Abstract
Introduction
HE4 is a novel biomarker for ovarian cancer. This study measured HE4 and CA125 levels in women with benign gynecologic disorders.
Methods
Sera were obtained from women prior to surgery for a pelvic mass and HE4 and CA125 levels were determined. The proportions of patients with elevated biomarker levels were compared.
Results
There were 1042 women with benign disease. HE4 levels were less often elevated than CA125 (8% versus 29%, p<0.001). A marked difference was observed in patients with endometriosis where HE4 was elevated in 3% of cases and CA125 in 67% (p<0.0001). Serous ovarian tumors were associated with elevated levels of HE4 in 8% of cases and CA125 in 20% (p=0.0002); uterine fibroids in 8% versus 26% (p=0.0083); dermoids in 1% versus 21% (p=0.0004); and inflammatory disease in 10% versus 37% (p=0.014).
Conclusion
HE4 is elevated less frequently than CA125 in benign disease, particularly in premenopausal patients.
Keywords: Biomarker, benign gynecologic disease, CA125, HE4, healthy women
Introduction
For nearly three decades, CA 125 has been used as a biomarker for monitoring the course of ovarian cancer (1;2). CA125 is a high molecular weight mucin (MUC 16; >1 million Daltons) that is enzymatically cleaved and shed from the surface of ovarian cancer cells. Approximately 80% of ovarian cancers express CA125. Only a few normal tissue express low levels of CA125 including the endometrium, fallopian tube epithelium, lung parenchyma and cornea. Significant levels of CA125 are found in deposits of endometriosis, during the first trimester of normal pregnancy and in some benign ovarian tumors (3-7). Any condition that irritates the peritoneum, pericardium or pleura can also elevate CA125. Consequently, CA125 levels can be elevated in pelvic inflammatory disease, cirrhosis with ascites and congestive heart failure with pleural effusions (3-7). False positive elevations of CA125 have been a particular problem in premenopausal women where endometriosis is more active, pregnancy occurs and CA125 can be modestly elevated by normal menstruation
As ovarian cancer occurs more frequently in postmenopausal women and the uterus and fallopian tubes are removed during cytoreductive surgery, CA125 has been a highly specific biomarker for monitoring women with epithelial ovarian cancer during treatment and subsequent followup for recurrent disease (2;8-11). Preoperative serum levels of CA125 and the rate of decline of CA125 in women undergoing chemotherapy treatment for epithelial ovarian cancer have been shown to be prognostic indicators for this disease (12-17). In differentiating benign from malignant pelvic masses, consideration of the CA125 level has improved prediction using ultrasound and age in a Risk of Malignancy Index (RMI)(18), but the limited specificity of CA125 has left room for improvement.
Recently, HE4 has been shown to be a promising marker for epithelial ovarian cancer with increased specificity over CA125 and improved sensitivity for distinguishing malignant from benign pelvic masses (19). HE4 is a whey acid protein (WAP) with a four disulfide core originally isolated from epithelial cells of the human epididymis. HE4 is expressed in numerous tissues throughout the body, including the female reproductive tract (20). Importantly, at least from the perspective of ovarian cancer detection, HE4 circulates in the bloodstream and is overexpressed in patients with serous and endometrioid epithelial ovarian carcinomas but is not expressed in normal ovarian surface epithelium (21). Several recent studies from our group have demonstrated that HE4 in combination with CA125 yields a higher specificity and sensitivity for distinguishing malignant from benign pelvic masses compared to either marker alone (19;22).
To date there are no large studies that have examined serum HE4 levels in healthy premenopausal and postmenopausal women with benign gynecologic disorders. In a complementary article reported in this issue of the American Journal of Obstetrics and Gynecology we have defined normal levels of HE4 in sera from healthy women and found that HE4 levels increase with age and decrease during pregnancy. In the present study we have documented that HE4 is less frequently elevated than CA125 in many, but not all benign conditions and diseases.
Materials and Methods
The Institutional Review Board (IRB) for Human Studies at Woman and Infants Hospital approved an analysis that utilized serum biomarker levels of HE4 and CA125 from three IRB prospective trials (WIH Pilot trial, FDI-03 trial, registered with the National Institute of Health clinical trial registry ClinicalTrial.gov identifier: NCT00315692 and the FDI-15 trial registered with the National Institute of Health clinical trial registry ClinicalTrial.gov identifier: NCT00987649) as well as serums obtained from IRB approved serum repositories at the Massachusetts General Hospital (MGH) and the MD Anderson Cancer Center (MDACC) to evaluate biomarker levels of HE4 and CA125. All serum samples were obtained from women in the prior to surgery for an ovarian cyst or pelvic mass. Serum HE4 levels were determined using the HE4 EIA assay kit (Fujirebio Diagnostics Inc. Malvern, PA) and serum CA125II levels were measured on the Abbott i2000 ARCHITECT® assay platform (Abbott Diagnostics Inc, Abbott Park, IL). Serum assays were run at three different laboratories (MGH, MDACC and Fujirebio Diagnostics Inc, Malvern, PA). All HE4 values were derived using cubic spline interpolation. Pathology reports were reviewed at the time of each study and used for histopathologic classification of the benign neoplasms.
Statistical Analysis
The primary endpoint of this study was to measure serum concentrations of HE4 (pM) in premenopausal and postmenopausal women with benign gynecologic neoplasms and compare HE4 levels to those of CA125 measured in the same samples. Women were stratified by age, menopausal status, and benign disease classification. In each group, the median, range, mean, standard deviation, percent coefficient of variation (%CV), and the 95th percentiles for serum HE4 levels were determined. Normal serum levels for HE4 were defined using a cut point at the upper 95th percentile as describe in a complementary article in this issue of the American Journal of Obstetrics and Gynecology. The upper limit of normal used for CA125 was 35 U/mL for both premenopausal and postmenopausal women as defined in the Abbott i2000 ARCHITECT® Food and Drug Agency (FDA) cleared package insert. The upper limit of normal using the 95th percentile for HE4 in healthy premenopausal women is 89 pM and for healthy postmenopausal women a 128 pM. The 95th percentile cut point for HE4 in pre and post-menopausal women combined is 114 pM. These reference points were determined in a study examining normative values for HE4 and published as a complementary article in this issue of the American Journal of Obstetrics and Gynecology. These cut points will be used as a reference and the basis of this report. P values for comparison of medians biomarker levels were derived using a continuity corrected Pearson's chi-square median test and the Wilcoxon rank sum method. P-values for comparison of the proportion of patients with elevations in HE4 versus CA125 in the various benign histopathologic classifications were determined with a two sample test for equality of proportions using large sample statistics. Log base 2 transformed scatter plots were generated for HE4 levels by decadal age group and menopausal status and by histopathological classification. All HE4 values were derived using cubic spline interpolation of HE4 standards.
Results
Serum HE4 and CA125 values were measured in 1042 women diagnosed with benign gynecologic conditions of which 449 (43%) were premenopausal and 593 (57%) were postmenopausal. There were 236 women (120 premenopausal, 116 postmenopausal) entered from a combined WIH and MGH pilot trial, 74 women (41 premenopausal, 33 postmenopausal) from the MDACC serum repository, 351 women (201 premenopausal, 150 postmenopausal) from the FDI-03 multicenter pelvic mass trial and 381women (231 premenopausal, 150 postmenopausal) from the FDI-15 multicenter low risk pelvic mass trial. The mean age of all women was 50 years (range: 18 to 89) with the mean age for premenopausal women 40 years (range: 18 to 56) and for postmenopausal women 62 years (range: 39 to 89).
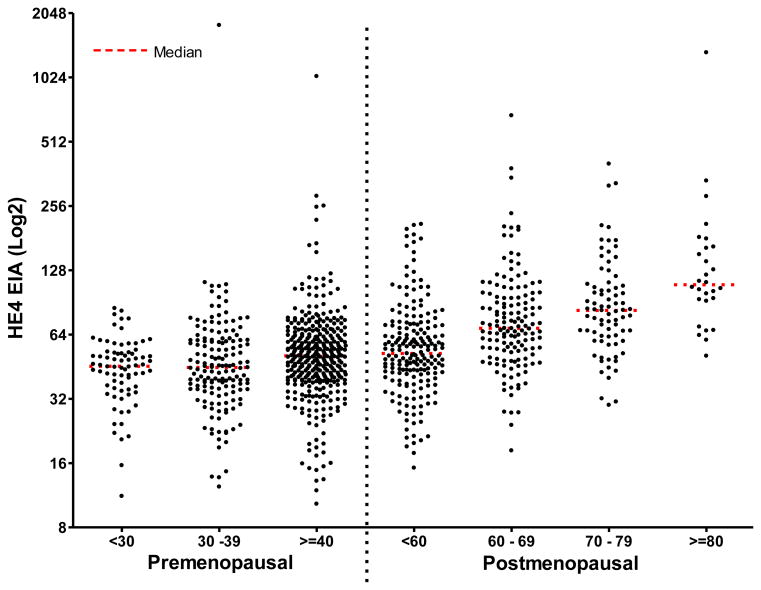
The mean, standard deviation, median and ranges for serum HE4 levels by age groups and menopausal status are shown in Table 1. A comparison of the median HE4 serum levels for both premenopausal and postmenopausal women by decade of age showed the median serum HE4 levels rise consistently with age. Significant differences between all age groups were observed (p≤0.01), with the exception of premenopausal women <30 years versus those between 30 and 39 years (median p=0.852, ranksum p = 0.7406). This finding is consistent with the description of HE4 normal levels in healthy women.
Table 1.
Statistical characteristics of serum HE4 levels (pM) for premenopausal and postmenopausal women with benign disease by age group.
| N=1042 | Pre-Menopausal | Post-Menopausal | |||||
|---|---|---|---|---|---|---|---|
| Age Group | <30 | 30 - 39 | ≥40 | <60 | 60 - 69 | 70 - 79 | ≥80 |
| N | 76 | 155 | 362 | 197 | 145 | 79 | 28 |
| Mean | 45.9 | 60.0 | 58.2 | 61.4 | 86.4 | 98.8 | 171.6 |
| Std Dev | 14.9 | 142.1 | 59.9 | 35.5 | 71.5 | 64.4 | 238.0 |
| Median | 45.5 | 44.9 | 50.9 | 52.2 | 68.5 | 83.0 | 109.5 |
| Range | 11.2 -84.9 | 12.4-1799 | 10.3-1037 | 15.2-210.3 | 18.3-678.6 | 29.9-403.2 | 50.8-1339 |
Histopathologic results were subcategorized into 11 separate groups. The category cysts included simple, paratubal, hemorrhagic, corpus luteal and follicular cysts. The category of benign other included normal ovaries with other benign gynecologic findings such as endosalpingiosis, endometrial hyperplasia and patients undergoing prophylactic oophorectomies. The category of benign not otherwise specified (NOS) comprised benign cysts where the pathologic report did not specify histology. Cystadenoma, adenofibroma and cystadenofibroma were all grouped together and divided by serous and mucinous histology when known. Inflammatory processes such as abscess, hydrosalpinx and pelvic inflammatory disease were grouped together. Other groupings included endometriosis and endometriomas, dermoid tumors, benign sex cord stromal tumors (fibrothecoma and thecomas), and leiomyomas.
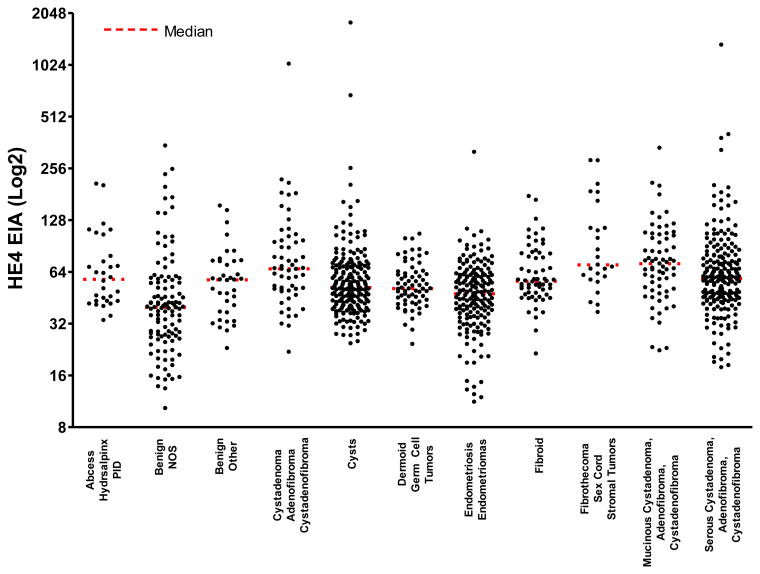
The serum HE4 levels for all 1042 cases grouped by menopausal status and age are shown in a scatter plot in Figure1, whereas Figure 2 displays a scatterplot of the serum HE4 levels for all 1042 samples grouped by benign pathological categories. The mean, median and ranges for HE4 and CA125 broken down by the specified histopathologic categories are shown in Table 2. Comparison of serum HE4 and CA125 levels for all benign tumors and sub-categories are displayed in Tables 3-6.
Figure 1.
Scatterplot of the serum HE4 levels for premenopausal and postmenopausal women with benign neoplasms by age groups.
Figure 2.
Scatterplot of the serum HE4 levels for women with benign neoplasms by histopathologic classification.
Table 2.
Analysis of HE4 and CA125 levels in selected pathologic groups of benign gynecologic disorders and neoplasms.
| Benign classification | N | HE4 (pM) | CA125 (U/mL) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | ||
| Ovarian Cysts | 204 | 70.8 | 52.0 | 24.5 - 1799 | 38.1 | 16.1 | 2.9 - 770.7 |
| Germ Cell Tumors (Mature Teratoma) | 67 | 55.8 | 51.2 | 24.3 - 106.4 | 25.8 | 17.9 | 3.9 - 100.7 |
| Sex Cord Stromal Tumors (Thecoma, Fibrothecoma) | 25 | 106.4 | 70.3 | 37.3 - 284.8 | 138.9 | 36.5 | 8.9 - 889.1 |
| Cystadenoma, Adenofibroma, Cystadenofibroma | 56 | 97.4 | 66.7 | 21.9 - 1037 | 62.4 | 17.1 | 3.7 - 1339 |
| Serous Epithelial Tumors | 206 | 76.3 | 58.6 | 17.8 - 1339 | 46.5 | 19.1 | 4.0 - 2260 |
| Mucinous Epithelial Tumors | 67 | 82.2 | 71.4 | 22.3 - 336.0 | 26.6 | 19.0 | 5.9 - 258.4 |
| Benign - NOS | 113 | 53.6 | 39.8 | 10.3 - 346.3 | 43.5 | 18.1 | 2.1 - 677.3 |
| Endometriosis / Endometriomas | 176 | 51.2 | 47.8 | 11.2 - 318.1 | 122.5 | 65.6 | 5.5 - 2409 |
| Abscess / Hydrosalpinx / PID | 30 | 72.4 | 58.0 | 33.5 - 208.0 | 148.7 | 17.9 | 5.2 - 2545 |
| Fibroid (Leiomyomas) | 61 | 66.5 | 56.5 | 21.4 - 176.1 | 38.3 | 20.4 | 5.7 - 240.9 |
| Benign – Other (Normal Ovaries) | 37 | 61.0 | 57.5 | 23.0 - 155.1 | 50.0 | 18.8 | 5.4 - 733.7 |
|
| |||||||
| All Benign Tumors | 1042 | 68.2 | 54.1 | 10.3 - 1799 | 60.4 | 20.2 | 2.1 - 2545 |
Table 3.
Percentage of time where serum HE4 levels are >114.8 pM and serum CA125 levels are >35 U/mL in premenopausal and postmenopausal women combined.
| Benign Classification (Pre- and Postmenopausal Women Combined) | N | HE4 > 95% tile | CA125 > 95% tile | P value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Ovarian Cysts | 204 | 11 | 5% | 29 | 14% | 0.0027 |
| Germ Cell Tumors (Mature Teratoma) | 67 | 0 | 0% | 14 | 21% | 0.0001 |
| Sex Cord Stromal Tumors (Thecoma, Fibrothecoma) | 25 | 8 | 32% | 13 | 52% | 0.1520 |
| Cystadenoma, Adenofibroma, Cystadenofibroma | 56 | 9 | 16% | 11 | 20% | 0.6217 |
| Serous Epithelial Tumors | 206 | 19 | 9% | 42 | 20% | 0.0014 |
| Mucinous Epithelial Tumors | 67 | 11 | 16% | 12 | 18% | 0.8188 |
| Benign - NOS | 113 | 9 | 8% | 31 | 27% | 0.0001 |
| Endometriosis / Endometriomas | 176 | 1 | 1% | 118 | 67% | 0.0000 |
| Abscess / Hydrosalpinx / PID | 30 | 3 | 10% | 11 | 37% | 0.0146 |
| Fibroid (Leiomyomas) | 61 | 3 | 5% | 16 | 26% | 0.0012 |
| Benign – Other (Normal Ovaries) | 37 | 3 | 8% | 8 | 22% | 0.1023 |
|
| ||||||
| All Benign Tumors | 1042 | 77 | 7% | 305 | 29% | 0.0000 |
Table 6.
Percentage of time where biomarker levels were above the 95th percentile in postmenopausal women: serum HE4 levels >128.0 pM and serum CA125 levels >35 U/mL.
| Benign Classification (Postmenopausal Women Only) |
N | HE4 > 95% tile | CA125 > 95% tile | P value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Ovarian Cysts | 48 | 6 | 13% | 6 | 13% | 1.0000 |
| Germ Cell Tumors (Mature Teratoma) | 24 | 0 | 0% | 6 | 25% | 0.0088 |
| Sex Cord Stromal Tumors (Thecoma, Fibrothecoma) | 20 | 5 | 25% | 10 | 50% | 0.1025 |
| Cystadenoma, Adenofibroma, Cystadenofibroma | 36 | 7 | 19% | 8 | 22% | 0.7717 |
| Serous Epithelial Tumors | 151 | 15 | 10% | 28 | 19% | 0.0323 |
| Mucinous Epithelial Tumors | 38 | 7 | 18% | 7 | 18% | 1.0000 |
| Benign - NOS | 50 | 7 | 14% | 9 | 18% | 0.5854 |
| Endometriosis / Endometriomas | 17 | 1 | 6% | 3 | 18% | 0.2871 |
| Abscess / Hydrosalpinx / PID | 15 | 2 | 13% | 5 | 33% | 0.1953 |
| Fibroid (Leiomyomas) | 27 | 2 | 7% | 2 | 7% | 1.0000 |
| Benign – Other (Normal Ovaries) | 23 | 1 | 4% | 4 | 17% | 0.1553 |
|
| ||||||
| All Benign Tumors | 449 | 53 | 12% | 88 | 20% | 0.0013 |
Analysis of all women premenopausal and postmenopausal combined
Elevation of HE4 and CA125 levels was analyzed by two methods. The first considered premenopausal and postmenopausal patients together with a 95th percentile cut point of 114.8 pM for HE4 and 35 IU/mL for CA125 (Table 3). However, because we have shown significant differences in the HE4 levels between premenopausal and postmenopausal women the second analysis used the two HE4 95th percentile cut points of 89.1 pM for premenopausal women and 128.0 pM for postmenopausal women. The results of this evaluation are displayed in Table 4-6 and are the focus of the remainder of this section.
Table 4.
Percentage of time where serum HE4 levels are >89.1 pM for premenopausal women and >128 pM for postmenopausal women and serum CA125 levels >35 U/mL in premenopausal and postmenopausal women.
| Benign Classification (Pre- and Postmenopausal Women Combined) | N | HE4 > 95% tile | CA125 > 95% tile | P value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Ovarian Cysts | 204 | 16 | 8% | 29 | 14% | 0.0399 |
| Germ Cell Tumors (Mature Teratoma) | 67 | 1 | 1% | 14 | 21% | 0.0004 |
| Sex Cord Stromal Tumors (Thecoma, Fibrothecoma) | 25 | 6 | 24% | 13 | 52% | 0.0414 |
| Cystadenoma, Adenofibroma, Cystadenofibroma | 56 | 11 | 20% | 11 | 20% | 1.0000 |
| Serous Epithelial Tumors | 206 | 16 | 8% | 42 | 20% | 0.0002 |
| Mucinous Epithelial Tumors | 67 | 9 | 13% | 12 | 18% | 0.4759 |
| Benign - NOS | 113 | 10 | 9% | 31 | 27% | 0.0003 |
| Endometriosis / Endometriomas | 176 | 6 | 3% | 118 | 67% | 0.0000 |
| Abscess / Hydrosalpinx / PID | 30 | 4 | 13% | 11 | 37% | 0.0369 |
| Fibroid (Leiomyomas) | 61 | 5 | 8% | 16 | 26% | 0.0083 |
| Benign – Other (Normal Ovaries) | 37 | 2 | 5% | 8 | 22% | 0.0413 |
|
| ||||||
| All Benign Tumors | 1042 | 86 | 8% | 305 | 29% | 0.0000 |
Analysis of 1042 women with benign disease showed that serum HE4 levels were significantly less frequently elevated in benign disease when compared to CA125. HE4 was found to be elevated in only 8% (86/1042) of benign cases compared to serum CA125 levels that were elevated in 29% (305/1042) of benign cases (p<0.0001). Examination of all subclassifications of benign disease revealed that there was a significant difference in the proportion of patients where CA125 was elevated compared with HE4 with the exception of mucinous and non-specified cystadenomas, adenofibromas, and cystadenofibroma. The most striking finding was for endometriosis and endometriomas where HE4 was elevated in only 3% (6/176) of the cases compared with CA125, which was elevated in 67% (118/176) of the cases (p<0.0001). Interestingly, serum HE4 levels in women with benign serous tumors were elevated in only 8% (16/206) of the cases compared to CA125, which was elevated in 20% (42/206) of the cases (p=0.0002). Additionally, HE4 was significantly less often elevated compared to CA125 in patients with fibroids (8% versus 26%, p=0.0083) and in patients with benign germ cell tumors (dermoid tumors) (1% versus 21%, p=0.0004). HE4 was also found to be less often elevated in patients with inflammatory disease (abscess, hydrosalpinx and PID) (13% versus 37%, p=0.0369).
Analysis of premenopausal women
Analysis of premenopausal women and pathologic sub-groupings using the premenopausal cut point of 89.1 pM for HE4 and 35 IU for CA125 revealed that HE4 was less often elevated than CA125 in benign disease (Table 5). Once again, significant differences were found when comparing elevations in HE4 and CA125 in the category of endometriosis and endometriomas, where HE4 was elevated in only 3% (5/159) of the cases compared with CA125, which was elevated in 72% (115/159) of the cases (p<0.0001). In benign serous tumors, HE4 was elevated in 2% (1/55) of the cases whereas CA125 was found to be elevated in 25% (14/55) of the cases (p=0.0003). Additionally, when compared to CA125, HE4 was significantly less often elevated in patients with fibroids (9% versus 41%, p=0.0021) and in patients with benign germ cell tumors (dermoid tumors) (2% versus 19%, p=0.0137). For the premenopausal group, there were no significant differences detected between proportions of patients with elevations in HE4 and CA125 for those patients with mucinous and non-specified cystadenomas, adenofibromas, and cystadenofibroma, sex cord stromal tumors, and inflammatory disease. Examination of all benign tumors together revealed that HE4 was elevated in only 6% (33/593) of the premenopausal cases compared to CA125 elevations in 37% (217/593) of the cases (p<0.0001).
Table 5.
Percentage of time where biomarker levels were above the 95th percentile in premenopausal women: serum HE4 levels >89.1 pM and serum CA125 levels >35 U/mL.
| Benign Classification (Premenopausal Women Only) |
N | HE4 > 95% tile | CA125 > 95% tile | P value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Ovarian Cysts | 156 | 10 | 6% | 23 | 15% | 0.0167 |
| Germ Cell Tumors (Mature Teratoma) | 43 | 1 | 2% | 8 | 19% | 0.0137 |
| Sex Cord Stromal Tumors (Thecoma, Fibrothecoma) | 5 | 1 | 20% | 3 | 60% | 0.1967 |
| Cystadenoma, Adenofibroma, Cystadenofibroma | 20 | 4 | 20% | 3 | 15% | 0.6773 |
| Serous Epithelial Tumors | 55 | 1 | 2% | 14 | 25% | 0.0003 |
| Mucinous Epithelial Tumors | 29 | 2 | 7% | 5 | 17% | 0.2266 |
| Benign - NOS | 63 | 3 | 5% | 22 | 35% | 0.0000 |
| Endometriosis / Endometriomas | 159 | 5 | 3% | 115 | 72% | 0.0000 |
| Abscess / Hydrosalpinx / PID | 15 | 2 | 13% | 6 | 40% | 0.0986 |
| Fibroid (Leiomyomas) | 34 | 3 | 9% | 14 | 41% | 0.0021 |
| Benign – Other (Normal Ovaries) | 14 | 1 | 7% | 4 | 29% | 0.1388 |
|
| ||||||
| All Benign Tumors | 593 | 33 | 6% | 217 | 37% | 0.0000 |
Analysis of postmenopausal women
Analysis of postmenopausal women and pathologic sub-groupings using the postmenopausal cut point of 128 pM for HE4 and 35 IU/mL for CA125 is illustrated in (Table 6). Unlike the premenopausal group, significant differences were seen only in the categories of serous cystadenomas (10% versus 19%, p=0.0003) and germ cell tumors (dermoid tumors) (0% versus 25%, p=0.0088). For the remainder of the categories, no significant differences were detected between the proportions of patients with elevated HE4 and CA125, which may have been due to smaller numbers of subjects in each of the individual categories. However, when examining all benign cases in postmenopausal women, HE4 was significantly less often elevated compared to CA125, with HE4 being elevated in 12% (53/449) of benign cases compared with 20% (88/449) for CA125 (p=0.0013).
Comment
HE4 is a novel biomarker that is expressed in epithelial ovarian cancers but not in normal surface epithelium. An assessment of HE4 expression with immunohistochemical staining revealed that HE4 is expressed in 100% of endometrioid adenocarcinomas, 93% of serous adenocarcinomas and 50% of clear cell epithelial ovarian cancers (21). Similar to CA125, serum HE4 levels in sera from women with epithelial ovarian cancer have been shown to be elevated in over 80% of cases (19;23). Several studies examining multiple serum biomarkers for epithelial ovarian cancer have found the combination of HE4 and CA125 has greater sensitivity and specificity than either marker alone (19;24). The Risk of Ovarian Malignancy Algorithm (ROMA) utilizes both serum HE4 and CA125 levels along with menopausal status to estimate the probability that an ovarian cyst or pelvic mass represents a malignancy. In two prospective multicenter trials, ROMA has achieved a sensitivity of 94% at a pre-set specificity of 75% for the detection of invasive epithelial ovarian cancer (22;25). Although HE4 has been shown to be a sensitive biomarker for epithelial ovarian cancer with an increased specificity over that of CA125, very little has been published with regards to HE4 expression in benign gynecologic tumors and disorders (19;26).
Endometriosis is a gynecologic disease that is well known to cause elevations in serum CA125, limiting the use of this biomarker in premenopausal women where this disease is most common. In a study evaluating HE4 levels in 129 women with endometriosis, Huttenen et al demonstrated that HE4 was not elevated in any stages of endometriosis, with a median serum level of 43.5 pM and a range of 15 to 111 pM, which is well within the normal reference limits for HE4(27). In the current study, only 3% of premenopausal and postmenopausal women diagnosed with endometriosis had elevated serum levels of HE4 compared to 67% of women with elevated serum CA125 levels. Serum CA125 levels have been suggested as a biomarker for the diagnosis and monitoring of treatment for endometriosis (28;29). However, the lack of specificity in premenopausal women limits the use of CA125 in differential diagnosis. A study examining the use of a panel of serum markers including CA125, interleukin 6 and 8, C reactive protein and tumor necrosis factor achieved a sensitivity of 92% to 94% and a specificity of 61% to 63% for diagnosing mild to moderate endometriosis (30). The finding that HE4 is rarely elevated in women with endometriosis will improve the specificity of multiple marker panels and assist in the differentiation of endometriosis from malignant disease. Inflammatory disorders, including pelvic inflammatory disease and pelvic abscesses, are also conditions where CA125 is frequently elevated (6;31). In a study examining the use of CA125 to predict the severity of pelvic infection, the investigators found that all patients had an elevated serum CA125 and that it was associated with the severity of disease (31). In our series, HE4 was elevated in 10% of cases compared to CA125 which was elevated in 37% of the cases. These findings are consistent with those reported by Dauod et al where a third of patients with PID had elevated CA125 levels (32).
Benign ovarian neoplasms can give rise to elevated serum CA125 in over 20% of cases (33). These findings are confirmed in the current study, where 29% of cases had elevated CA125 levels in contrast to 8% of the patients with elevated levels of HE4. One of the most common neoplasms found in both premenopausal and postmenopausal women are serous cystadenomas and cystadenofibroma. These tumors often present as cystic and solid ovarian masses that are difficult to differentiate from ovarian malignancies using standard imaging techniques. Analysis of premenopausal and postmenopausal women showed that very few patients had an elevated HE4 level compared to women with an elevated CA125 levels especially in the premenopausal age group. The contrary was found in mucinous tumors, where there was no significant difference in the proportion of women where either biomarker was elevated. Other subgroups such as benign germ cell tumors, simple ovarian cysts and leiomyomas also showed a significantly smaller fraction of cases with elevated HE4 levels compared to CA125.
Both HE4 and CA125 have similar sensitivities for detecting epithelial ovarian cancers; one or both markers can be elevated in over 90% of cases. The improvement in specificity without loss of sensitivity that HE4 brings to the dual marker algorithm provides increased accuracy over CA125 alone for distinguishing malignant from benign pelvic masses with marked improvement in the premenopausal group and is responsible for the additive effect of HE4 to CA125 in risk prediction models such as ROMA (19;22;25;34;35). As well, HE4 was shown not to be elevated in gravid women, suggesting a potential role for HE4 in pregnant women found to have an ovarian cyst or mass.
Finally, the cut point of 35 IU/mL for CA125 is based on the accepted clinical standard used for both premenopausal and postmenopausal patients. Proportions of patients with elevated levels were compared between the two markers to indicate the types of benign conditions for which HE4 is significantly less often elevated. A finer analytic comparison, although less clinically relevant, would adjust for differences between CA125 distributions between premenopausal women and postmenopausal women.
The current observations provide an explanation for the contribution that HE4 makes to the dual marker combination of HE4 and CA125 in distinguishing malignant and benign pelvic masses.
Acknowledgments
RGM is partially supported by NCI 1 RO1 CA136491-01 grant and RCB is partially supported by MDACC Ovarian SPORE 1 P50 grant CA83639. SJS is partially supported by U01-CA152990 as part of NCI's Early Detection Research Network.
R.G.M. receives research funding from Fujirebio Diagnostics Inc. and Abbott Diagnostics Inc. G.L.-M. receives research funding from Fujirebio Diagnostics Inc., Beckman Coulter Inc., and Abbott Diagnostics Inc. M.C.M. receives consulting fees from Fujirebio Diagnostics Inc. R.C.B. receives royalties for CA125 from Fujirebio Diagnostics Inc. and serves on Scientific Advisory Boards of Vermillion Inc. and Illumina Inc.
Footnotes
Disclosure: The remaining authors report no conflict of interest.
Potential conflict of interest: Richard G. Moore, MD: receives research funding from Fujirebio Diagnostics Inc. and Abbott Diagnostics Inc.
Geralyn Lambert-Messerlian, PhD: receives research funding from Fujirebio Diagnostics Inc., Beckman Coulter Inc. and Abbott Diagnostics Inc.
Michael Craig Miller, BSn: receives consulting fees from Fujirebio Diagnostics Inc.
Robert C. Bast Jr, MD: receives royalties for CA125 from Fujirebio Diagnostics Inc. and serves on Scientific Advisory Boards of Vermillion Inc. and Illumina Inc.
Steven J. Skates, PhD: No conflicts to declare
Karen H. Lu, MD: No conflicts to declare
Margaret M. Steinhoff, MD: No conflicts to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–7. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–7. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 3.Buamah P. Benign conditions associated with raised serum CA-125 concentration. J Surg Oncol. 2000 Dec;75(4):264–5. doi: 10.1002/1096-9098(200012)75:4<264::aid-jso7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Buamah PK, Skillen AW. Serum CA 125 concentrations in patients with benign ovarian tumours. J Surg Oncol. 1994 Jun;56(2):71–4. doi: 10.1002/jso.2930560204. [DOI] [PubMed] [Google Scholar]
- 5.Fuith LC, Daxenbichler G, Dapunt O. CA 125 in the serum and tissue of patients with gynecological disease. Arch Gynecol Obstet. 1987;241(3):157–64. doi: 10.1007/BF00931312. [DOI] [PubMed] [Google Scholar]
- 6.Meden H, Fattahi-Meibodi A. CA 125 in benign gynecological conditions. Int J Biol Markers. 1998 Oct;13(4):231–7. doi: 10.1177/172460089801300411. [DOI] [PubMed] [Google Scholar]
- 7.Niloff JM, Knapp RC, Schaetzl E, Reynolds C, Bast RC., Jr CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol. 1984 Nov;64(5):703–7. [PubMed] [Google Scholar]
- 8.Buller RE, Berman ML, Bloss JD, Manetta A, DiSaia PJ. Serum CA125 regression in epithelial ovarian cancer: correlation with reassessment findings and survival. Gynecol Oncol. 1992 Oct;47(1):87–92. doi: 10.1016/0090-8258(92)90082-t. [DOI] [PubMed] [Google Scholar]
- 9.Canney PA, Moore M, Wilkinson PM, James RD. Ovarian cancer antigen CA125: a prospective clinical assessment of its role as a tumour marker. Br J Cancer. 1984 Dec;50(6):765–9. doi: 10.1038/bjc.1984.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkins RE, Roberts K, Wiltshaw E, Mundy J, McCready VR. The clinical correlates of serum CA125 in 169 patients with epithelial ovarian carcinoma. Br J Cancer. 1989 Oct;60(4):634–7. doi: 10.1038/bjc.1989.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Burg ME, Lammes FB, Verweij J. The role of CA 125 in the early diagnosis of progressive disease in ovarian cancer. Ann Oncol. 1990 Jul;1(4):301–2. doi: 10.1093/oxfordjournals.annonc.a057754. [DOI] [PubMed] [Google Scholar]
- 12.Nagele F, Petru E, Medl M, Kainz C, Graf AH, Sevelda P. Preoperative CA 125: an independent prognostic factor in patients with stage I epithelial ovarian cancer. Obstet Gynecol. 1995 Aug;86(2):259–64. doi: 10.1016/0029-7844(95)00126-c. [DOI] [PubMed] [Google Scholar]
- 13.Prat A, Parera M, Peralta S, Perez-Benavente MA, Garcia A, Gil-Moreno A, et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol. 2008 Feb;19(2):327–31. doi: 10.1093/annonc/mdm495. [DOI] [PubMed] [Google Scholar]
- 14.Riedinger JM, Wafflart J, Ricolleau G, Eche N, Larbre H, Basuyau JP, et al. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006 Aug;17(8):1234–8. doi: 10.1093/annonc/mdl120. [DOI] [PubMed] [Google Scholar]
- 15.Riedinger JM. Prognostic value of CA125 half-life and early normalization during chemotherapy in advanced ovarian tumors: results of a multicentric French study. Bull Cancer. 2007 Mar 1;94(3):287–95. [PubMed] [Google Scholar]
- 16.Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996 May;14(5):1545–51. doi: 10.1200/JCO.1996.14.5.1545. [DOI] [PubMed] [Google Scholar]
- 17.Tate S, Hirai Y, Takeshima N, Hasumi K. CA125 regression during neoadjuvant chemotherapy as an independent prognostic factor for survival in patients with advanced ovarian serous adenocarcinoma. Gynecol Oncol. 2005 Jan;96(1):143–9. doi: 10.1016/j.ygyno.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990 Oct;97(10):922–9. doi: 10.1111/j.1471-0528.1990.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2007 Nov 30;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Galgano MT, Hampton GM, Frierson HF., Jr Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006 Jun;19(6):847–53. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 21.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005 Mar 15;65(6):2162–9. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 22.Moore RG, McMeekin DS, Brown AK, Disilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009 Jan;112(1):40–6. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003 Jul 1;63(13):3695–700. [PubMed] [Google Scholar]
- 24.Nolen B, Velikokhatnaya L, Marrangoni A, De GK, Lomakin A, Bast RC, Jr, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010 Jun;117(3):440–5. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Women With a Pelvic Mass. Obstet Gynecol. 2011 Aug;118(2, Part 1):280–8. doi: 10.1097/AOG.0b013e318224fce2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellstrom I, Raycraft J, Kanan S, Sardesai NY, Verch T, Yang Y, et al. Mesothelin variant 1 is released from tumor cells as a diagnostic marker. Cancer Epidemiol Biomarkers Prev. 2006 May;15(5):1014–20. doi: 10.1158/1055-9965.EPI-05-0334. [DOI] [PubMed] [Google Scholar]
- 27.Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009 Apr 21;100(8):1315–9. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrelli TS, Berretta R, Gizzo S, Pezzuto A, Franchi L, Lukanovic A, et al. CA 125 serum values in surgically treated endometriosis patients and its relationships with anatomic sites of endometriosis and pregnancy rate. Fertil Steril. 2011 Jan;95(1):393–6. doi: 10.1016/j.fertnstert.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 29.Kitawaki J, Ishihara H, Koshiba H, Kiyomizu M, Teramoto M, Kitaoka Y, et al. Usefulness and limits of CA-125 in diagnosis of endometriosis without associated ovarian endometriomas. Hum Reprod. 2005 Jul;20(7):1999–2003. doi: 10.1093/humrep/deh890. [DOI] [PubMed] [Google Scholar]
- 30.Mihalyi A, Gevaert O, Kyama CM, Simsa P, Pochet N, De SF, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. 2010 Mar;25(3):654–64. doi: 10.1093/humrep/dep425. [DOI] [PubMed] [Google Scholar]
- 31.Moley KH, Massad LS, Mutch DG. Pelvic inflammatory disease. Correlation of severity with CA-125 levels. J Reprod Med. 1996 May;41(5):341–6. [PubMed] [Google Scholar]
- 32.Daoud E, Bodor G. CA-125 concentrations in malignant and nonmalignant disease. Clin Chem. 1991 Nov;37(11):1968–74. [PubMed] [Google Scholar]
- 33.Malkasian GD, Jr, Knapp RC, Lavin PT, Zurawski VR, Jr, Podratz KC, Stanhope CR, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988 Aug;159(2):341–6. doi: 10.1016/s0002-9378(88)80081-4. [DOI] [PubMed] [Google Scholar]
- 34.Kim YM, Whang DH, Park J, Kim SH, Lee SW, Park HA, et al. Evaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean population. Clin Chem Lab Med. 2011 Feb 15; doi: 10.1515/CCLM.2011.085. [DOI] [PubMed] [Google Scholar]
- 35.Montagnana M, Danese E, Ruzzenente O, Bresciani V, Nuzzo T, Gelati M, et al. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med. 2011 Feb 3; doi: 10.1515/CCLM.2011.075. [DOI] [PubMed] [Google Scholar]