Abstract
Using 51V magic angle spinning solid-state NMR, SSNMR, spectroscopy and quantum chemical DFT calculations we have characterized the chemical shift and quadrupolar coupling parameters of a series of 8 hydroxylamido vanadium(V) dipicolinate complexes of the general formula VO(dipic)(ONR1R2)(H2O) where R1 and R2 can be H, CH3, or CH2CH3. This class of vanadium compounds was chosen for investigation because of their seven coordinate vanadium atom, a geometry for which there is limited 51V SSNMR data. Furthermore, a systematic series of compounds with different electronic properties are available and allows for the effects of ligand substitution on the NMR parameters to be studied. The quadrupolar coupling constants, CQ, are small, 3.0 to 3.9 MHz, but exhibit variations as a function of the ligand substitution. The chemical shift tensors in the solid state are sensitive to changes in both the hydroxylamide substituent and the dipic ligand, a sensitivity which is not observed for isotropic chemical shifts in solution. The chemical shift tensors span approximately 1000 ppm, and are nearly axially symmetric. Based on DFT calculations of the chemical shift tensors, one of the largest contributors to the magnetic shielding anisotropy is an occupied molecular orbital with significant vanadium dz2 character along the V=O bond.
Keywords: Solid-State NMR, Magic Angle Spinning, MAS, 51V, hydroxylamido complexes, dipicolinic acid, quadrupolar, chemical shift, magnetic shielding, EFG, CSA
Introduction
Studies have shown that vanadium, while a trace element in biochemical systems, can play an important role in metalloenzymes and impact insulin regulation.1, 2, 3 One class of compounds that are insulin enhancing agents are vanadium dipicolinate complexes.4, 5, 6, 7, 8 In these compounds the vanadium likely plays an important role in determining the biological activities9, 10, 11 and ligand functionalization can be used to tune the properties of these complexes.12 The parent five-coordinate dipicolinate vanadium complexes can form adducts with small ligands such as peroxides and hydroxylamines.1, 13, 14 Studies of systematic series of ternary vanadium complexes that are known to contain two different coordinating ligand systems can assist in gauging the effect of ligand substitution on the chemistry at the vanadium center. Solid-state 51V NMR has the potential to yield a three-dimensional description of the local vanadium environment and of the possible changes that take place in the molecular orbitals and electrostatic structure at the vanadium when the coordinating ligands are changed.3, 15, 16, 17, 18, 19, 20,
Many inorganic vanadium–containing coordination complexes have been studied using 51V SSNMR.21, 22, 23 The investigations of vanadium organometallic and coordination compounds have illustrated that valuable information about the molecular and electronic structure can be obtained by measuring the anisotropy of the 51V chemical shift tensor and the electric field gradient (EFG) tensor; 51V is a spin I = 7/2 quadrupolar nucleus.24, 25, 26 The 51V chemical shifts span a range of approximately 3500 ppm making it a sensitive probe of structure.19 Typical quadrupolar coupling constants, CQ, are small compared to many other half-integer quadrupolar nuclei because of the small nuclear quadrupole moment of 51V, −4.8 fm2;27 CQ values for highly coordinated vanadium sites are typically less than 15 MHz.19, 21, 28 51V solid-state NMR spectroscopic studies of the active site of vanadium chloroperoxidase enzymes have provided insights such as details about the protonation mechanism of the peroxide.3, 20 In general, many of these studies have illustrated that the anisotropy of the 51V chemical shift tensor is more informative than the isotropic chemical shifts alone.28
Modern quantum chemical calculations have proven invaluable for understanding the origins of the NMR parameters in transition metal complexes.29, 30, 31 Calculations of 51V NMR parameters have been performed for a range of vanadium compounds and have employed both single-molecule and extended-structure models.26, 27, 32, 33, 34 These studies have suggested that in non-ionic coordination complexes, both the magnetic shielding and the EFG tensors are predominantly determined by the local structure about the vanadium, and that the extended crystal lattice plays only a minor role. Good agreement between experimental and calculated NMR parameters has been obtained for vanadium.26 To better understand the relationships between the NMR parameters and the reactivity at the vanadium center, more experimental and computational NMR investigations of systems with variations in the electronic properties of ligands and vanadium are required.
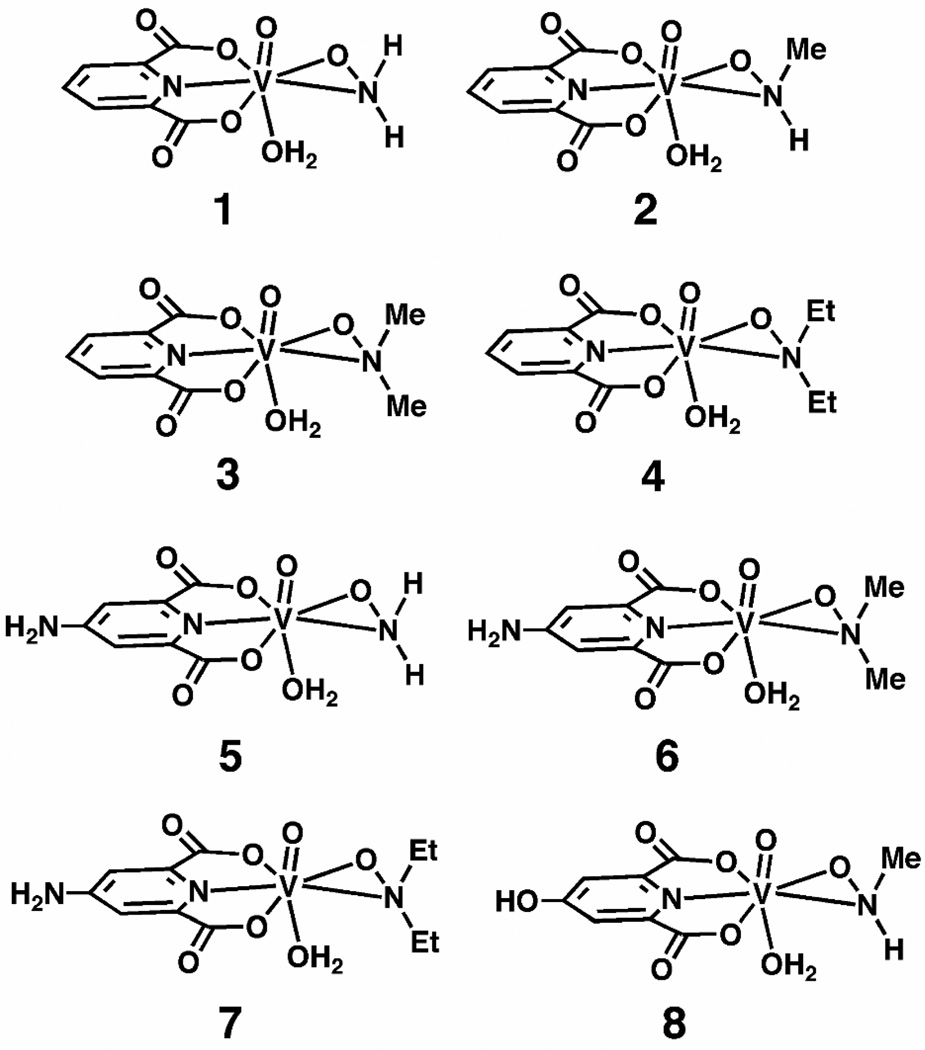
In this work, we have investigated a series of eight seven-coordinate hydroxylamido vanadium(V) dipicolinate (dipic) complexes, Figure 1, using 51V solid-state and solution NMR spectroscopy, and quantum chemical DFT calculations. The compounds differ in the functionality at either the hydroxylamido ligand (hydrogen replaced with methyl, Me, or ethyl, Et, groups) or the dipic ligand, which was substituted in the para position with respect to the nitrogen of the ring. Single-crystal x-ray diffraction studies reveal that there is one unique molecule in the unit cell, and that in addition to the dipic and hydroxylamido ligands, a water molecule is trans to the V=O bond with a bond length of approximately 2.45 Å.37 This is a very long bond, and likely to be weak. Interestingly, the comparison of experimental solid-state and solution NMR spectroscopic chemical shifts demonstrates that ligand-associated changes lead to a greater variation in the anisotropic tensors than in the isotropic values. The combined experimental and computational results reveal that in this series, the quadrupolar coupling constants and chemical shift anisotropies display a relatively small variation when the ligand is substituted.
Figure 1.
Schematic representations of the eight vanadium(V) compounds under investigation. [VO(dipic)(ONH2)(H2O)] (1), [VO(dipic)(ONHMe)(H2O)] (2), [VO(dipic)(ONMe2)(H2O)]·0.5H2O (3), [VO(dipic)(ONEt2)(H2O)] (4), [VO(dipic-NH2)(ONH2)(H2O)] (5), [VO(dipic-NH2)(ONMe2)(H2O)]·H2O (6), [VO(dipic-NH2)(ONEt2)(H2O)] (7), [VO(dipic-OH)(ONHMe)(H2O)]·H2O (8).
Experimental
Sample preparation
Sodium metavanadate (96%), potassium permanganate and hydroxylamine hydrochloride (NH2OH·HCl) were purchased from Fisher Scientific. Ammonium metavanadate (99%), potassium metavanadate (98%), dipicolinic acid (also referred to as 2,6-pyridinedicarboxylic acid, H2dipic), N-methylhydroxylamine hydrochloride (MeNHOH·HCl), N, N-dimethylhydroxylamine hydrochloride (Me2NOH·HCl) and aqueous N, N-diethylhydroxylamine (Et2NOH, 85% w/w) were purchased from Aldrich. Chelidamic acid (4-hydroxy-2,6-dipicolinic acid) monohydrate was purchased from TCI America. The NH4[VO2(dipic)] complex,35 Na[VO2(dipic-OH)]·2H2O,36 and [VO(dipic)(ONH2)(H2O)]37 were prepared as previously reported. The (NH4)2dipic-NH2 was prepared modifying a previously reported synthesis.38 Sodium 4-aminodipicolinatooxovanadate(V) dihydrate, Na[VO2(dipic-NH2)]·2H2O was prepared similarly as reported previously for the NH4+ salt.4 IR spectroscopy on the compounds was carried out (either as KBr pellets, or as Nujol mulls on NaCl plates) on an Avatar 320 FTIR. Elemental analysis (C, H, N) was carried out by Desert Analytics in Tucson, Arizona.
Aquadipicolinatohydroxylamidooxovanadium(V) [VO(dipic)(ONH2)(H2O)] (1)
The compound was prepared as described previously.37 1H NMR (D2O, ppm): 8.63 (t), 8.39 (dd, 2H). 51V NMR (D2O, ppm): –681.
Aquadipicolinatomethylhydroxylamidooxovanadium(V), [VO(dipic)(ONHMe)(H2O)] (2)
The compound was prepared as described previously by first preparing the NH4[VO2(dipic)] complex35 from NaVO3 and H2dipic.13 1H NMR (D2O, ppm): 8.64 (t, 1H), 8.39 (dd, 2H), 3.90 (s, 3H), 3.43 (s, 3H). 51V NMR (D2O, ppm): –634, –647.
Aquadipicolinatodimethylhydroxylamidooxovanadium(V) 0.5 hydrate [VO(dipic)(ONMe2)(H2O)]·0.5H2O (3)
The compound was prepared analogous to 2 using Me2NOH·HCl in place of MeHNOH·HCl as described previously by first preparing the NH4[VO2(dipic)] complex35 from NaVO3 and H2dipic.13 1H NMR (D2O, ppm): 8.62 (t, 1H), 8.38 (dd, 2H), 3.95 (s, 3H), 3.42 (s, 3H). 51V NMR (D2O, ppm): –614.
Aquadipicolinatodiethylhydroxylamidooxovanadium(V) [VO(dipic)(ONEt2)(H2O)] (4)
The compound was prepared analogous to 2 using Et2NOH in place of MeHNOH·HCl as described previously by first preparing the NH4[VO2(dipic)] complex35 from NaVO3 and H2dipic13 1H NMR (D2O, ppm): 8.62 (t, 1H), 8.38 (dd, 2H), 3.87 (m, 2H), 3.44 (m, 2H), 1.59 (t, 3H), 1.41 (t, 3H). 51V NMR (D2O, ppm): –598.
Ammonium 4-aminodipicolinate, (NH4)2(dipic-NH2)
The ligand was prepared modifying the literature procedure38 as described in by Smee et. al..13 1H NMR (D2O, pH 7.0, ppm): 7.13 (s, py-H, 2H).
Sodium 4-aminodipicolinatodioxovanadate dihydrate, Na[VO2(dipic-NH2)]·2H2O
Na[VO2(dipic-NH2)]·2H2O was prepared similarly as previously reported for the NH4+ salt.4, 13 1H NMR (D2O, ppm): 7.1 (s, py-H, 2H). 51V NMR (D2O, ppm): –525.
Aqua-4-aminodipicolinatohydroxylamidooxovanadium(V) [VO(dipic-NH2)(ONH2)(H2O)] (5)
The compound was prepared as described previously from Na[VO2(dipic-NH2)]·2H2O.13 1H NMR (D2O, ppm): 7.20 (s, 2H). 51V NMR (D2O, ppm): –688.
Aqua-4-aminodipicolinatodimethylhydroxylamidooxovanadium(V) monohydrate [VO(dipic-NH2)(ONMe2)(H2O)]·H2O (6)
The compound was prepared using an analogous procedure to 5 using Me2NOH·HCl instead of H2NOH·HCl as described previously from Na[VO2(dipic-NH2)]·2H2O.13 1H NMR (D2O, ppm): 7.20 (dd, 2H), 3.81 (s, 3H), 3.28 (s, 3H). 51V NMR (D2O, ppm): –619.
Aqua-4-aminodipicolinatodiethylhydroxylamidooxovanadium(V) [VO(dipic-NH2)(ONEt2)(H2O)] (7)
The compound was prepared using an analogous procedure to 5 using Et2NOH instead of H2NOH·HCl as described previously from Na[VO2(dipic-NH2)]·2H2O. 13 1H NMR (D2O, ppm): 7.18 (dd, 2H), 3.71 (m, 2H), 3.35 (m, 2H), 1.43 (t, 3H), 1.26 (t, 3H). 51V NMR (D2O, ppm): –604.
Aqua-4-hydroxydipicolinatomethylhydroxylamidooxovanadium(V) [VO(dipic-OH)(ONHMe)(H2O)]·H2O (8)
The [VO(dipic-OH)(ONMe2)(H2O)] was prepared most consistently by using Na[VO2(dipic-OH)]·2H2O prepared as described previously.36 Na[VO2(dipic-OH)]·2H2O (0.32 g, 0.99 mmol) was dissolved in H2O (50 ml). MeHNOH·HCl (0.083 g, 1.0 mmol) was added to it and the clear solution was allowed to stir for 1 hour. A light yellow solution of pH ~3 was kept at 4°C for 72 hrs. The precipitate, which had formed, was filtered off and vacuum dried. Yield: 0.15 g (50%). IR (NaCl, ν(COO), ν(V=O)), cm−1): 1653 (br,s), 1461 (s), 1377 (vs), 1064 (vs), 994 (m). 1H NMR (D2O, pH 2.68): 7.61 (dd, 2H), 3.88 (s, 3H), 3.4 (s, 3H). 51V NMR (D2O, pH 2.68): –637, –650. Calcd for C8H9N2O8V: C 25.60, H 3.76, N 8.05. Found: C 27.63, H 3.49, N 7.89.
Solution Preparation and Solution State 51V NMR Spectroscopy
The 1H and 51V spectra were recorded on a Varian INOVA-300 spectrometer (7.0 T) at 300 MHz for 1H and 78.9 MHz for 51V. Routine parameters were used for the 1H NMR spectra and DSS (3-(trimethylsilyl)-propanesulfonic acid, sodium salt) was used as the external reference for the 1H chemical shifts. The 51V NMR spectra were generally acquired with a spectral window of 83.6 kHz, a pulse angle of 60° and an acquisition time of 0.096 s with no relaxation delay. The 51V chemical shifts were obtained using an external reference of VOCl3, δiso = 0.00 ppm.
Solid-State NMR spectroscopy
Solid-state 51V NMR spectra were acquired on a Tecmag Discovery spectrometer, interfaced with a wide bore 9.4 T Magnex magnet, and operating at a frequency of 105.23 MHz for 51V. Vanadium chemical shifts were referenced to neat VOCl3, δiso = 0.0 ppm, as an external reference.39 This sample was also used to determine the 90° pulse widths of 4.0 µs (δB1/2π ≈ 62 kHz). The magic angle, MA, was set by maximizing the number of rotational echoes observed in the 23Na NMR free induction decay of solid NaNO3. The angle was set for each sample; accurate setting of the MA is critical when acquiring spectra from the satellite transitions of quadrupolar nuclei over a broad frequency range.21 A 4 mm Doty XC4 MAS probe was used for acquiring all the spectra. For each sample, spectra at several MAS frequencies ranging from 11 to 17 kHz were collected. The MAS frequency was controlled by an automated Tecmag MAS Control Unit to within 5 Hz. All spectra were acquired using a one-pulse experiment with a pulse of 1µs, a spectral width of 2 MHz, and a recycle time of 1 s. Proton decoupling did not significantly improve the spectra and was therefore not used. The NMR spectra were processed with Gaussian line broadening functions of 100 Hz, and baseline corrections. Spectra of the entire manifold of central and satellite transitions were simulated with SIMPSON,40 using 1 µs excitation pulses at a 62 kHz B1 field; these settings match the experimental conditions.
Computations
Gaussian0341 was used to calculate the vanadium magnetic shielding and EFG tensors. The B3LYP42, 43 functional and the 6-311+G(p,d) basis set were employed for all atoms, as available in the Gaussian package; molecular orbitals and electrostatic potential surfaces were displayed using GaussView3.09.44 Natural bond order, NBO,45 analysis and natural chemical shielding, NCS,46 analysis were also performed using Gaussian03. The calculated principal components of the magnetic shielding tensor, σii (i = 1, 2, or 3), were converted to the principal components of the chemical shift tensor, δii, using the relation δii = σiso (ref) − σii, where σiso (ref) = −2286 ppm and is the calculated isotropic magnetic shielding of VOCl3. The structures used in the calculations were obtained from single-crystal x-ray diffraction data37, 13 or the geometry optimized structure of VOCl3; optimization was performed using B3LYP/6-311+G(d,p). Carbon-hydrogen bond lengths were set to 1.08 and 1.09 Å for carbons bonded to three and four other atoms, respectively. Nitrogen-hydrogen and oxygen-hydrogen bond lengths were set to 1.06 and 0.96 Å, respectively. 47
Results
Hydroxylamido Vanadium(V) Dipicolinate Complexes
A number of vanadium(V) hydroxylamido complexes have been prepared, all containing seven-coordinate vanadium atoms. Although the vanadium complex is susceptible to hydrolysis and reduction, preparation of the parent vanadium dipicolinate complex prior to addition of hydroxyl amine significantly reduces the amount of redox chemistry and side-product formation. All the complexes described in this manuscript have been prepared by this method. Details of the preparation and solution properties of this class of compounds have been described elsewhere.13 However, in this manuscript we provide sufficient information to allow a comparison of the aqueous solution and solid state NMR chemical shifts.13
Solid-State 51V NMR spectroscopy
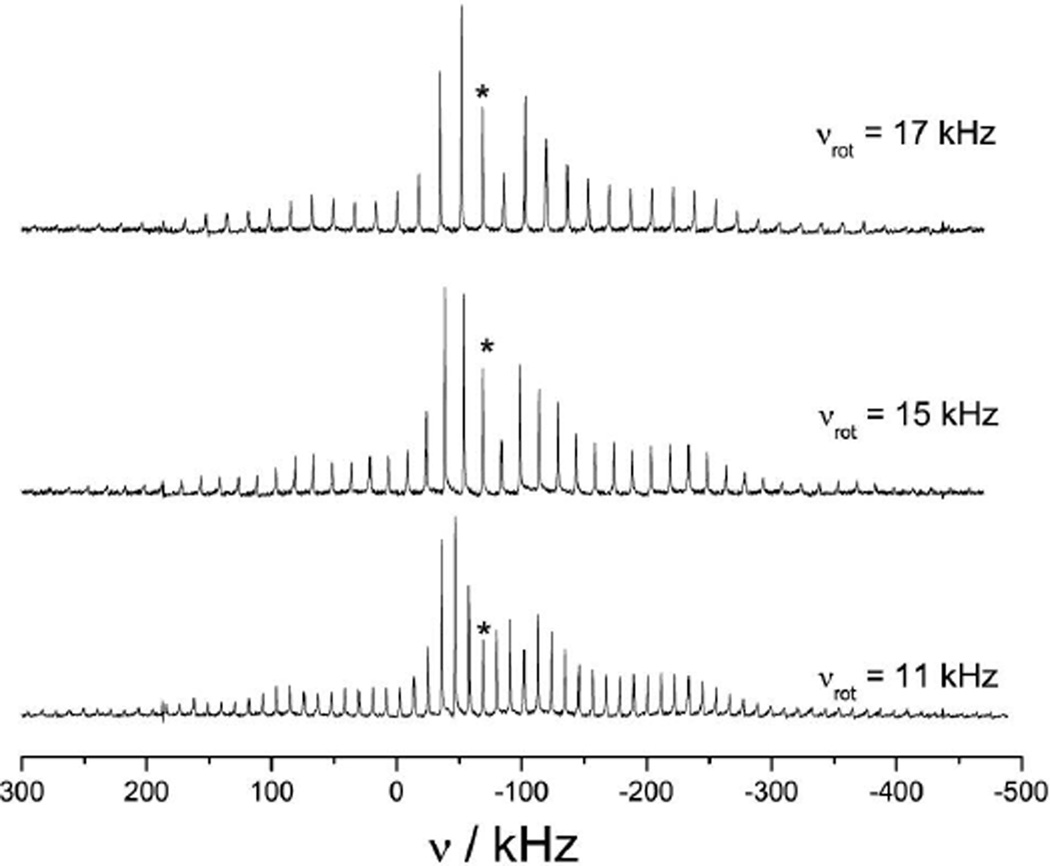
For each of the samples, 51V NMR spectra were acquired at 9.4 T using several MAS rates (νrot). Figure 2 shows the spectra acquired of V(V)O(dipic-OH)(ONHMe)(H2O), 8, when the sample was spun at 11 kHz, 15 kHz and 17 kHz. The isotropic chemical shifts are indicated with asterisks in Figure 2. The use of different MAS rates allows for accurate determination of the anisotropic observables from numerical simulations. Figure 3 shows a representative spectrum of VO(dipic)(ONH2)(H2O), 1, acquired at the MAS frequency of 11 kHz, along with an expansion of the central region of the spectrum. From the expansion it can be seen that each spinning side band has a nearly isotropic lineshape, and the center-band, δiso, is near −71 kHz (−677 ppm). The isotropic lineshapes of the individual spinning side bands indicate that the 51V CQ is small, probably less than 4 MHz, which results in no noticeable second-order quadrupolar lineshape at 9.4 T. The small CQ values also lead to small second-order quadrupolar shifts which cause the center-bands from the various transitions to overlap; the small shoulder on the high frequency sides of the peaks, Figure 3 inset, is from the small difference in the shifts of the different transitions. The 51V δiso values can therefore be easily obtained from the centerband because any second order quadrupolar shifts are small. However, the small CQ values mean that the quadrupolar coupling constants cannot be obtained by fitting the lineshapes of the individual central-transition spinning side bands as is commonly done for quadrupolar nuclei, but rather the entire spinning side band manifold, spanning the central and satellite transitions, needs to be examined as discussed below.
Figure 2.
51V SSNMR spectra of 8 acquired at 9.4 T with MAS rates of 11, 15 and 17 kHz. Each spectrum is the sum of 8192 scans. The asterisk indicates the center-band of the spectrum.
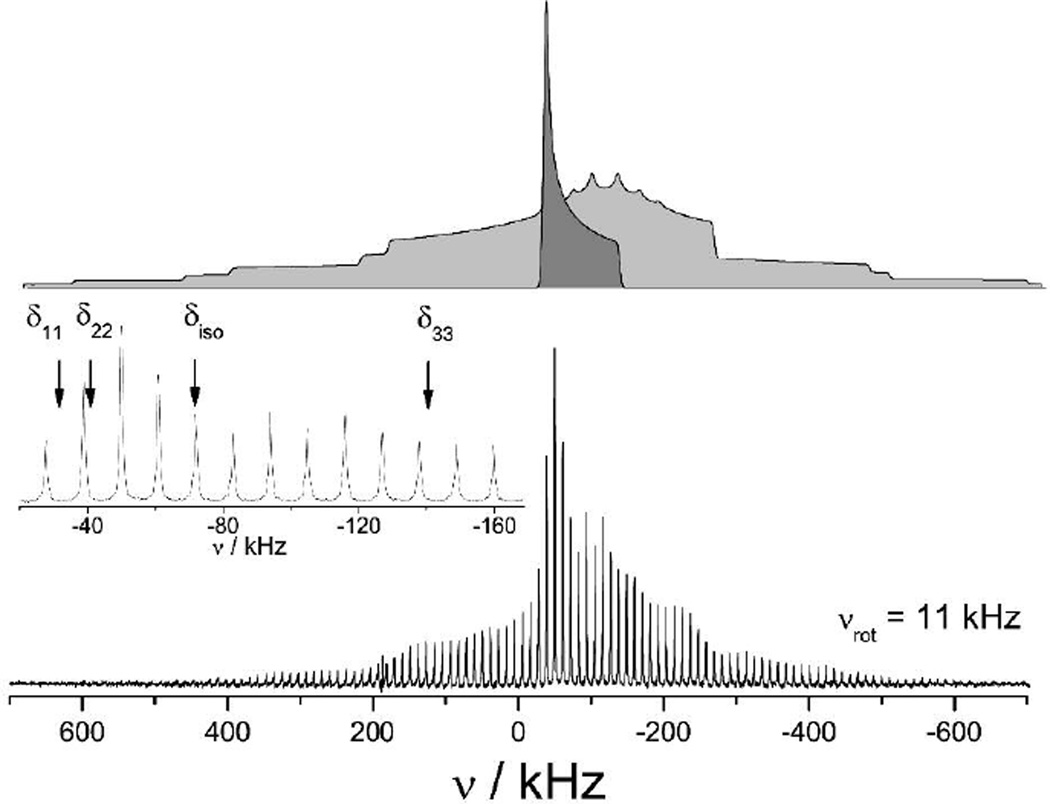
Figure 3.
Bottom: 51V SSNMR spectrum of 1 acquired at 9.4 T with an MAS rate of 11 kHz. The inset spectrum is an expansion of the central transition region of the spectrum illustrating the lack of second-order quadrupolar lineshapes. Top: a stationary spectrum calculated using the parameters in table 1 illustrating the contributions from the central transition (dark grey) and the satellite transitions (light grey). The spinning side band envelope in the experimental spectrum mimics the overall lineshape of these calculated spectra.
The overall shape of the spinning side band envelope is dominated by the contribution of the first-order quadrupolar interaction to the 51V NMR satellite transitions. Due to the small CQ, the 51V NMR signal in this series under investigation originates from both the satellite transitions (mI = ±7/2↔±5/2, ±5/2↔±3/2, ±3/2↔±1/2), light grey spectrum in Figure 3, and the central transition (mI =+1/2↔−1/2), the dark grey spectrum in Figure 3. The central transition is not affected by the quadrupolar interaction to first order and consequently, the influence of the chemical shift anisotropy is readily apparent in this region.48 The spinning side bands from the central transition span approximately 100 kHz and display a pattern typical of an axially symmetric chemical shift tensor.
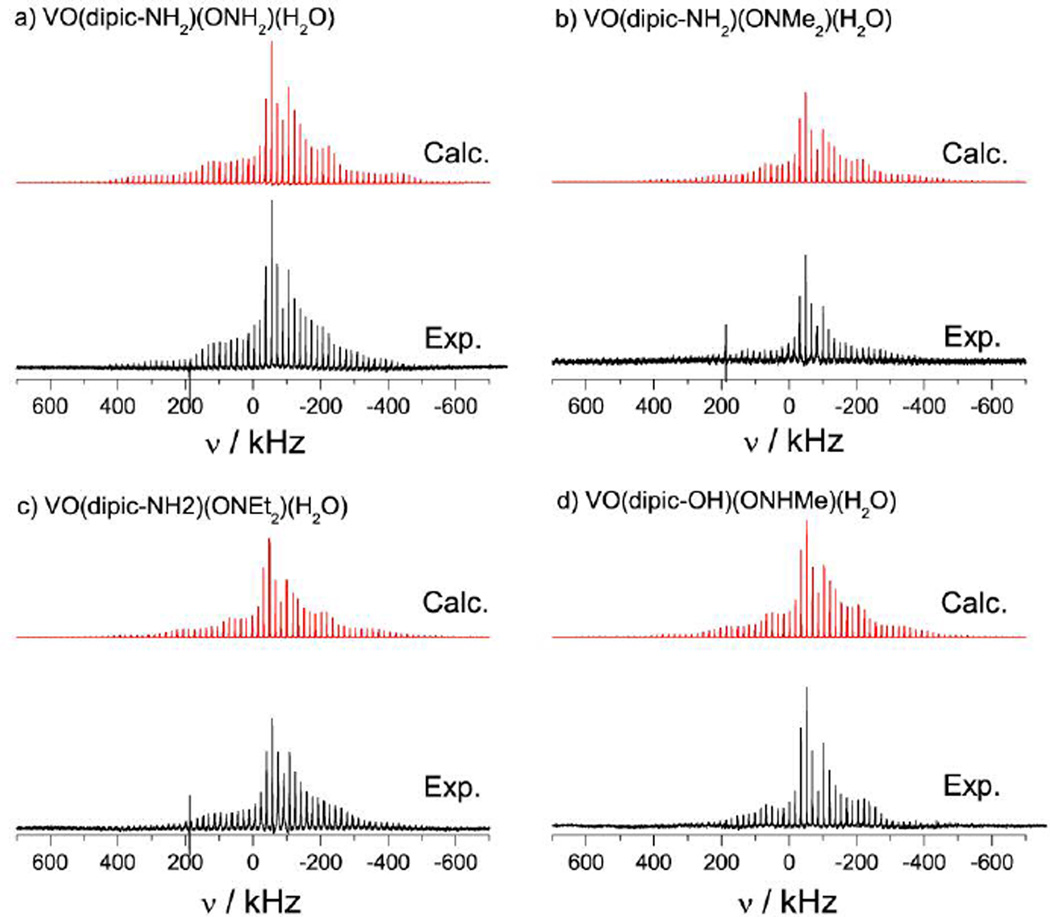
By fitting calculated spectra to the experimental spectra, Figure 4 and 5, the 51V NMR parameters can be obtained: the CQ and ηQ parameters can be determined primarily from the satellite transitions, which exhibit only small affects of the chemical shift parameters, while the chemical shift parameters can be determined from the central transition, which exhibits only minor contributions from the second-order quadrupolar interaction. The 51V NMR observables for the eight samples studied here are presented in Table 1; definitions of the NMR parameters used can be found in the footnote of Table 1. The chemical shift tensors are nearly axially symmetric (δ11 ≈ δ22) for all complexes with spans (δ11 - δ33) of approximately 930 to 1120 ppm. The principal components of the chemical shift tensor are indicated in the expansion of the spectrum acquired for VO(dipic)(ONH2)(H2O) at 17 kHz spinning frequency, see Figure 3. The CQ values are all between 3.0 and 3.9 MHz with ηQ values between 0.4 and 0.8.
Figure 4.
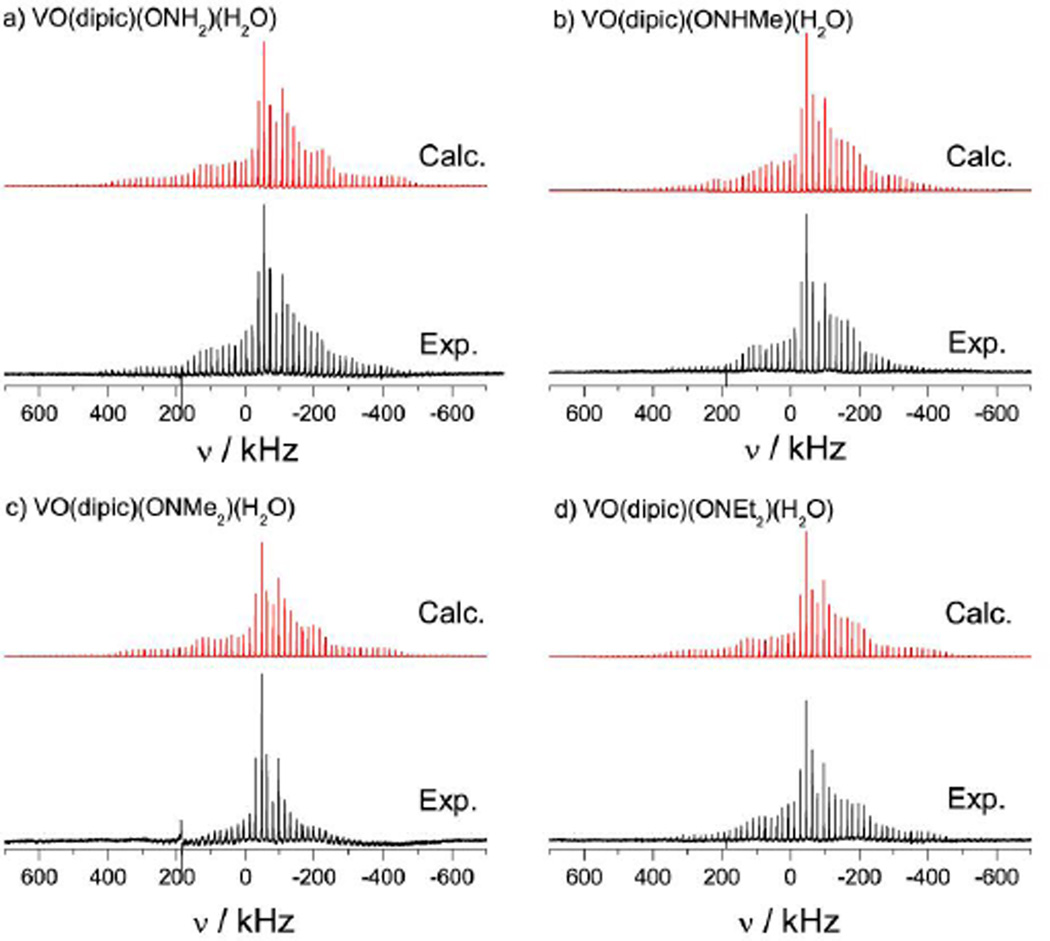
51V SSNMR spectra of samples of a) 1, b) 2, c) 3, and d) 4, acquired at 9.4 T with the MAS rate of 17 kHz. The calculated spectra were obtained using the parameters presented in Table 1. Each experimental spectrum is the sum of 8192 scans except for c) which is a sum of 16,384 scans.
Figure 5.
51V SSNMR spectra of samples of a) 5, b) 6, c) 7, and d) 8, acquired at 9.4 T with MAS rates of 17 kHz. The calculated spectra were obtained using the parameters presented in Table 1.
Table 1.
| X | L | CQ /MHz ± 0.1 |
ηQ ± 0.0 |
δiso / ppm ± 5 |
δ11 / ppm ± 30 |
δ22 / ppm ± 30 |
δ33 / ppm ± 30 |
α4/° ± 30 |
β4/° ± 20 |
δiso (solution) / ppm |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | ONH2 | 3.4 | 0.8 | −678 | −315 | −381 | −1338 | 20 | 90 | −681 |
| 2 | H | ONHMe | 3.0 | 0.5 | −612 | −293 | −351 | −1192 | 20 | 90 | −634 / −647 |
| 33 | H | ONMe2 | 3.2 | 0.8 | −597 | −256 | −314 | −1217 | 20 | 90 | −614 |
| 4 | H | ONEt2 | 3.3 | 0.7 | −590 | −280 | −280 | −1210 | 10 | 90 | −598 |
| 5 | NH2 | ONH2 | 3.9 | 0.8 | −710 | −314 | −386 | −1430 | 40 | 90 | −688 |
| 6 | NH2 | ONMe2 | 3.4 | 0.4 | −627 | −242 | −312 | −1327 | 35 | 90 | −619 |
| 73 | NH2 | ONEt2 | 3.5 | 0.7 | −621 | −236 | −306 | −1321 | 25 | 90 | −604 |
| 8 | OH | ONHMe | 3.2 | 0.4 | −666 | −270 | −342 | −1386 | 35 | 90 | −637 / −650 |
The chemical shift parameters are defined such that δ33 ≤ δ22 ≤ δ11 and δiso = (δ11 + δ22 + δ33)/3.56 The components of the chemical shift tensor are δii = (vii - vref)/vref (ii = 11, 22, 33).
The EFG parameters are CQ = eQVZZ/h and ηQ = (VXX-VYY)/VZZ where |VZZ| |VYY| |VXX|, e is the electron charge and h is Planck’s constant.
Due to low signal to noise the errors are approximately 50% greater than reported in the table headings.
The Euler angle γ had no noticeable affect on the NMR lineshapes.
The two chemical shifts listed for samples 2 and 8 arise from the compounds adopting two different conformations in solution.
he relative orientation of the two tensors also plays a pronounced role in determining the lineshape of the spinning side band envelope, particularly the angle between δ33 and VZZ, β (see reference 49 for a discussion of the Euler angle convention used). In general the lineshapes were fit best using an α angle of 10 to 50° ± 30° for different samples while the β angle was 90°± 20°;50 γ had no significant effect due to the near axial symmetry of the chemical shift tensor.
Quantum chemical calculations
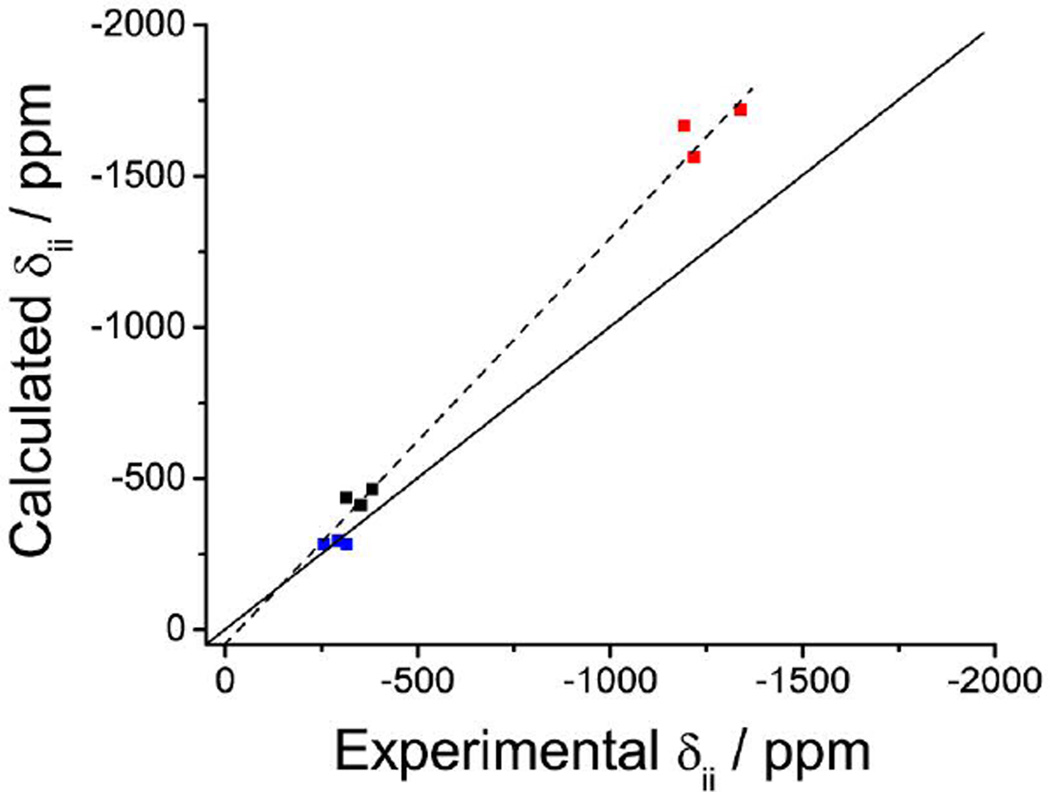
Crystal structures obtained from single-crystal x-ray diffraction were available for three of the eight complexes.13, 37 These structures were used to calculate the magnetic shielding and the EFG tensors at the vanadium nucleus. Overall, the results presented in Table 2 indicate that the calculations are in reasonable agreement with experiment. The CQ values are all within 0.8 MHz of the experimental values; however, the asymmetry parameter of the EFG tensor is not well reproduced by the calculations. This is not a surprising result in light of prior reports demonstrating that DFT calculations at the single molecule or small cluster level may not be able to define the principal components of the EFG tensor well.29, 30 In the compounds under investigation, since the CQ is so small, any errors in calculating the principal components of the EFG tensor could result in large errors in the calculated asymmetry. In contrast, the axial symmetry and isotropic values of the magnetic shielding tensor are reproduced by the calculations while the calculated anisotropy of the tensor is consistently greater than that observed experimentally. By comparing the principal components of the chemical shift tensor, see Figure 6, it can be seen that most of the discrepancy between calculated and experimental chemical shift parameters lies in the most shielded component, δ33. Note that δ11 has a deviation within experimental error, δ22 deviates by 100 to 150 ppm, while δ33 deviates by 330 to 430 ppm. Previously, quantum chemical calculations of the chemical shift anisotropy of vanadium coordination complexes have shown good agreement with experiment for complexes with O and N coordinated to the V.26
Table 2.
Calculated 51V NMR parameters for solid VO(dipic) L (H2O) compounds.
| L | CQ / MHz | ηQ | δiso / ppm | δ11 / ppm | δ22 / ppm | δ33 / ppm | |
|---|---|---|---|---|---|---|---|
| 1 | ONH2 | 3.43 | 0.68 | −822 | −282 | −464 | −1721 |
| 2 | ONHMe | 2.82 | 0.36 | −790 | −294 | −412 | −1666 |
| 3 | ONMe2 | 4.09 | 0.38 | −768 | −283 | −438 | −1564 |
Figure 6.
Plots of the experimental and calculated δii values (ii = 11, 22, 33). Solid black line corresponds to perfect agreement, while the dashed black line is the best fit line; δcalc(ppm) = 1.4 δexp(ppm) + 76, R2 = 0.99.
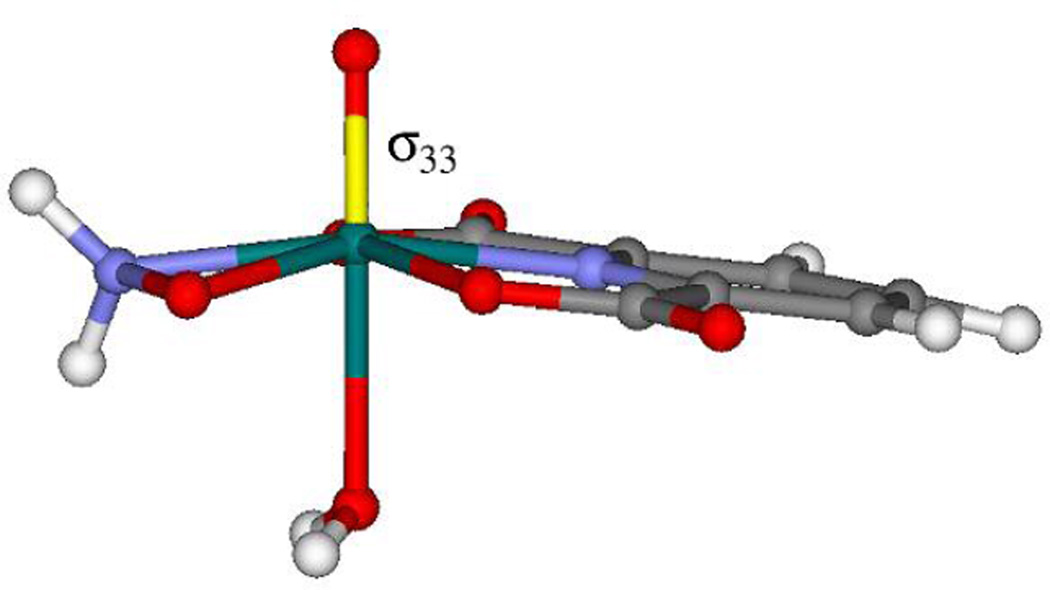
The orientation of the tensors in the molecular frame, which is not available from the NMR experiments, has also been obtained from the calculations. However, given the errors in the EFG asymmetry parameter the reliability of the EFG orientation obtained from the calculations is questionable. On the other hand, since the axial symmetry of the magnetic shielding is well reproduced by the calculations it is expected that the orientation of the tensor is also qualitatively correct, despite the errors in δ33 noted above. Only the unique component of the nearly axially symmetric tensor needs to be specified to fix the orientation of the tensor in the molecular frame. Figure 7 shows the orientation of σ33 in compound 1; calculations indicate that the orientation is the same in all three complexes for which calculations were performed. σ33 is perpendicular to the dipic plane and approximately coincident with the V=O bond. This orientation is similar to that previously observed for vanadium in (NH4)[VO(O2)(H2O)(dipic)].26
Figure 7.
The molecular structure of 1 obtained from single-crystal x-ray diffraction with the orientation of σ33 as predicted by the quantum chemical calculations indicated in yellow.37 Carbons are grey, hydrogens are white, oxygens are red, nitrogens are blue and vanadium is green.
The sign of CQ is another parameter not available from 51V NMR spectroscopy but available from the DFT calculations. The calculations indicate that CQ is positive for all three complexes.
Discussion
In the drive to optimize the biological activity of vanadium containing compounds the coordinating ligands can be functionalized to tune properties such as solubility, physiological uptake, and the stability of the complex under physiological conditions. Defining how the electronic properties at the vanadium center change, will assist in fine-tuning complex design. By characterizing a series of ternary complexes, changes in two different ligand systems can be monitored and the influence of each substituent on the vanadium center can be established. These hydroxylamido vanadium(V) dipicolinate complexes are of interest because the ternary complexes contain hydroxylamido and dipicolinate ligands, and the vanadium atom is seven coordinate; previous SSNMR studies have mostly focused on other coordination geometries. Furthermore, the solution 51V NMR studies of these compounds demonstrated that the isotropic chemical shifts change upon modification of the hydroxylamido ligand, whereas only small changes were observed when the dipicolinate group was functionalized. However, knowledge of the isotropic chemical shifts in solution are insufficient for deriving information about the three-dimensional electronic and geometric structure of the vanadium site,20, 26 and therefore the anisotropic EFG and chemical shift tensor parameters measured here by SSNMR and calculated using DFT are important.
The compounds studied here can be divided into two sets for comparison, compounds where the protons on the hydroxylamine are changed with alkyl groups, and compounds containing different substituents in the para position of the dipic ligand. For compounds 1 to 4 the hydroxylamido ligand is ONH2, ONHMe, ONMe2, and ONEt2, respectively, see Figure 4. In general, there is a very minor change in the electric field gradient upon alkylation of the hydroxylamine: the CQ values are 3.4, 3.0, 3.2, and 3.3 MHz; the differences are close to the experimental error. Compounds 5 to 7 also have different hydroxylamido groups but have a functionalized dipic (dipic-NH2). For these ONH2, ONMe2, and ONEt2 complexes the CQ values are 3.9, 3.4 and 3.5 MHz, respectively, also revealing a very small variation in the EFG tensor at the vanadium site. Similarly, we observed only a small change in the EFG tensor when the substituent on the dipic ligand is modified. Samples 3, 6 and 8 have H, NH2, and OH groups para to the nitrogen of the dipic ring, and their CQ values were found to be 3.4, 3.9, and 3.2 MHz, respectively, see Figure 5 and Table 1. These results indicate that changing the functionality of the hydroxylamine or the dipic has a relatively small effect on the electrostatic environment at the vanadium.
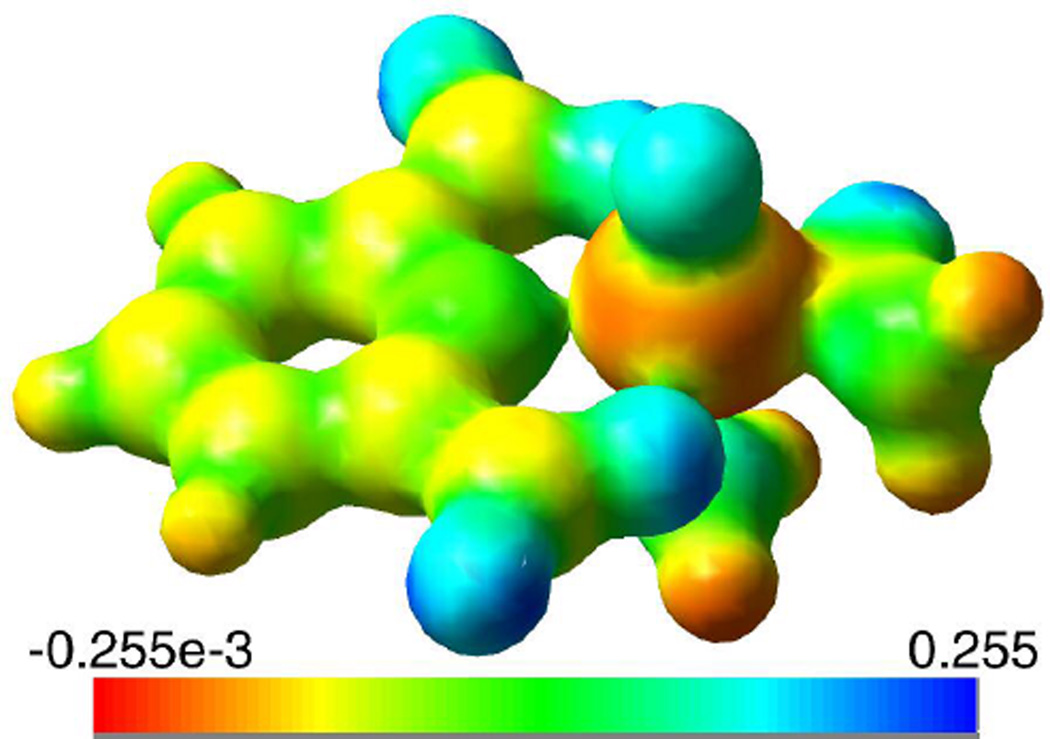
We note that the EFG is the second derivative of the electric potential and the tensor is determined by the partial charge distribution around the vanadium atom, and has an 〈r−3〉 dependence.22, 26 As mentioned, the quadrupolar couplings in these compounds are relatively small considering they do not adopt octahedral or tetrahedral coordination geometries; perfect octahedral and tetrahedral geometries would produce no EFG at the vanadium.51 By examining the electrostatic potential surface insights into the origin of the small EFGs can be gained. Figure 8 shows the ESP surface of compound 1. As can be seen the five coordinated oxygens create similar potentials about them (the blue in Figure 8 indicates regions that attract negative charge). In comparison, the potentials are much smaller in the proximity of the nitrogen atoms (green color). Given the small potentials at the nitrogens, and despite the 7-coordinate bicapped-pentagonal coordination geometry the potential surface in the immediate coordination environment about the vanadium can be best described as either a distorted trigonal prism or capped square planar arrangement. Point charge geometries such as trigonal prism, trigonal-bipyramidal and capped square planar produce small to moderate EFGs.51 The small additional charges from the nitrogens and the extended structure will serve to increase the spherical symmetry of the charge about the vanadium, decreasing the EFG even further.
Figure 8.
An image of the electrostatic potential surface of [VO(dipic)(ONH2)(H2O)] (1) created from the total SCF density calculated in Gaussian. The regions of the positive charges are colored in blue while the regions of the negative charge are depicted in red. The color legend is shown at the bottom.
The small variations in the EFG between the different compounds suggest that the changes in functionality produce almost no change in the charge distribution of the coordinating O and N atoms from the dipic or hydroxylamine ligands; there was negligible change in the electrostatic potential surfaces near the vanadium in compounds 1, 2 and 3. For the complexes that have different hydroxylamine ligands this observation may not be surprising given the fact that the substituent groups have similar electronegativity, H, CH3 and CH2CH3. It is interesting that substitution on the dipic ligand, with the NH2 and OH replacing the H, does not perturb the EFG at the vanadium site, again indicating very minor changes in the charge distribution close to the vanadium. These observations have important consequences because they suggest that ligand functionality in this class of compounds can be tuned for specific biological applications without causing a large change in the electrostatic environment of the vanadium.
The anisotropy of the vanadium CS tensors shows stronger variation than the EFG tensors. Of the three principal components of the CS tensor, δ33 exhibits the greatest variation, 238 ppm, while δ11 and δ22 show variations of 79 and 106 ppm, respectively, see Table 1. We note that the experimental error is approximately ± 30 ppm. In general, δ33 increases as the hydroxylamine is changed through the series ONH2, ONMe2, and ONEt2. This trend was observed for the dipic, 1, 3, and 4, and dipic-NH2, 5–7, series of compounds, where δ33 varies by 128 and 109 ppm, respectively. Comparison of the results for the two sets of compounds indicates that δ33 decreases by approximately 100 ppm when the dipic is changed to dipic-NH2, see Table 1. A larger decrease in δ33 of 194 ppm is observed when the dipic is replaced by dipic-OH, compounds 2 and 8. Since δ33 arises from the mixing of orbitals perpendicular to the V=O bond, vide infra, the changes in δ33 arise from changes in the molecular orbitals in the dipic plane as the functionality of the dipic and oxyamide ligands are changed. This means that δ33 can provide insights into the molecular orbitals that are responsible for the ligand-V binding in these compounds.
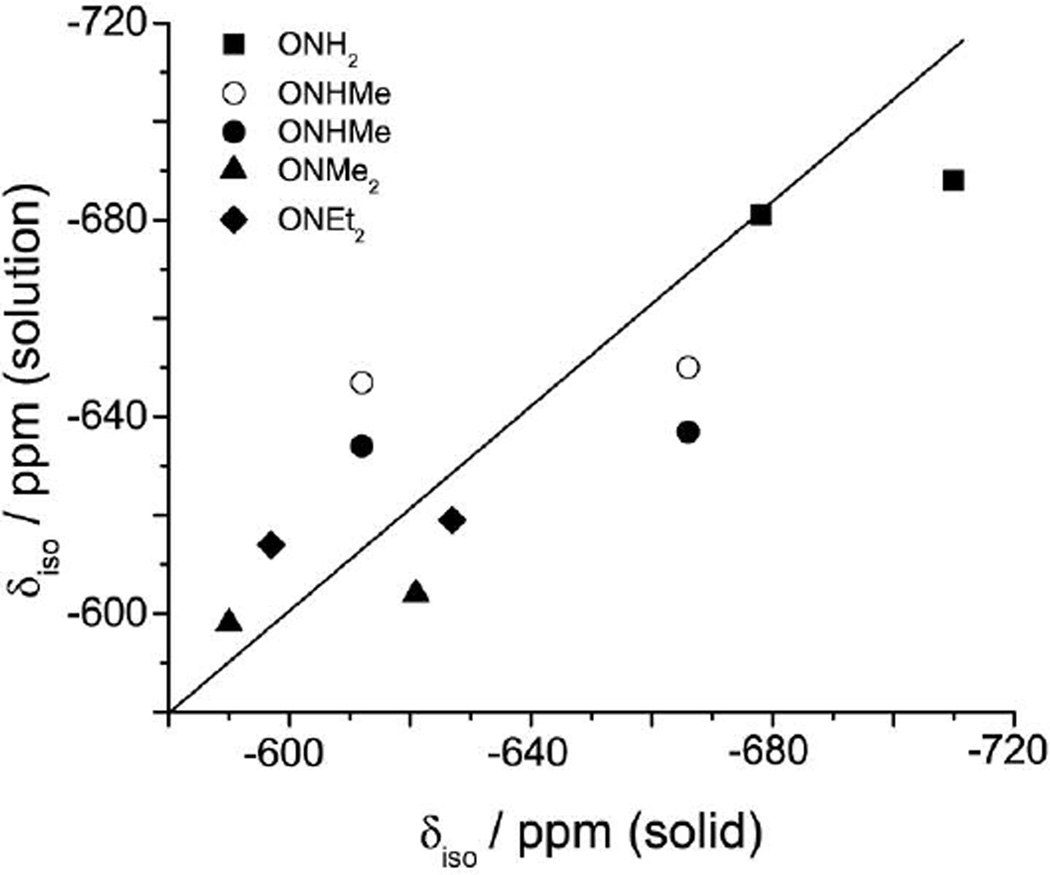
In biological environments, these vanadium compounds are exposed to solvent, metabolites and other biological components. Therefore, a comparison of the solid-state and solution chemical shifts is relevant; Table 2 contains selected δiso values measured in aqueous solution.13 Figure 9 shows a plot of solution vs. solid-state δiso and reveals that the differences are all less than 30 ppm, likely due to solvent effects. It has been suggested that for vanadium complexes with weakly bound ligands, such as the H2O in these compounds, thermal and solvent effects are on the order of a few dozen ppm.34 The pairs of compounds that have the same hydroxylamine ligand are illustrated using the same symbols in Figure 9. This plot clearly illustrates the small changes in the solution δiso values when the dipic ligand is varied whereas the solid-state δiso values are more sensitive to dipic substitution. Both the solution and solid-state δiso values are sensitive to changes in the hydroxylamine. Interestingly, there is a smaller difference in δiso between the methyl and ethyl substituted complexes than between the H and alkyl complexes.
Figure 9.
A plot of the 51V isotropic chemicals shift obtained from solutions NMR vs. that obtained from SSNMR for a series of hydroxylamido vanadium dipicolinate complexes. The compounds are shown presented by the symbol (solid square) are 1 and 4, (solid circle) are the major component in solution of 2 and 8, (open circle) are the minor component in solution of 2 and 8, (solid diamond) are 3 and 5 and (solid triangle) are 4 and 7. The two symbols solid and open circles of 2 and 8 reflect the fact that there are two isomers of these complexes in aqueous solution. Solid black line corresponds to perfect agreement between solid and solution NMR values.
For all the compounds studied, the vanadium chemical shift tensors are axially symmetric within experimental error. Upon examining the structures of the compounds, we see no obvious reason for this symmetry since the vanadium is not on a Cn rotation axis, n ≥ 3. However, the quantum chemical calculations of the magnetic shielding tensor reproduce the axial symmetry observed experimentally and, as mentioned, the orientation of the tensor is such that the unique component of the tensor, δ33, is approximately perpendicular to the plane of the dipic ligand, Figure 7.
To properly understand the chemical shift tensor one must examine the contributions of the individual molecular orbitals, MOs, to the magnetic shielding. According to the Ramsey’s theory, the magnetic shielding can be divided into diamagnetic and paramagnetic terms.52 The diamagnetic contribution to shielding, which is normally positive, depends on the ground electronic state of the molecule and is fairly constant for a given nucleus. The paramagnetic contribution to shielding, σp, which is normally negative, is related to the magnetic-dipole allowed mixing between symmetry-appropriate occupied and unoccupied MOs.52, 53 Efficient mixing requires that the symmetries of the occupied MOs after a rotation about the axis of the applied magnetic field be the same as those of the unoccupied MOs. For example, when the applied magnetic field is directed along the X-axis, paramagnetic shielding is created when the occupied MOs with dxz character mix with unoccupied MOs of dxy character.53 The energy separating the occupied and unoccupied MOs is also important, with the σp being inversely related to the energy difference; therefore only the MOs near the HOMO-LUMO gap are typically important for determining σp. Also, σp contains a L̂/〈r3〉 term, implying that only orbitals near the vanadium need to be considered. Therefore, by examining the symmetry of the occupied and unoccupied MOs near the HOMO-LUMO gap that contain significant metal d-character, the magnetic shielding and the orientation of the tensor can sometimes be explained.30, 54, 55
The natural bond order and natural chemical shielding analysis as available in Gaussian03 were used to obtain the contributions of various MOs to the isotropic vanadium magnetic shielding of [VO(dipic)(ONH2)(H2O)] as well as the contributions from the various atomic orbitals to the MOs.41, 45, 46 The symmetry of the MOs and percent vanadium d-orbital character were then compared to the MOs generated from the DFT magnetic shielding calculations to gain an understanding of which MOs contribute to the magnetic shielding.
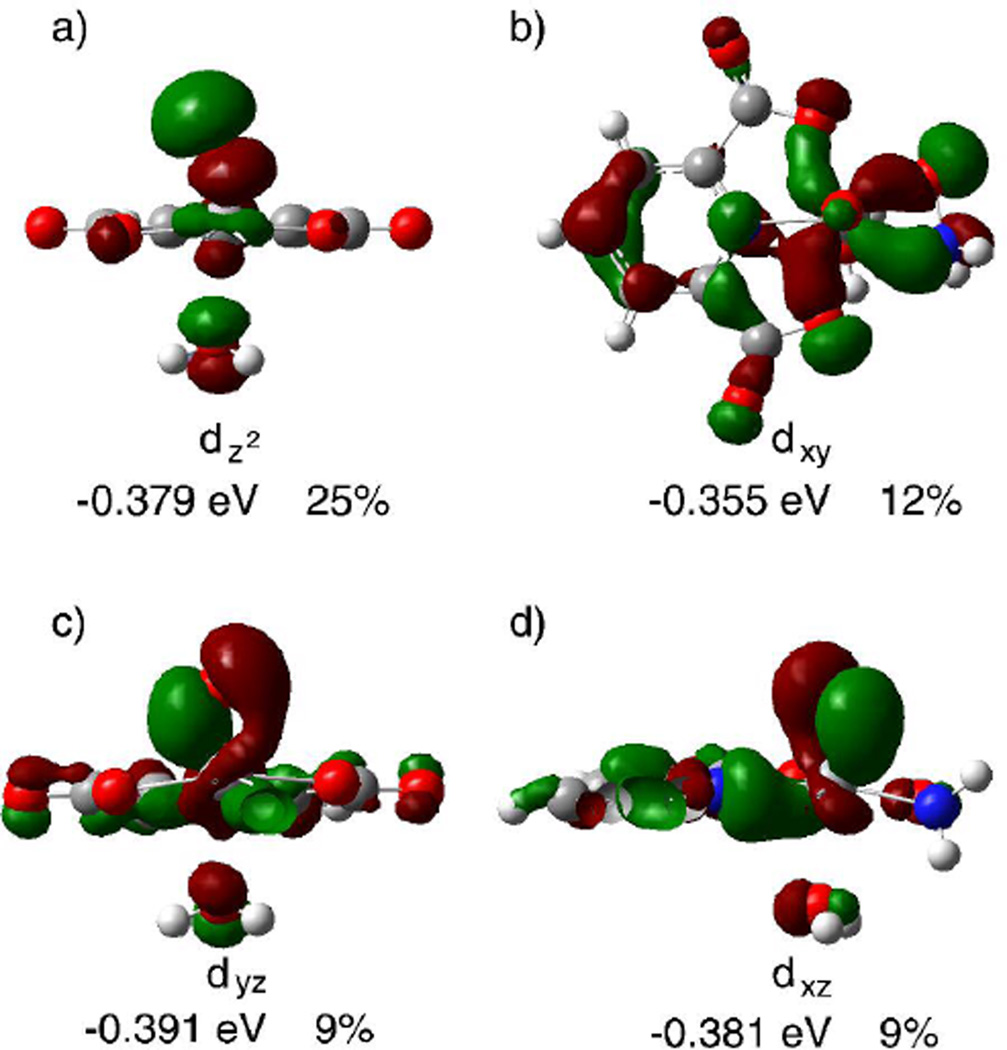
Figure 10 shows the four occupied [VO(dipic)(ONH2)(H2O)] MOs that contribute the most to the vanadium σpiso. As illustrated in the figure, the MOs can be said to have symmetries closely related to the vanadium d-orbitals. The largest single contributor to the shielding is an occupied MO that displays significant dz2 character, Figure 10a; the axis system is fixed such that Z-axis is perpendicular to the dipic plane. Mixing of the MO depicted in Figure 10a with unoccupied MOs of the appropriate symmetry accounts for 25% of the total σpiso. The dz2 orbital is unique among the d-orbital symmetries in that it does not produce paramagnetic shielding when the magnetic field is applied along the Z-axis.53 This has an important impact on the anisotropy of the magnetic shielding as the MO in Figure 10a will contribute to σp in the X and Y directions but not in the Z direction. It is therefore likely that this orbital is responsible for a major portion of the chemical shift anisotropy observed experimentally. The four MOs contributing the most to σpiso also qualitatively illustrate why the CS tensor is close to being axially symmetric. The MOs in Figure 10a and 10b are nearly symmetric about the Z-axis while the MOs in Figure 10c and 10d are approximately related by rotational symmetry about the Z-axis. Therefore, these four MOs will contribute equally to shielding in both the X and Y directions; low-lying unoccupied MOs with contributions from all five d-orbitals are available to mix with the occupied MOs.
Figure 10.
Four of the MOs obtained from the Gaussian03 calculations that contribute significantly to the calculated paramagnetic shielding and contain significant vanadium d-orbital character; contributions are given as a % of the total paramagnetic shielding. The energies of the orbital and % contributions are obtained from the NBO/NCS analysis. The orientation of the molecule is as follows: a) and c) viewed along the V-(dipic-N) bond, b) viewed down the V=O bond, d) viewed perpendicular to both the V=O and V-(dipic-N) bonds.
In addition to qualitatively explaining the CS anisotropy and orientation, the MO analysis also suggests that the magnetic shielding is dictated primarily by the V=O bond rather than binding to the dipic and hydroxylamine ligands. In fact, most of the occupied and unoccupied MOs that have significant vanadium d-orbital character are primarily centered on the V=O, Figure 10. Except for the MO depicted in Figure 10b, the high energy occupied MOs that represent “bonds” between vanadium and the dipic or hydroxylamine contain very little vanadium d-orbital character and are more characteristic of O or N “lone pair” orbitals. This can explain why dipic substitution causes relatively small changes in the vanadium chemical shift tensors. Furthermore, substitution of the hydroxylamido ligands coordinated to the vanadium atom in the V-N-O three membered ring-arrangement is likely to impact the V=O bond more significantly.
Conclusions
Using solid-state NMR spectroscopy we have shown that the electronic environment at the vanadium center in seven coordinate VO(dipic)(ONH2)(H2O) complexes is only slightly perturbed when the functionality of the coordinating ligands is changed. While the chemical shift interaction displays measurable yet small variations, the EFG tensor is less responsive. The lack of variation in the EFG tensor suggests that there is minimal change in the local charge distribution close to the vanadium site, possibly suggesting that there is little redistribution of charge in the dipic or hydroxylamine ligands even when the electronic nature of the ligands is significantly altered. These results suggest that ligand functionality in these seven-coordinate vanadium compounds can be tuned without causing a large change in the electrostatic environments. The CS tensor shows minor variations due to ligand substitution which has been attributed to the dominance of the V=O bond in determining the magnetic shielding of the vanadium. Despite this the CS tensor shows a greater sensitivity than the isotropic chemical shifts obtained from solution 51V NMR.
BRIEFS 51V MAS NMR spectroscopy and DFT calculations have been employed to investigate the vanadium environments in a series of hydroxylamido VO-dipicolinate complexes. The computed electric field gradient and chemical shift tensors are in good agreement with the experimental results. The vanadium dz2 orbital along the V=O bond is a major contributor to the magnetic shielding anisotropy.
Acknowledgements
T.P. acknowledges financial support of the National Science Foundation (NSF-CAREER CHE-0237612) and the National Institutes of Health (P20-17716, COBRE individual subproject). K.J.O thanks the Natural Sciences and Engineering Research Council of Canada for financial support. D.C.C acknowledges financial support of the Institute of General Medicine at the National Institutes of Health (GM40525) and the National Science Foundation (CHE-0314719).
References
- 1.Crans DC, Smee JJ, Gaidamauskas E, Yang L. Chem. Rev. 2004;104:849–902. doi: 10.1021/cr020607t. [DOI] [PubMed] [Google Scholar]
- 2.Thompson KH, Orvig C. Dalton Trans. 2006:761–764. doi: 10.1039/b513476e. [DOI] [PubMed] [Google Scholar]
- 3.Polenova T, Pooransingh-Margolis N, Renirie R, Wever R, Rehder D. In: Vanadium, The Versatile Metal. Kustin K, Crans DC, Costa Pessoa J, editors. Washington: ACS Publications; 2007. In Press. [Google Scholar]
- 4.Crans DC, Mahroof-Tahir M, Johnson MD, Wilkins PC, Yang L, Robbins K, Johnson A, Alfano JA, Godzala ME, III, Austin LT, Willsky GR. Inorg. Chim. Acta. 2003;356:365–378. [Google Scholar]
- 5.Časný M, Rehder D. Chem. Commun. 2001:921–922. [Google Scholar]
- 6.Gätjens J, Meier B, Adachi Y, Sakurai H, Rehder D. Eur. J. Inorg. Chem. 2006:3575–3585. [Google Scholar]
- 7.Parajón-Costa BS, Piro OE, Pis-Diez R, Castellano EE, González-Baró AC. Polyhedron. 2006;25:2920–2928. [Google Scholar]
- 8.González-Baró AC, Castellano EE, Piro OE, Parajón-Costa BS. Polyhedron. 2005;24:49–55. [Google Scholar]
- 9.Zampella G, Fantucci P, Pecoraro VL, De Gioia L. Inorg. Chem. 2006;45:7133–7143. doi: 10.1021/ic060555g. [DOI] [PubMed] [Google Scholar]
- 10.Zampella G, Fantucci P, Pecoraro VL, De Gioia L. J. Am. Chem. Soc. 2005;127:953–960. doi: 10.1021/ja046016x. [DOI] [PubMed] [Google Scholar]
- 11.Schneider CJ, Zampella G, Greco C, Pecoraro VL, De Gioia L. Eur. J. Inorg. Chem. 2007:515–523. [Google Scholar]
- 12.Sakurai H, Tamura A, Takino T, Ozutsumi K, Kawabe K, Kojima Y. Inorg. React. Mech. 2000;2:69–77. [Google Scholar]
- 13.Smee JJ, Epps JA, Teissedre G, Maes M, Harding N, Yang L, Miller SM, Anderson OP, Willsky GR, Crans DC. Manuscript Submitted to Inorg. Chem. doi: 10.1021/ic701233y. [DOI] [PubMed] [Google Scholar]
- 14.Butler A, Clague MJ, Meister G. Chem. Rev. 1994;94:625–638. [Google Scholar]
- 15.Huang W, Todaro L, Francesconi LC, Polenova T. J. Am Chem. Soc. 2003;125:5928–5938. doi: 10.1021/ja029246p. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Todaro L, Yap GPA, Beer R, Francesconi LC, Polenova T. J. Am Chem. Soc. 2004;126:11564–11573. doi: 10.1021/ja0475499. [DOI] [PubMed] [Google Scholar]
- 17.Skibsted J, Nielsen N Chr, Bildsøe H, Jakobsen HJ. Chem. Phys. Lett. 1992;188:405–412. [Google Scholar]
- 18.Nielsen UG, Jakobsen HJ, Skibsted J. J. Phys. Chem. B. 2001;105:420–429. [Google Scholar]
- 19.Rehder D, Polenova T, Bühl M. Annu. Rep. NMR Spectrosc. 2007:50–116. In Press. [Google Scholar]
- 20.Pooransigh-Margolis N, Renirie R, Hasan Z, Wever R, Vega AJ, Polenova T. J. Am. Chem. Soc. 2006;128:5190–5208. doi: 10.1021/ja060480f. [DOI] [PubMed] [Google Scholar]
- 21.Shubin AA, Lapina OB, Bondareva VM. Chem. Phys. Lett. 1999;302:341–346. [Google Scholar]
- 22.Skibsted J, Jacobsen CJH, Jakobsen HJ. Inorg. Chem. 1998;37:3083–3092. [Google Scholar]
- 23.Mackenzie KJD, Smith ME. Multinuclear Solid-State NMR of Inorganic Materials. Oxford: Pergomon; 2002. [Google Scholar]
- 24.Crans DC, Felty RA, Chen H, Eckert H, Das N. Inorg. Chem. 1994;33:2427–2438. [Google Scholar]
- 25.Rehder D, Paulsen K, Basler W. J. Magn. Reson. 1983;53:500–502. [Google Scholar]
- 26.Pooransingh N, Pomerantseva E, Ebel M, Jantzen S, Rehder D, Polenova T. Inorg. Chem. 2003;42:1256–1266. doi: 10.1021/ic026141e. [DOI] [PubMed] [Google Scholar]
- 27.Hansen MR, Madsen GKH, Jakobsen HJ, Skibsted J. J. Phys. Chem. B. 2006;110:5975–5983. doi: 10.1021/jp0555549. [DOI] [PubMed] [Google Scholar]
- 28.Rehder D. In: Multinuclear NMR. Mason J, editor. New York: Plenum; 1989. pp. 488–493. [Google Scholar]
- 29.Ooms KJ, Wasylishen RE. J. Am. Chem. Soc. 2004;126:10972–10980. doi: 10.1021/ja0400887. [DOI] [PubMed] [Google Scholar]
- 30.Feindel KW, Ooms KJ, Wasylishen RE. Phys. Chem. Chem. Phys. 2007;9:1226–1238. doi: 10.1039/b616821c. [DOI] [PubMed] [Google Scholar]
- 31.Kaupp M, Bühl M, Malkin VG, editors. Calculation of NMR and EPR Parameters. Weinheim: Wiley-VCH; 2004. [Google Scholar]
- 32.Gee BA. Solid State Nucl. Magn. Reson. 2006;30:171–181. doi: 10.1016/j.ssnmr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Bühl M, Hamprecht FA. J. Comp. Chem. 1998;19:113–122. [Google Scholar]
- 34.Bühl M, Parrinello M. Chem. Eur. J. 2001;7:4487–4494. doi: 10.1002/1521-3765(20011015)7:20<4487::aid-chem4487>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.Wieghardt K. Inorg. Chem. 1978;17:57–64. [Google Scholar]
- 36.Yang L, La Cour A, Anderson OP, Crans DC. Inorg. Chem. 2002;41:6322–6331. doi: 10.1021/ic0201598. [DOI] [PubMed] [Google Scholar]
- 37.Nuber B, Weiss J. Acta Crystallogr. 1981;B37:947–948. [Google Scholar]
- 38.Bradshaw JS, Maas GE, Lamb JD, Izatt RM, Christensen JJ. J. Am Chem. Soc. 1980;102:467–474. doi: 10.1021/ja00449a070. [DOI] [PubMed] [Google Scholar]
- 39.Harris RK, Becker ED, De Menezes SMC, Goodfellow R, Granger P. Pure Appl. Chem. 2001;73:1795–1818. [Google Scholar]
- 40.Bak M, Rasmussen JT, Nielsen NC. J. Magn. Reson. 2000;147:296–330. doi: 10.1006/jmre.2000.2179. [DOI] [PubMed] [Google Scholar]
- 41.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Wallingford, CT: Gaussian, Inc; 2004. [Google Scholar]
- 42.Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- 43.Lee CT, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 44.Dennington R, II, Keith T, Millam J, Eppinnett K, Hovell WL, Gilliland R. GaussView, Version 3.09. Shawnee Mission, KS: Semichem, Inc.; 2003. [Google Scholar]
- 45.Glendening ED, Reed AE, Carpenter JE, Weinhold F. NBO Version 3.1 [Google Scholar]
- 46.Bohmann JA, Weinhold F, Farrar TC. J. Chem. Phys. 1997;107:1173–1184. [Google Scholar]
- 47.Stark RE, Haberkorn RA, Griffin RG. J. Chem. Phys. 1978;68:1996–1997. [Google Scholar]
- 48.Abragam A. The Principles of Nuclear Magnetism. Oxford: Oxford University Press; 1961. [Google Scholar]
- 49.Duer M, editor. Solid-State NMR Spectroscopy: principles and applications. Oxford: Blackwell Science; 2002. [Google Scholar]
- 50.The α angle obtained directly from SIMPSON ranged from 110 to 140°. The 90° phase shift is a result of SIMPSON defining the EFG components as |VZZ| |VXX| |VYY|.
- 51.Koller H, Engelhardt G, Kentgens APM, Sauer J. J. Phys. Chem. 1994;98:1544–1551. [Google Scholar]
- 52.Ramsey NF. Phys. Rev. 1950;78:699–703. [Google Scholar]
- 53.Jameson CJ, Gutowsky HS. J. Chem. Phys. 1964;40:1714–1724. [Google Scholar]
- 54.Juranić N. Coord. Chem. Rev. 1989;96:253–290. [Google Scholar]
- 55.Ooms KJ, Wasylishen RE. Can. J. Chem. 2006;84:300–308. [Google Scholar]
- 56.Mason J. Solid State Nucl. Magn. Reson. 1993;2:285–288. doi: 10.1016/0926-2040(93)90010-k. [DOI] [PubMed] [Google Scholar]