus) J Biol Rhythms. 1999;14:62–71. doi: 10.1177/074873099129000452. [DOI] [PubMed] [Google Scholar]
- 42.Deboer T, Tobler I. Shortening of the photoperiod affects sleep distribution, EEG and cortical temperature in the Djungarian hamster. J Comp Physiol A. 1996;179:483–92. doi: 10.1007/BF00192315. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 44.Paul KN, Turek FW, Kryger MH. Influence of sex on sleep regulatory mechanisms. J Womens Health (Larchmt) 2008;17:1201–8. doi: 10.1089/jwh.2008.0841. [DOI] [PubMed] [Google Scholar]
- 45.Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734:275–85. [PubMed] [Google Scholar]
- 47.Bartness TJ. Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiol Behav. 1996;60:517–29. doi: 10.1016/s0031-9384(96)80027-8. [DOI] [PubMed] [Google Scholar]
- 48.Durazzo A, Proud K, Demas GE. Experimentally induced sickness decreases food intake, but not hoarding, in Siberian hamsters (Phodopus sungorus) Behav Processes. 2008;79:195–8. doi: 10.1016/j.beproc.2008.07.009. [DOI] [PubMed] [Google Scholar]