Abstract
Background
Liver cirrhosis is recognized with long-term follow-up of patients after the Fontan procedure. The effect of liver cirrhosis on the use of heart transplant (HT) and on post-HT outcomes is unknown.
Methods
We reviewed Fontan patients evaluated for HT from 2004 to 2012 with hepatic computed tomography (CT) imaging, classified as normal, non-cirrhotic changes, or cirrhosis. The primary outcome was 1-year all-cause mortality, and the secondary outcome was differences in serial post-HT liver evaluation.
Results
CT imaging in 32 Fontan patients evaluated for HT revealed 20 (63%) with evidence of liver disease, including 13 (41%) with cirrhosis. Twenty underwent HT, including 5 non-cirrhotic and 7 cirrhosis patients. Characteristics at listing between normal or non-cirrhotic (n = 13) and cirrhosis (n = 7) groups were similar, except cirrhosis patients were older (median 17.6 vs 9.6 years, p = 0.002) and further from Fontan (median 180 vs 50 months, p < 0.05). Serial liver evaluation was similar, including aspartate aminotransferase, alanine aminotransferase, bilirubin, albumin, and tacrolimus dose at 1, 3, 6, 9, and 12 months. Overall patient survival was 80% at 1 year, with no difference between cirrhosis and non-cirrhosis patients (86% vs 77%, p = 0.681). Liver biopsies were performed in 7 patients before HT, and all specimens showed architectural changes with bridging fibrosis.
Conclusions
Most patients evaluated for HT had abnormal liver findings by CT, with cirrhosis in 41%. One-year mortality and serial liver evaluation were similar between groups after HT. Liver cirrhosis identified by CT imaging may not be an absolute contraindication to HT alone in this population.
Keywords: liver cirrhosis, Fontan procedure, heart transplant, pediatric cardiology, liver transplant
The Fontan procedure remains the standard final stage of surgical palliation for complex congenital heart disease with single-ventricle physiology. With long-term follow-up of patients undergoing the Fontan procedure, several studies have found most patients have abnormal liver findings by computed tomography (CT) imaging or by analysis of liver biopsy specimens.1–3 The prevalence of liver cirrhosis varies by study and diagnostic modality, with cirrhosis noted in up to 25% of Fontan patients.4 The development of liver fibrosis and cirrhosis is thought to be multifactorial but likely partly related to passive venous congestion, volume overload, peri-operative insults, and/or low cardiac output in Fontan patients.5–7
Although liver changes are frequently noted in Fontan patients, how the presence of liver cirrhosis affects outcomes in children undergoing heart transplant (HT) after a previous Fontan operation is unknown. Some centers have reported the successful use of combined heart and liver transplantation in children with heart failure and liver cirrhosis, even in patients with apparent intact synthetic liver function, but no consensus exists regarding patient selection for heart and liver transplantation in this population.8 We conducted a single-center retrospective review to compare post-HT outcomes in pediatric Fontan patients based on evidence of liver cirrhosis, including 1-year mortality and serial liver evaluation.
Methods
Study population
A retrospective analysis of all Fontan patients evaluated for HT at Saint Louis Children's Hospital between 2004 and 2012 was performed. During this period, CT imaging of the chest and liver was routinely performed in all Fontan patients during HT evaluation. Outcomes were analyzed by the presence of liver cirrhosis on CT imaging at the time of HT evaluation. The primary outcome was 1-year all-cause mortality, and the secondary outcome included differences in serial post-HT liver evaluation between groups.
CT imaging
Analysis of all available CT imaging was performed by an experienced staff pediatric radiologist (G.K.) who was blinded to patient outcomes. Liver findings were described using a classification modified from previously published description of liver imaging in Fontan patients.9 Liver findings on CT were classified as normal, non-cirrhotic liver changes (mild to severe reticular pattern of enhancement ranging from peripheral to >50% involvement), or cirrhosis (irregular and nodular liver contour with or without segmental atrophy or caudate hypertrophy; Figure 1).
Figure 1.
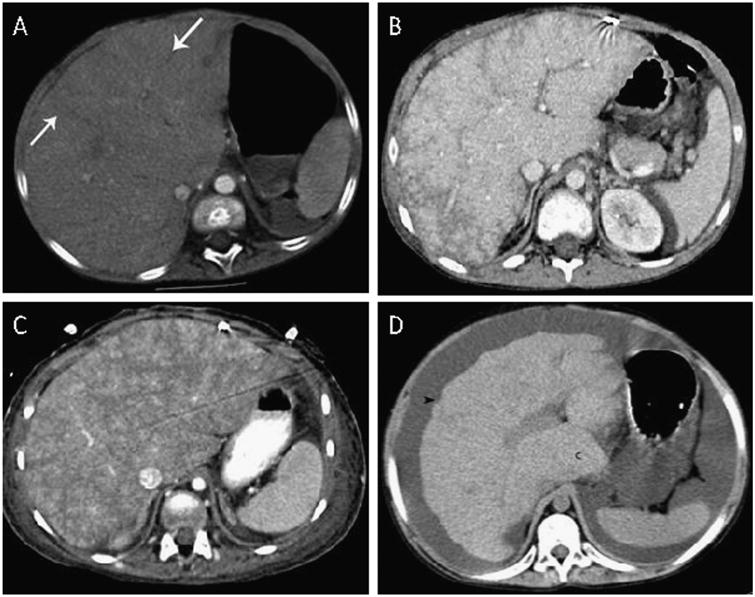
Grading of abnormal liver changes on computed tomography imaging. (A) Reticular enhancement of the liver with hypodense bands (arrows) in the peripheral third of the liver. (B) Reticular enhancement of the liver involving approximately 50% of the liver. (C) Reticular enhancement involves most of the liver. (D) Cirrhosis with noted nodular contour of the liver (arrowhead) and hypertrophy of the caudate lobe (c).
Liver biopsy
All available liver biopsy samples were reviewed by a senior experienced staff pathologist (F.W.) using a modified staging method based on previously described liver histology in Fontan patients.10 Findings on liver specimens were scored by the extent of architectural changes present, including (1) cord atrophy and/or sinusoidal fibrosis, (2) focal parenchymal collapse and/or approximation of portal tracts and central veins, (3) focal bridging fibrosis involving central veins but less than 50% of core, (4) extensive bridging fibrosis that involved greater than 50% of core but not diffuse, and (5) diffuse bridging fibrosis (Figure 2). Focal regenerative nodules were included in scores 4 or 5 depending on associated fibrosis.
Figure 2.
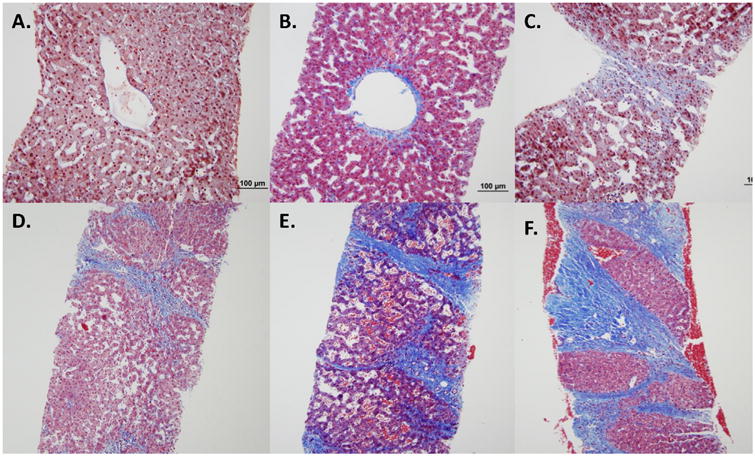
Liver biopsy samples illustrating liver scoring based on extent of architectural changes present. All are low power views with H&E staining. (A) Normal central vein without fibrosis and mild centrilobular congestion (stage 0). (B) Noted thickened central vein wall, early sinusiodal fibrosis and mild cord atrophy (stage 1). (C) Focal area of parenchymal collapse (stage 2). (D) Bridging fibrosis involving central veins but <50% of parenchyma (stage 3). (E) Extensive bridging fibrosis involving >50% of parenchyma (stage 4). (F) Diffuse bridging fibrosis with noted regenerative nodule (stage 5).
Serial liver evaluation
Serial laboratory evaluation was routinely performed at time of initial evaluation for HT and at standardized intervals after HT in all Fontan patients. We analyzed results of blood alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and albumin levels at HT evaluation as well as at 1, 3, 6, 9, and 12 months after HT.
Our institution uses tacrolimus as part of our standard maintenance immunosuppression regimen after HT, with adjustment of dosage to reach standardized goal trough levels. Tacrolimus is completely metabolized in the liver, and dosing adjustment is required in patients with liver dysfunction to reach the same trough levels.11 We therefore used the tacrolimus dose (mg/kg/dose) as another marker for liver function and evaluated patient doses at the same serial time points post-HT.
Statistical analysis
Statistical analysis was performed using SPSS 20 (IBM Corp, Armonk, NY) and GraphPad Prism 4 (GraphPad Software Inc, La Jolla, CA) software. Continuous variables are described as means ± standard deviation for normally distributed data and median with interquartile range (IQR) for non-parametric data. Categoric data were compared using the Fisher exact test. Kaplan-Meier survival analysis was used to compare the difference in survival between groups. A p-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics and hemodynamics at evaluation
A total of 32 Fontan patients were evaluated for HT, and all had CT imaging of the liver at time of HT evaluation per our clinical protocol during the study period. Patient characteristic are reported in Table 1. Patients were a mean age of 13 ± 6.4 years at evaluation, with cirrhotic patients significantly older than non-cirrhotic patients (17.7 vs 9.6 years, p < 0.05). Overall median time from the Fontan procedure to HT evaluation was 102 months (IQR, 37–180 months) and significantly longer in the cirrhotic patients (180 vs 50 months, p < 0.05). Protein-losing enteropathy (PLE) was noted in 47% and pre-sensitization (panel reactive antibody screen > 10%) was present in 38%, with no difference based on presence of liver cirrhosis.
Table 1. Patient Characteristics at Time of Heart Transplant Evaluationa.
| Variableb | Total | Non-cirrhotic | Cirrhotic | p-value |
|---|---|---|---|---|
|
| ||||
| (N = 32) | (n = 19) | (n = 13) | ||
| Protein-losing enteropathy | 15 (46.9) | 7 (37) | 8 (62) | 0.28 |
| Age, years | 13 ± 6.4 | 9.6 ± 5.6 | 17.7 ± 4 | <0.05c |
| Time from Fontan, months | 102 (37–180) | 50 (27–98) | 180 (126–196) | <0.05c |
| Fontan pressure, mm Hg | 16.8 ± 3.3 | 16.6 ± 3.9 | 17.2 ± 2.3 | 0.63 |
| PVRi, WU × m2 | 2.35 (2–3) | 2.2 (2–3) | 3 (1.8–3.5) | 0.594 |
| Cardiac index, liters/min/m2 | 3 (2–3.6) | 3 (2–4.7) | 3 (2–3) | 0.213 |
| Pre-sensitized (>10% PRA) | 12 (37.5) | 8 (42) | 4 (31) | 0.713 |
| Bilirubin, mg/dl | 0.8 (0.4–1.3) | 0.7 (0.5–1.1) | 0.9 (0.4–1.3) | 0.734 |
| AST, units/liter | 32 (26–44) | 32 (26–45) | 22 (15–27) | 0.404 |
| ALT, units/liter | 23 (17–32) | 31 (24–38) | 30 (20–40) | 0.059 |
| Albumin, g/dl | 3.4 (2.4–4.1) | 3.1 (2.4–3.8) | 3.4 (2.3–4.3) | 0.734 |
| INRd | 1.26 (1.2–1.4) | 1.31 (1.2–1.4) | 1.18 (1.1–1.3) | 0.088 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; PRA, panel reactive antibody; PVRi, indexed pulmonary vascular resistance.; WU, Woods units.
Age and evaluation and time from Fontan procedure to evaluation were significantly higher in patients with liver cirrhosis (p < 0.05).
Continuous data are shown as median (interquartile range) or mean ± standard deviation and categoric data as number (%).
Statistically significant (p < 0.05).
INR values included only for 13 non-cirrhotic and 10 cirrhotic patients not taking warfarin.
Median (IQR) total bilirubin, AST, ALT, and albumin levels for the group were 0.8 (0.4–1.3 mg/dl), 32 (26–44 U/liter), 23 U/liter (17–32 U/liter), and 3.4 (2.4–4.1 g/dl), respectively, with no difference between cirrhotic and non-cirrhotic patients. The median international normalized ratios in the 24 patients who were not taking warfarin therapy at time of HT evaluation were also not significantly different between non-cirrhotic and cirrhotic groups (1.31 vs 1.18, p = 0.088). Overall group hemodynamics revealed a mean Fontan pressure of 16.8 ± 3.3 mm Hg, median indexed pulmonary vascular resistance (PVRi) of 2.35 Woods unit (WU) × m2 (IQR, 2–3 WU × m2), and median cardiac index of 3 liters/min/m2 (IQR, 2–3.6 liters/min/m2), with no significant difference according to cirrhosis status.
CT imaging
CT imaging showed abnormal liver changes in 20 of the 32 patients (63%) who underwent HT evaluation, and 13 (41%) of these had liver cirrhosis (Figure 3). Twenty patients underwent HT, including 7 with liver cirrhosis, 5 with non-cirrhotic changes, and 8 with normal liver findings by CT. The remaining 12 patients did not undergo HT. Of these, 5 patients died before HT, 3 of progressive heart failure and 2 of cardiac arrest. One patient was actively listed and awaiting transplant at the time of this review. Another patient was evaluated, but the family and patient deferred HT. One patient with PLE symptomatically improved after optimization of medications and aggressive coiling of collateral vessels. Two patients were not listed due to unfavorable anatomy for HT, including 1 with severe pulmonary vein stenosis and 1 with single-lung anatomy. Finally, 2 patients were not listed due to concerns of liver disease, including a patient with irreversible hepatopulmonary syndrome and another with precancerous hepatic nodules.
Figure 3.
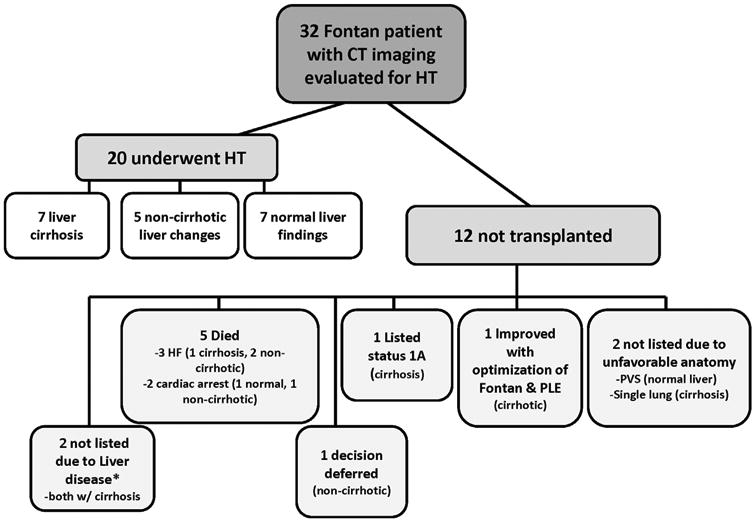
Outcomes for Fontan patients evaluated for heart transplantation (HT), including reasons for not listing for HT in 12 patients who did not undergo HT. *Patients not listed because of percutaneous hepatic nodules (n = 1) and irreversible hepatopulmonary syndrome (n = 1). CT, computed tomography; HF, heart failure; PLE, protein-losing enteropathy; PVS, pulmonary vein stenosis.
Baseline characteristics at HT evaluation did not differ significantly between patients who did and did not undergo HT, including median age (12.5 vs 15.5 years, p = 0.604), months from Fontan (100 vs 102 months, p = 0.951), mean Fontan pressure (16.5 vs 17.5 mm Hg, p = 0.699), PVRi (2.4 vs 2 WU × m2, p = 0.451), cardiac index (3 vs 3.2 liters/min/m2, p = 0.328), presence of PLE (50% vs 42%, p = 0.726), and pre-sensitization (35% vs 42%, p = 0.724). Laboratory evaluation at the HT assessment was also not statistically different, including median international normalized ratio (1.3 vs 1.3, p = 0.19), total bilirubin (0.65 vs 1.2 mg/dl, p = 0.136), albumin (3.5 vs 3.1 g/dl, p = 0.774), and ALT (22 vs 25.5 U/liter, p = 0.182). Although median AST was statistically lower in transplant recipients (28.5 vs 43.5 U/liter, p = 0.17), neither group had median levels outside the normal range and the difference was not likely to be clinically significant.
Liver biopsies
Liver biopsies were performed in 9 patients at the discretion of the managing physician to further evaluate severity of liver cirrhosis (n = 5) or nodules (n = 4) on liver imaging. Hematoxylin and eosin stains were available on all samples, and additional trichrome and orcein staining was performed in 8 samples. The observed liver histology was consistent across all staining methods in all samples when available. All patients showed abnormal architectural changes with bridging fibrosis. Two patients with biopsy results did not undergo HT due to anatomic limitations. Of the remaining 7 patients who underwent HT, 3 had focal areas of fibrosis (3 with cirrhosis by CT), 2 had extensive bridging fibrosis (1 with cirrhosis by CT), and 2 had diffuse (both with cirrhosis by CT) bridging fibrosis.
Transplantation
Statistical analysis was performed on data of the 20 HT patients. Results were compared between patients with normal or non-cirrhotic liver changes and those with cirrhosis. Liver evaluation after HT, including AST, ALT, albumin, total bilirubin, and tacrolimus dose per kilogram did not significantly differ over serial time points after HT (Table 2).
Table 2. Serial Liver Evaluations in Fontan Patients Who Underwent Heart Transplantation Compared by Presence of Liver Cirrhosis on Computed Tomography at Heart Transplant Evaluationa.
| Variable | Albumin,b g/dl | AST,b units/liter | ALT,b units/liter | Total bilirubin,b mg/dl | Tacrolimus dose,b mg/kg/dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| NC | C | p-value | NC | C | p-value | NC | C | p-value | NC | C | p-value | NC | C | p-value | |
| Listing | 3.5 (2.3–3.9) | 3.8 (3.2–4.4) | 0.54 | 28 (25–37) | 34 (21–37) | 1 | 22 (13–28) | 23 (16–51) | 0.4 | 0.6 (0.4–0.8) | 0.9 (0.4–1.3) | 0.39 | N/A | N/A | N/A |
| 1 month | 4 (3.2–4.1) | 3.3 (2.7–3.8) | 0.11 | 30 (25–42) | 23 (22–42) | 0.34 | 20 (16–32) | 23 (22–42) | 0.53 | 0.5 (0.3–0.7) | 0.9 (0.5–1.8) | 0.28 | 0.04 (0.02–0.07) | 0.02 (0.01–0.06) | 0.41 |
| 3 months | 3.7 (3.4–4.2) | 3.2 (2.9–) | 0.29 | 35 (28–38) | 48 (33–110) | 0.11 | 26 (19–36) | 42 (35–101) | 0.07 | 0.3 (0.2–0.4) | 0.3 (0.1–) | 0.71 | 0.04 (0.03–0.15) | 0.05 (0.02–) | 0.5 |
| 6 months | 4 (3.4–4.3) | 3 (2–) | 0.25 | 38 (31–45) | 48 (43–) | 0.14 | 29 (24–33) | 64 (32–) | 0.07 | 0.5 (0.3–0.6) | 0.2 (–) | 0.27 | 0.04 (0.03–0.8) | 0.06 (0.03–) | 1 |
| 9 months | 4.7 (3.8–) | 3.2 (2–) | 0.2 | 32 (15–) | 39 (32–) | 0.4 | 28 (28–) | 37 (13–) | 1 | 0.4 (0.2–) | 0.3 (0.2–) | 1 | 0.05 (0.02–0.09) | 0.08 (0.02–) | 1 |
| 12 months | 3.4 (3.3–) | 3.7 (2.4–) | 1 | 32 (26–) | 33 (27–) | 1 | 18 (18–) | 38 (25–) | 0.2 | 0.4 (0.4–) | 0.3 (0.2–) | 0.4 | 0.03 (0.02–) | 0.04 (0.02–) | 1 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, cirrhotic group; NA, not applicable; NC, non-cirrhotic group.
There was no significant difference in results between groups at serial time points.
Data are shown as the median (interquartile range)
Post-HT survival and group comparison
Overall 1-year post-HT survival was 80% for all Fontan patients. Kaplan-Meier analysis of 1-year all-cause mortality revealed no statistical difference between cirrhotic and non-cirrhotic groups (86% vs 77%, log-rank p = 0.681). Longterm survival overall was 75%, with no statistical difference between cirrhotic and non-cirrhotic groups by Kaplan-Meier survival analysis (71% vs 77%, log-rank p = 0.9488; Figure 4). In the cirrhosis group, 1 patient died at 3 months secondary to multiorgan failure after a massive intraoperative hemorrhage during HT, and an additional late death occurred at 15 months due to rejection. Causes of death in the non-cirrhotic group included rejection in 2 and infection in 1, all within 1 year after HT.
Figure 4.
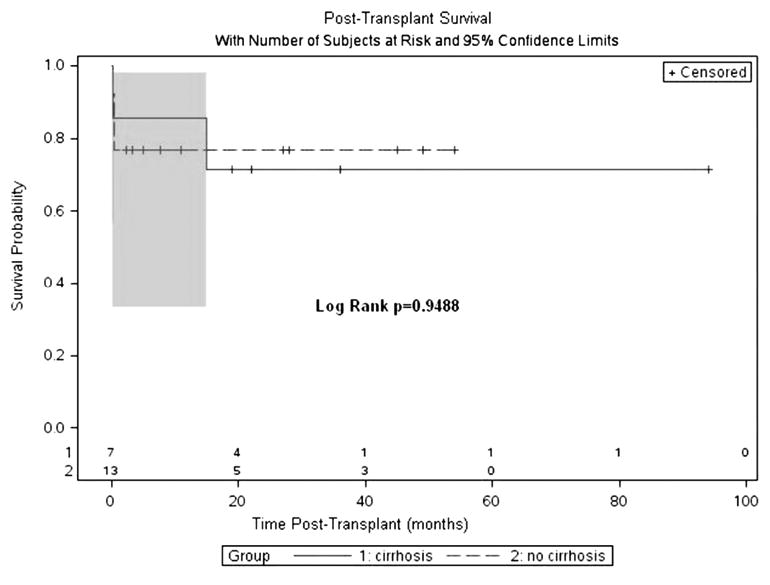
Kaplan-Meier survival analysis of long-term post-transplant survival by group. Overall survival was 75%, with no significant difference between cirrhotic and non-cirrhotic groups (71% vs 71%; p = 0.9488). Plots with 95% confidence limits are denoted by shaded area during time frame of patient events. Number of patients at risk for serial time points is shown.
Baseline characteristics at time of evaluation are reported in Table 3. Patients with cirrhosis were older (mean age 17.6 ± 2.6 vs 9.6 ± 5.3 years, p = 0.002) and had a longer median interval from the Fontan procedure (184 [IQR, 146–187] vs 48 [IQR, 28.5–187] months; p < 0.05) compared with non-cirrhotic patients. Cirrhotic patients also had a significantly higher PVRi (3 [IQR 1.9–4] vs 1.99 [IQR 1.2–2.4] WU × m2; p = 0.046), although the cardiac index and mean Fontan pressure were similar between groups. The difference in the prevalence of PLE or pre-sensitization between groups was not significant.
Table 3. Pre-Transplant Characteristics of Patients Who Underwent Transplant Compared by Cirrhosis Status at Heart Transplant Evaluationa.
| Variable | Non-cirrhotic | Cirrhoticb | p-value |
|---|---|---|---|
| (n = 13) | (n = 7) | ||
| Protein losing enteropathy | 6 (46) | 4 (57) | 1 |
| Age at listing, years | 9.6 ± 5.3 | 17.6 ± 2.6 | 0.002c |
| Time from Fontan to listing, months | 48 (28.5–100) | 184 (146–187) | <0.05c |
| Fontan pressure, mm Hg | 15 (13.5–18.5) | 17 (16–20) | 0.211 |
| PVRi, WU × m2 | 1.99 (1.2–2.4) | 3 (1.9–4) | 0.046c |
| Cardiac index, liters/min/m2 | 2.5 (2.2–4.2) | 2.37 (2.28–3.22) | 1 |
| Ischemic time, min | 270 (217–302) | 210 (165–276) | 0.21 |
| Pre-sensitized (PRA > 10%) | 4 (31) | 3 (43) | 0.651 |
PRA, panel reactive antibody; PVRi: indexed pulmonary vascular resistance; WU, Woods units.
Cirrhotic Fontan heart transplant patients were significantly older, further from time of Fontan, and had a higher PVRi at listing.
Categoric data are shown as number (%) and continuous data as mean ± standard deviation or median (interquartile range).
Statistically significant (p < 0.05).
Discussion
Our study found that most Fontan patients evaluated for HT had abnormal liver findings by CT imaging, including a substantial number with cirrhosis, consistent with previously reported findings in Fontan patients.1,3 All liver biopsy specimens reviewed showed varying degrees of fibrosis, with extensive or diffuse bridging fibrosis in 4 patients who underwent HT. However, results of CT liver findings and specimen analyses were not consistent in severity, with several biopsy samples revealing fibrosis to a lesser degree than suggested by CT imaging. This highlights the difficulty in accurately assessing the severity of liver disease in Fontan patients by CT imaging alone.
Similar to prior reports, patients with cirrhosis were significantly older and further from time of Fontan.1,3,4 Cirrhosis patients in our study who underwent HT also had a significantly higher PVRi than the non-cirrhotic group; however, the clinical significance of this is unknown because PVRi is known to be difficult to accurately measure in Fontan patients.12 Contrary to data reported by Camposilvan et al,13 however, measured cardiac index was not significantly different between groups in our study, although the prior study showed only a weak correlation (r2 = 0.34) between reduced cardiac index and increased liver disease. Kiesewetter et al3 found that hepatic vein pressure, but not hepatic vein wedge pressure, was higher in Fontan patients with cirrhosis. However, hepatic pressures were measured in only 1 patient in our study, and these values have not been routinely obtained at our institution.
Our data revealed no significance in 1-year all-cause mortality after HT between groups. Crespo-Leiro et al14 reported reversal of liver cirrhosis secondary to congestive heart failure in an adult with dilated cardiomyopathy after HT, but we could not find previous reports of post-HT mortality in Fontan patients based on the presence of liver cirrhosis. The overall 80% 1-year survival in our study was also consistent with previous post-HT outcomes in Fontan patients.15
Results of serial liver laboratory evaluation was also similar between groups and, in fact, laboratory values were within the normal reference range in most of our patients. Furukawa et al16 also found that most routine liver laboratory values on serial evaluation were within normal ranges in children after Fontan, even in patients with elevated serum markers of cirrhosis. This is contrary to reports of liver laboratory abnormalities in a substantial number of Fontan patient cohorts in previous studies.4,13 Differences may be explained by the relatively older patient population evaluated in those studies who were further from the Fontan procedure.
Liver fibrosis has been suggested to develop over time in Fontan patients, remaining mainly asymptomatic in children without derangement of standard laboratory values. There is likely a period of time before patients reach a threshold of liver damage that leads to liver dysfunction in adulthood. Indeed, Guha et al17 reported that the degree of liver fibrosis in Fontan patients was disproportional to the degree of actual liver dysfunction. Some reports have also suggested that liver fibrosis is reversible up to a point, although data are mainly from rodent models of chronic liver disease.18 Still, it may be possible that intervening on the cause of liver damage, such as by HT in a failing Fontan patient, will prevent progression to irreversible liver changes and dysfunction.
Because the physiology after the Fontan operation places patients at risk for developing liver disease long-term, the evaluation of liver disease should be part of a routine transplant evaluation in Fontan patients. This is especially important in patients with suspected liver nodules because hepatocellular carcinoma has been reported in Fontan patients.5,7,19 However, the best modality for evaluating the severity of liver disease in Fontan patients remains unclear. Although a biopsy specimen is considered the gold standard for the diagnosis of liver cirrhosis, questions regarding sampling error, invasiveness of the biopsy procedure, and differing interpretations by pathologists have been recognized.19,20 CT imaging may be limited as well to accurately define severity and determine prognosis. Serial CT imaging may also be less than ideal, with the potential risk of increasing radiation exposure in children. More recently, newer imaging modalities, such as ultrasound or magnetic resonance elastography, have shown promise as a possibly more reliable way to evaluate liver cirrhosis. Elastography may be a non-invasive way to evaluate development of liver disease, without the use of radiation, in children with Fontan over time and to offer prognostic potential. Further large, prospective multi-center studies are required to evaluate long-term outcomes in these patients and to assess if there is evidence of improvement in liver disease and cirrhosis after HT.
This study had several limitations due to the retrospective nature of the data collection over a long interval. Our results also represent a small patient sample size and single institutional experience. In addition, follow-up was limited in some patients, and no repeat liver imaging or biopsy sample was available in patients after HT. Other markers of possible liver dysfunction after HT, such as differences in peri-operative bleeding, were also difficult to accurately assess due to multiple clinical confounders and the retrospective data review.
In conclusion, overall, our pilot data suggest liver cirrhosis identified by CT imaging at HT evaluation may not be an absolute contraindication to HT alone in this population. We also showed that patients with frank cirrhosis identified on a biopsy specimen appear to have equivalent outcomes at 1 year after HT. However, the magnitude of liver disease by biopsy specimen or CT that would preclude HT alone is yet to be determined.
Acknowledgments
This publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 and by grant TL1TR000449 from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Ginde S, Hohenwalter MD, Foley WD, et al. Noninvasive assessment of liver fibrosis in adult patients following the Fontan procedure. Congenit Heart Dis. 2012;7:235–42. doi: 10.1111/j.1747-0803.2012.00632.x. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MC, Sullivan LM, Glatz AC, et al. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol. 2013;34:135–42. doi: 10.1007/s00246-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 3.Kiesewetter CH, Sheron N, Vettukattill JJ, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–84. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek JS, Bae EJ, Ko JS, et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010;96:1750–5. doi: 10.1136/hrt.2010.201772. [DOI] [PubMed] [Google Scholar]
- 5.Khanna G, Bhalla S, Krishnamurthy R, Canter C. Extracardiac complications of the Fontan circuit. Pediatr Radiol. 2012;42:233–41. doi: 10.1007/s00247-011-2225-x. [DOI] [PubMed] [Google Scholar]
- 6.Asrani SK, Asrani NS, Freese DK, et al. Congenital heart disease and the liver. Hepatology. 2012;56:1160–9. doi: 10.1002/hep.25692. [DOI] [PubMed] [Google Scholar]
- 7.Rychik J, Veldtman G, Rand E, et al. The precarious state of the liver after a Fontan operation: Summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–12. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander SA, Reinhartz O, Maeda K, Hurwitz M, NR D, Bernstein D. Intermediate-term outcomes after combined heart-liver transplantation in children with a univentricular heart. J Heart Lung Transplant. 2013;32:368–70. doi: 10.1016/j.healun.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Wallihan DB, Podberesky DJ. Hepatic pathology after Fontan palliation: spectrum of imaging findings. Pediatr Radiol. 2013;43:330–8. doi: 10.1007/s00247-012-2531-y. [DOI] [PubMed] [Google Scholar]
- 10.Kendall TJ, Stedman B, Hacking N, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: A detailed morphological study. J Clin Pathol. 2008;61:504–8. doi: 10.1136/jcp.2007.052365. [DOI] [PubMed] [Google Scholar]
- 11.Rodighiero V. Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet. 1999;37:399–431. doi: 10.2165/00003088-199937050-00004. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell MB, Campbell DN, Boucek MM. Heart transplantation for the failing Fontan circulation. Seminars in thoracic and cardiovascular surgery. Pediatr Cardiac Surg Annu. 2004;7:56–64. doi: 10.1053/j.pcsu.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Camposilvan S, Milanesi O, Stellin G, Pettenazzo A, Zancan L, D'Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177–82. doi: 10.1016/j.athoracsur.2008.03.077. [DOI] [PubMed] [Google Scholar]
- 14.Crespo-Leiro MG, Robles O, Paniagua MJ, et al. Reversal of cardiac cirrhosis following orthotopic heart transplantation. Am J Transplant. 2008;8:1336–9. doi: 10.1111/j.1600-6143.2008.02227.x. [DOI] [PubMed] [Google Scholar]
- 15.Lamour JM, Kanter KR, Naftel DC, et al. Cardiac Transplant Registry D, Pediatric Heart Transplant S. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol. 2009;54:160–5. doi: 10.1016/j.jacc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa T, Akimoto K, Ohtsuki M, et al. Non-invasive assessment of liver fibrosis in patients after the Fontan operation. Pediatr Int. 2011;53:980–4. doi: 10.1111/j.1442-200X.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 17.Guha IN, Bokhandi S, Ahmad Z, et al. Structural and functional uncoupling of liver performance in the Fontan circulation. Int J Cardiol. 2013;164:77–81. doi: 10.1016/j.ijcard.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 18.Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171–80. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Wu FM, Ukomadu C, Odze RD, Valente AM, Mayer JE, Jr, Earing MG. Liver disease in the patient with Fontan circulation. Congenit Heart Dis. 2011;6:190–201. doi: 10.1111/j.1747-0803.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]


