Abstract
A benchtop miniature mass spectrometer system, Mini 12, with ambient ionization source and tandem mass spectrometry capabilities has been developed and characterized. This instrument was developed as a self-contained system to produce quantitative results for unprocessed samples of small volumes including nonvolatile analytes. The ion processing system, vacuum system, and control system are detailed. An integrated sample loading system facilitates automated operation. A user interface has been developed to acquire and to interpret analytical results for personnel who have limited mass spectrometry knowledge. Peak widths of Δm/z 0.6 Th (full width at half-maximum) and a mass range of up to m/z 900 are demonstrated with the rectilinear ion trap mass analyzer. Multistage experiments up to MS5 are accomplished. Consumable cartridges have been designed for use in ambient paper spray ionization, and the recently developed extraction spray ionization method has been employed to improve the quantitative performance. Monitoring of trace-levels of chemicals in therapeutic drugs, as well as in food safety and environmental protection operations is demonstrated. Dual MS/MS scans are implemented to obtain the intensities of the fragment ions from the analyte and its internal standard, and the ratio is used in quantitative analysis of complex samples. Limits of quantitation (LOQ) of 7.5 ng/mL, with relative standard deviations below 10%, have been obtained for selected therapeutic drugs in whole blood throughout their individual therapeutic ranges.
Mass spectrometry (MS) is widely recognized for its high sensitivity and high selectivity in chemical analysis. In the application fields of drug discovery, clinical analysis, food safety, and environmental monitoring, mass spectrometry plays an important role in both qualitative and quantitative trace analysis. Standard analytical procedures include multiple steps of sample pretreatment and chromatographic separation prior to MS analysis, and these preliminary steps can only be performed in analytical laboratories. The miniaturization of mass spectrometry systems started more than a decade ago with the clear aim of performing chemical analysis in its entirety in the field and providing results rapidly.1
Early in the miniaturization effort, significant research effort went into the miniaturization of the mass analyzer.2−9 This had direct and indirect impact on the size of the entire MS system. Later, miniature mass spectrometer systems were developed using different mass analyzers. Among these systems, the ion trap was preferred because of its modest vacuum requirements and intrinsic MS/MS capabilities. A configuration with an ion trap as mass analyzer and a pumping system composed of a Pfeiffer TPD 011 (later model HiPace 10, Pfeiffer Vacuum, Inc.) and a two-stage KNF diaphragm pump (model 1091-N84.0-8.99 or similar, KNF Neuberger, Inc.) became popular. This configuration was used in the Purdue University Mini 10,10 the Griffin 600 (Griffin Analytical Technologies),11 the Guardion-7 (Torion Technologies),12 and the MMS-1000 (1st Detect Corp.).13 Ion traps can perform MS analysis in the millitorr pressure range, significantly higher than for other mass analyzers, and the miniature pumping system provides adequate pumping speed to maintain vacuum for in-vacuum ionization, which is typically used for analysis of gas samples. The development of the discontinuous atmospheric pressure interface (DAPI) enabled the coupling of miniature mass spectrometers to atmospheric pressure ionization sources,14 including electrospray ionization (ESI),15 atmospheric pressure chemical ionization (APCI),16 and various ambient ionization sources.14,17−19
For volatile analytes, membrane introduction systems20 are useful interfaces for in situ analysis using portable mass spectrometers. However, analysis of nonvolatile analytes requires interfacing miniature mass spectrometers to external ionization sources. The ambient ionization methods were developed to bypass several steps in the analytical procedures for sample pretreatment and chromatographic separation that are traditionally required for MS analysis of complex samples.21,22 They allow analytes in complex matrices to be directly sampled and ionized for MS analysis.23,24 Qualitative analysis at low limits of detection (LODs) has been demonstrated with more than 30 ambient ionization methods, and the recently developed method of paper spray ionization has provided good limits of quantitation (LOQ) together with high accuracy in quantitative analysis.25−28 Development of disposable sample cartridges was also demonstrated to be feasible with paper spray.29 Our recent efforts in developing miniature MS systems have resulted in backpack and benchtop MS systems that share the same in-house control electronics, but which are operated using different ambient ionization sources and have different physical configurations. The backpack MS, described in an accompanying manuscript,30 typically uses a low-temperature plasma probe for screening nonvolatile compounds on surfaces. The benchtop miniature MS system described here, the Mini 12, was designed as a proof-of-concept prototype for targeted analysis. It is specifically aimed at point-of-care applications.
Minimizing the size and weight of the system has been a major goal in the development of miniature MS systems, and a complete mass spectrometer has been developed that weighed only 4 kg.31 The goal for the development of the Mini 12, however, was primarily to have a system that can perform direct chemical analysis without sample pretreatment and can be operated by untrained personnel. Use of ambient ionization to avoid sample preparation and chemical purification is a major convenience of such a system. Autonomous analysis, including recording of MS and MS/MS spectra, data analysis, and direct reporting, becomes possible with systems designed for specialized analysis. Specific scan functions and calibration data can be preset and loaded during operation and for data analysis, respectively.
The challenge to achieving analytical performance qualified for point-of-care or other types of regulated applications is to obtain adequate sensitivity and precision in quantitation. Rectilinear ion traps (RIT)8 have been used in the earlier Mini 1010 and 11(31) and also now in the Mini 12 instrument developed at Purdue. MS/MS analysis is performed to increase the chemical specificity as well as to increase the signal-to-noise ratios for characteristic ions in the direct analysis of complex mixtures.32 Quantitation at high precision of particular analytes in condensed phase samples, such as blood or food samples, has not been extensively investigated with miniature mass spectrometers, due to the technical challenges and practical difficulties in achieving highly reproducible results while maintaining the simplicity in the instrumentation and operating procedure. The use of internal standards (IS) with paper spray has been shown to improve the quantitation significantly, and several nontraditional methods of adding the standards have been reported.33,34 Simple procedures for introducing IS for ambient ionization are also required for POC or similar in situ applications of miniature MS systems. Several solutions exist, including the use of IS-coated capillaries for transferring samples.35 Transfer of quantitative performance to the miniaturized system involves some other technical difficulties, which will be further discussed later and favor ionization sources with stable signals.
System Design and Instrumentation
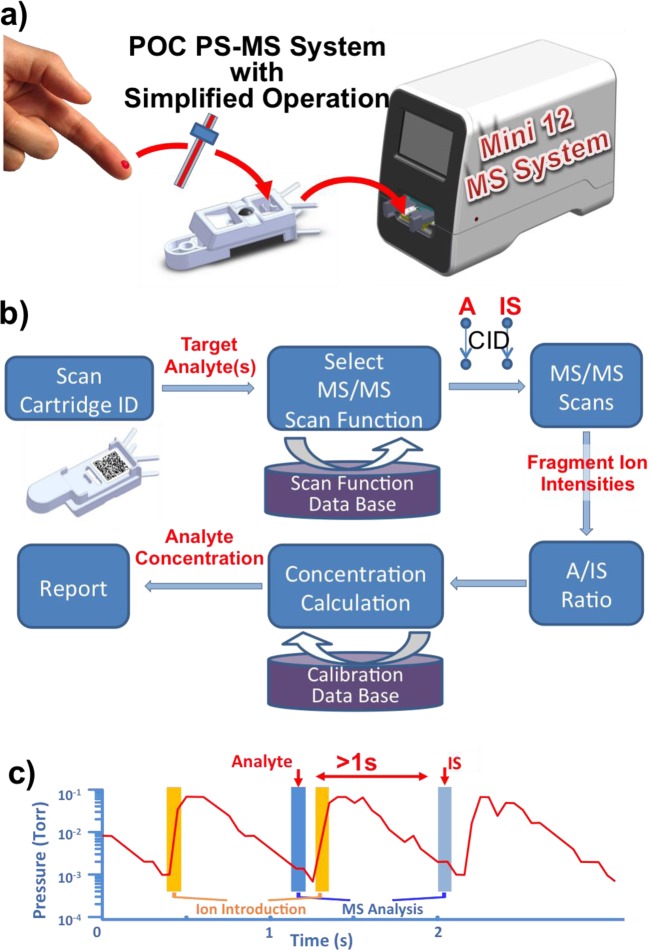
An exemplary analytical procedure intended for a miniature MS chemical analysis system is shown in Figure 1a for the particular case of drugs in blood. A small volume of blood is quantitatively taken up using a capillary and transferred to a sample cartridge, which is subsequently inserted into the MS system for automatic analysis. Capillaries with internal standard coated on the inside wall are used to transfer samples of about 1 μL during which the internal standard mixes into the sample. Highly accurate quantitative data (RSD < 3% at 10 ng/mL drug in blood) have previously been obtained using commercial triple quadrupole mass spectrometer for direct analysis using several ambient ionization methods.35 To minimize the need of human intervention, an autonomous procedure for analysis and data processing was designed for the Mini 12 (Figure 1b). Once the sample cartridge is inserted into the instrument, a bar code on the cartridge is read and used to search the instructions for analysis of target chemicals, which include the type of sample, target analytes, scan functions, and the calibration curve stored in the database. Using therapeutic drug monitoring as an example, two successive product ion scan functions for MS/MS analysis of the drug and the internal standard are executed; that is, the intensity of the target fragment ion generated from each precursor is recorded (Figure 1c). The ratio of these ion abundances is subsequently used to calculate the concentration based on a calibration curve made and saved for the particular drug of interest in a blood sample. A report of the analytical results can then be generated automatically without requiring the user to analyze the mass spectra. For each scan, the DAPI opens for 15 ms, and the ions introduced are trapped in the RIT at elevated pressure (up to 500 mTorr). After a delay of 800 ms, an MS or MS/MS scan is performed at 1 mTorr (Figure 1c).
Figure 1.
(a) Mini 12 system that provides a simplified operational protocol. (b) Flowchart for automated quantitative analysis using an internal standard. (c) Operation of the dual MS/MS scan function in the time domain.
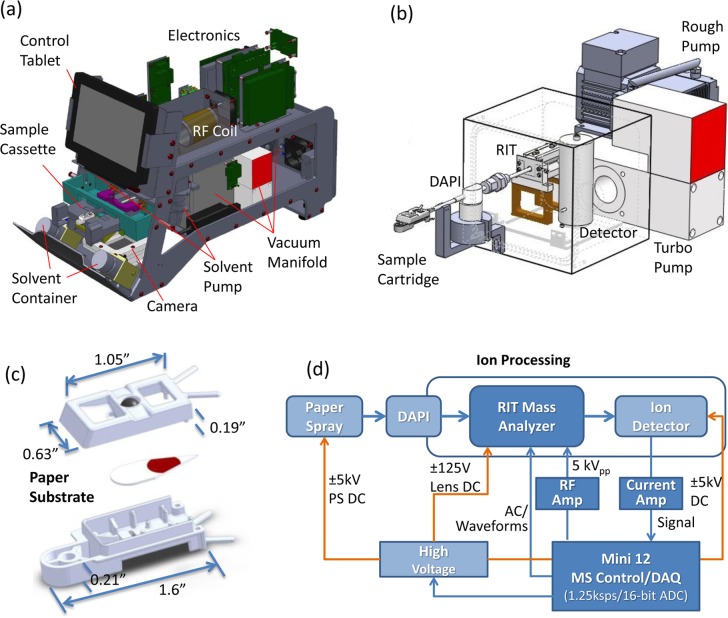
The integrated Mini 12 system weighs 25 kg, has dimensions of 19.6 × 22.1 × 16.5 in. (outer case measurements), consumes less than 100 W of power, and operates with 110 V AC or from a battery. The system configuration is shown in Figure 2a,b, and a schematic of the instrument control is shown in Figure 2d. The design of the ion source and the sample cartridges adapted to different ambient ionization methods were evaluated. NanoESI and APCI have also been used as ionization methods to characterize the instrument. As a demonstration of the possible use for POC or similar applications, the loading system shown in Figure 2a was developed to accept a single paper spray sample cartridge (Figure 2c) for analysis. A paper substrate with a sharp tip (∼30°) was sandwiched between top and bottom plastic covers made from polyoxymethylene (Ultraform N2320, BASF SE, Ludwigshafen, Germany) using 3D printing. Two openings on the top cover of the cartridge allow deposition of the sample and feed of the spray solvent. A stainless steel ball inserted into the top cover of the cartridge allows application of the high voltage to the paper substrate. Two solvent pumps (STH0CTCLF17, Fluid Metering, Inc., NY) with a minimum dispensing volume of 2 μL were installed, each connected to a 60 mL bottle filled with a spray solvent. This system allows a choice of spray solvent.
Figure 2.
(a) Overview of Mini 12 system and (b) selected subsystems showing ion generation, introduction, and analysis. (c) Design of paper spray sample cartridge used with the Mini 12 system. (d) Schematic diagram of the control electronics in the Mini 12 system.
A schematic of the system control is shown in Figure 2d, and more details on the MS control electronics are provided in the accompanying paper.30 A tablet computer (HP Slate 2) with a touch screen was integrated into the system to operate the user interface and to allow data processing. The tablet communicated with the MS control board through a USB 2.0 connection. Preoptimized scan functions and calibration functions for different types of targeted analysis were stored on the tablet computer. The scan function parameters were optimized using an expert user interface, as shown in Figure S1. A simpler and more user-friendly interface (Figure S2) was developed for end users. During operation, a user could press a button on the screen to open the cartridge tray, which was controlled through a solenoid actuator. Once the paper spray sample cartridge was placed on the tray and inserted into the system, a magnetic switch closed and triggered the analysis. A digital camera (NLV-2101, Opticon, Inc., WA) installed underneath the plastic tray read the bar code on the bottom of the paper cartridge. Identification of the needed target analysis was made reading the bar code into the program running on the tablet, and the corresponding scan functions were then automatically downloaded to the scan control board. In the particular case of analysis of a drug in blood with the use of an internal standard and paper spray ionization, a solvent was selected and dispensed on the basis of the target compound for optimal elution/ionization efficiency. Two MS/MS scan functions, each of which isolated a different precursor ion, were then executed in sequence. After the spectra data were recorded and transferred back to the tablet, the ratio of the selected fragment ions generated from the ionized drug and internal standard was calculated and used for determination of the drug concentration based on the corresponding calibration function, which was constructed using the same drug and internal standard and the same type of matrix.
The vacuum manifold was fabricated with dimensions of 4.65 × 5.00 × 4.74 in. (length × width × height) and with inside dimensions of 3.95 × 4.30 × 4.04 in. It was pumped using a 2 kg turbomolecular pump (HiPace 10, Pfeiffer Vacuum, Inc., Nashua, NH) and a 500 g two-stage diaphragm pump (MPU 1091-N84.0–8.99, KNF Neuberger, Inc., Trenton, NJ). An ultimate vacuum pressure below 1 × 10–5 Torr was achieved with the DAPI closed. The DAPI system used a normally closed pinch valve (390NC24330, ASCO Valve, Inc., Florham Park, NJ) with conductive silicone tubing (i.d. 1/16 in., o.d. 1/8 in., and length 2 in., Simolex Rubber Corp., Plymouth, MI) connecting two stainless steel tubes (Supelco, Bellefonte, PA). The pinch valve controlled open/closed status of sampling. The stainless steel tube leading into the trap had dimensions of 0.03 in. i.d., 1/16 in. o.d., and a 10 cm length, and the other tube had dimensions of 0.02 in. i.d., 1/16 in. o.d., and a 5 cm length. Both stainless steel tubes were electrically grounded during operation. A 24 V dc pulse signal was used to open the pinch valve. An rf coil of 1 in. i.d. was used to provide a rf amplitude higher than 5.5 kVp–p at 1 MHz. Resonance ejection was performed using an AC signal with the amplitudes of the AC and the main rf trapping signals ramping simultaneously to record mass spectra. A scan speed up to 10 000 mass/charge units per second was used, unless otherwise specified. The SWIFT (stored waveform inverse Fourier transform) waveform36 with a notch width of 7 kHz was used for isolation of the precursor ion prior to an MS/MS scan. The detector assembly (model 397, DeTech Detector Technology, Inc., MA) with an electron multiplier and conversion dynode was used.19
Experimental Section
Whatman chromatography papers (Grade 1, ET31, and silica-coated grade SG81) were used to prepare sample substrates for paper spray (Figure S3a) and extraction spray analysis37 (Figure S3b). All chemicals were commercially available and used without purification. The internal standard, amitriptyline-d6 was purchased from CDN isotopes (Pointe-Claire, Quebec, Canada). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Bovine whole blood stabilized with EDTAK2 was purchased from Innovative Research (Novi, MI). Methanol and water were purchased from Mallinckrodt (Hazelwood, MO) and used to prepare the spray solvent mixture (50/50, v/v). Tissue of a tomato was homogenized using the protocol described in a previous study38 to serve as a sample for quantitative analysis. Stock solutions of analytes were directly spiked into solvents or sample matrices to make standard samples for quantitative measurements.
NanoESI, APCI, paper spray, and extraction paper spray were used as ionization methods in the characterization of the instrument performance. For paper spray analysis, 2.5 μL of sample was deposited onto the paper substrate using IS-coated capillary samplers35 and air-dried for up to 2 h. Methanol (40 μL) was then applied to the paper to elute compounds from the matrix, followed by the application of a high voltage of 4.3 kV to the paper substrate for ionization.25 For extraction spray, the analyte prepared in blood was preloaded onto a paper strip (grade 1, length of 10 mm, width of 0.6 mm), which was inserted into a nanoESI tube made in-house from a borosilicate glass tube. After air drying (for up to 30 min), 5 μL of methanol was added into the glass tube, and a high voltage of 1.8 kV was applied to the electrode for compound elution and ionization. Further descriptions of the experimental conditions used for paper spray ionization and extraction spray ionization can be found in Supporting Information.
Performance Characterization and Discussion
During the years the Mini 12 was in development, many features of the system were tested and improved. The discussion here focuses on the performance of the MS analysis and the quality of the system when used for quantitation of analytes in complex mixtures.
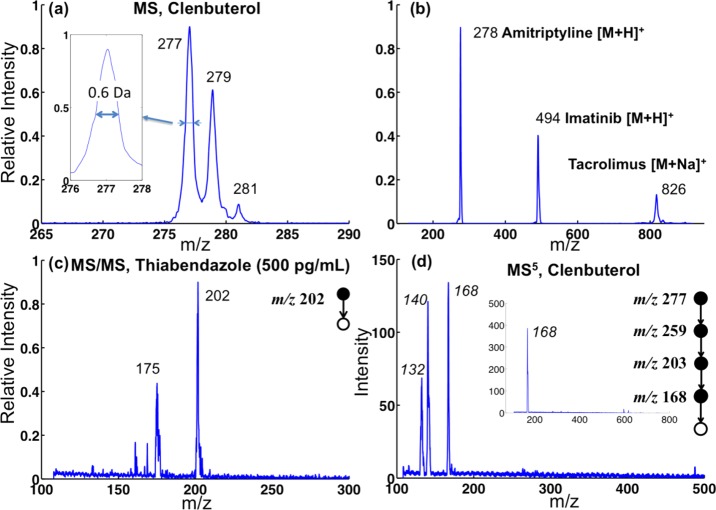
The resolution and mass range of the system were characterized with nanoESI using methanol/water (50/50) solutions containing pure compounds. As shown in the mass spectrum of 1 μg/mL clenbuterol (Figure 3a), isotopic peaks m/z 277, 279, and 281 were clearly separated in the mass selective instability scan performed at a scan rate of 3000 m/z per second and with a resonance ejection signal at 350 kHz (q = 0.80, AC amplitude scanned from 1.5 to 3.0 Vp–p). The FWHM (full width at half-maximum) was Δm/z = 0.6, for a resolution m/Δm of 500. The nanoESI spectrum of a mixture containing three therapeutic drugs (Figure 3b), tacrolimus (5 μg/mL, [M + H]+ at m/z 826), imatinib (1 μg/mL, [M + H]+ at m/z 494), and amitriptyline (1 μg/mL, [M + H]+ at m/z 278), was recorded at a scan rate of 10 000 m/z per second and with a resonance ejection at 300 kHz (q = 0.73, AC amplitude scan 3 .5–7.5 Vp–p). A maximum m/z above 850 was obtained.
Figure 3.
(a) Mass spectrum of 1 ppm clenbuterol recorded using nanoESI with RIT operated at rf frequency 1000 kHz with resonance ejection at 350 kHz (q = 0.80) and ac amplitude ramped from 1.5 to 3.0 Vp–p. (b) Mass spectrum of 5 ppm tacrolimus, 1 ppm imatinib, and 1 ppm amitriptyline mixture recorded using nanoESI and RIT rf frequency 1000 kHz and resonance ejection at 300 kHz and 3.5–7.5 Vp–p. (c) Mass spectrum of MS2 of 500 ppt thiabendazole in 50/50 MeOH/H2O using nanoESI. (d) Mass spectrum of MS5 of 20 ppm clenbuterol in 50/50 MeOH/H2O using nanoESI (inset shows the isolated peak of ions with m/z 168).
As discussed earlier in this paper, MS/MS capabilities are essential for the deployment of a system like the Mini 12, which is aimed at direct analysis of complex samples without traditional sample pretreatment or chemical separation. Higher sensitivity can be obtained due to the elimination of chemical noise and the identification of the target analytes can be confirmed by appropriate fragmentations corresponding to structural features of the analyte. The scan function for MS/MS is shown in Figure S4. The MS/MS spectrum of 500 pg/mL thiabendazole ([M + H]+ at m/z 202, Figure 3c) shows a good signal-to-noise ratio for the fragment ion m/z 175. In analysis of extremely complex mixtures, it is possible that multiple stages (>2) of mass analysis might be necessary to confirm chemical identity or to avoid interference in quantitation.39 As a demonstration of this capability, multiple stage MS analysis (up to five stages) has been performed starting with 20 μg/mL clenbuterol ([M + H]+ at m/z 277). The MS5 spectrum shown in Figure 3d displays very good S/N ratios for fragment ions m/z 132 and m/z 140. The spectra for the intermediate stages of this experiment (viz., MS2, MS3, and MS4) are shown in Figure S6. The MS/MS capability of the MS 12 has also been characterized using APCI using a gas mixture sample containing vapors of dimethyl methylphosphonate (DMMP), methyl salicylate, and N,N-diethyl-meta-toluamide (DEET) (Figure S7).
The ultimate goal in developing the Mini 12 MS system was to enable the direct analysis of nonvolatile analytes in samples with complex matrices. The sensitivity and the quantitative accuracy that can be achieved with ambient ionization are critical for the intended applications. Paper spray was selected as one of the main methods to be used with the Mini 12, due to the sensitivity achieved for complex mixture analysis and the potential for high-precision quantitation that has been demonstrated with lab-scale triple quadrupole mass spectrometers.25,26 Another ambient ionization method used for performance development and characterization with the Mini 12 was extraction spray. Instead of spraying the solvent directly from the paper substrate, a paper strip containing the sample was inserted into a nanoESI tube, and a solvent was added to extract the analyte, which was sprayed from the tip of the nanoESI emitter (Figure S3b). Good sensitivity can be achieved with both methods.
The MS/MS spectra recorded for analysis of amitriptyline at 50 ng/mL in blood using these two methods are shown in Figure 4a,b. Similar S/N ratios were observed for the peaks for the fragment ions of m/z 117, 155, and 191 in both spectra. Sample amounts used for preparing the dried blood spots (DBS) on paper substrates were different, with 2.5 μL being used for paper spray but only 1 μL for extraction spray. The most significant difference in the experimental conditions is the consumption of the spray solvent and the analysis time. About 1 min spray time was obtained in paper spray using 40 μL of methanol, whereas more than 10 min spray time was available with only 5 μL of methanol in the extraction spray. A much lower spray voltage of 1.8 kV was used for extraction spray, in comparison with 4.3 kV for paper spray. The stability of the signal was also much better with extraction spray, as previously observed.37 Figure S5 shows a MS/MS spectrum for analysis of 10 ng/mL verapamil in DBS prepared with 1 μL of blood sample, and the S/N ratios observed for the fragment ions m/z 165 and m/z 303 indicate that an LOD better than 10 ng/mL can be easily achieved. On the other hand, at the current stage of development, extraction spray involves more complex manipulations and is less amenable to automation and array mode operation.
Figure 4.
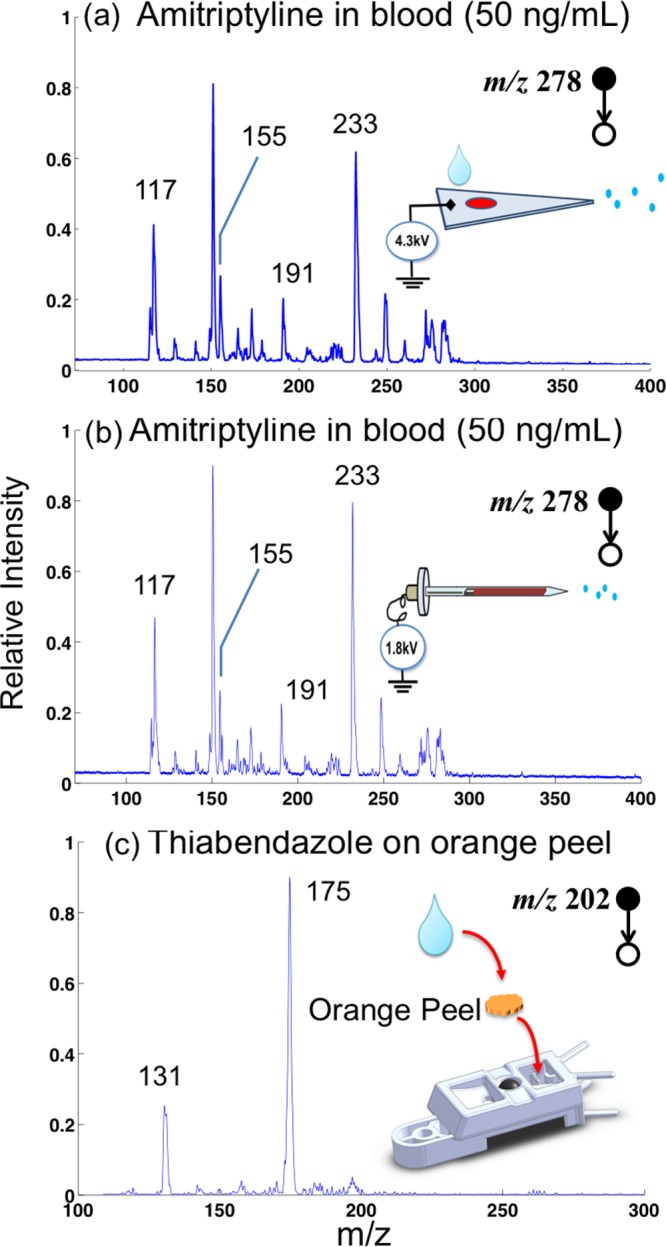
MS/MS spectra of (a) 50 ng of amitriptyline in blood recorded with paper spray ionization and (b) extraction spray ionization. MS/MS spectra of (c) thiabendazole on an orange peel obtained using paper spray ionization.
One of the advantages of using paper spray with the sample cartridge designed for the Mini 12 is the flexibility in analysis of complex samples in forms other than dried spots typically prepared from blood, homogenates, or other liquid samples. As a demonstration, a small piece (about 1 g) of the peel from an nonorganic orange was purchased from a local grocery store and dropped onto the paper spray cartridge, as shown in Figure 4c. Instead of feeding the spray solvent from the other opening behind the stainless steel ball, 40 μL of methanol was directly added on top of the sample. The spray voltage of 4.3 kV was then turned on, and MS/MS analysis was performed targeting thiabendazole (precursor ion m/z 202), which is one of the most commonly used fruit fungicides. A silica-coated paper substrate was used in this case, as these have been demonstrated to be effective for paper spray due to less retention of water-soluble organic compounds. The typical fragment ions of protonated thiabendazole at m/z 131 and 175 were observed with high peak intensities (Figure 4c).
In many of the potential applications of the Mini 12 system, mandatory performance criteria will need to be met for regulatory purposes. The LOQ, rather than the LOD, as well as the RSD over the entire calibration range are important characteristics in the analytical performance of the system. Ambient analysis using paper spray or extraction spray with a commercial triple quadrupole instrument has been demonstrated to give LOQs in the low ng/mL level and quantitative precision (RSD) of better than 8% for drug compounds in whole blood. The use of internal standards (IS) was also found to be critical,26 just as for LC-MS/MS. Although relatively good external calibration could be obtained27 with tightly controlled experimental conditions, for point-of-care type applications, the use of the analyte-to-IS (A/IS) ratios could help significantly to minimize the potential errors associated with variations in operation by personnel not trained in laboratory analytical techniques. When transferring multireaction monitoring (MRM) procedures for measuring the A/IS ratios to a system like the Mini 12, some challenges were foreseen. Therefore, the A/IS ratio was measured with dual MS/MS scans in which two product ion scans were performed sequentially for the analyte and IS. The analyte-to-IS (A/IS) ratio obtained was used to calculate the analyte concentration based on the calibration curve. A delay in the signal recording exists between these two scans, which could cause a deviation in A/IS ratio especially when fluctuations in the ion current are relatively large. The delay between the scans is only about 100 ms for a commercial triple quadrupole instrument; however, it is more than 1s for Mini 12 operation with a DAPI (Figure 1c), which represents a potential problem for quantitative analysis using the Mini12.
To explore this issue, a set of tests was run using blood samples containing 200 ng/mL amitriptyline and 100 ng/mL amitriptyline-d6 as internal standards to characterize drug quantitation using Mini 12 with paper spray and extraction spray methods. MS/MS scans for amitriptyline and amitriptyline-d6 were run alternately, and the intensity of fragment ion m/z 233 recorded was plotted as function of scan number, as shown in Figure 5. Greater fluctuations in the signal were observed for paper spray than for extraction spray. The A/IS ratios calculated for each pair of adjacent scans for amitriptyline and amitriptyline-d6 are plotted against the scan numbers (Figure 5c). The comparison of the stability in the A/IS ratios certainly predicts a better opportunity for achieving high precision in quantitative analysis with extraction spray. The variance in ratio was 200% for paper spray but only 17% for extraction spray. An average of the signals recorded over multiple scans could significantly improve the accuracy in the calculation of the A/IS ratio.
Figure 5.
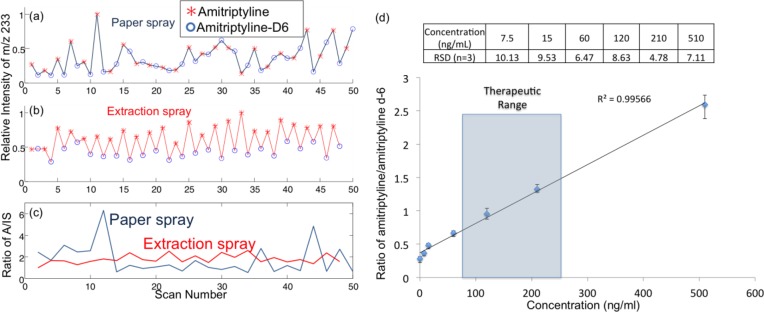
Ion intensity of 200 ng/mL amitriptyline and 100 ng/mL amitriptyline-d6 (IS) in blood with (a) paper spray ionization with silica-coated paper and (b) extraction spray ionization. (c) Ratio of amitriptyline/amitriptyline-d6 in adjacent MS/MS scans using extraction spray ionization and paper spray ionization. (d) Calibration curve showing ratio of amitriptyline/amitriptyline-d6 in blood with extraction spray ionization (product ion m/z of 233 was monitored).
A series of DBS samples containing the IS amitriptyline-d6 at 100 ng/mL but the analyte amitriptyline at different concentrations from 7.5 to 510 ng/mL were analyzed to develop a calibration curve for quantitation. For each point in the curve, the samples were analyzed and the A/IS ratio was calculated by averaging 50 pairs of scans for each sample. The calibration curve was linear over the range from 15 ng/mL to 510 ng/mL (Figure 5d), which covers the therapeutic range (80–250 ng/mL) for amitriptyline, but most importantly, RSD values of better than 10% were achieved over the entire range. As a means of monitoring the potential carryover between samples or assessing contamination problems, MS/MS analysis of blank paper substrates was performed using methanol/water (50/50, v/v) solvent between the analyses of two blood samples containing amitriptyline. No carryover problem was observed. An advantage of using paper spray or extraction spray is that the majority of the sample stays on the paper substrate and only a small portion of the analytes are eluted and ionized for analysis.
Conclusion
A point-of-care miniature mass spectrometry system, the Mini 12, has been developed, built, and characterized. This system includes an RIT miniature mass spectrometer and an ambient ionization source, as well as a user-friendly interface that requires no special training in MS. This system can perform direct mass spectrometry analysis on such complex samples as whole blood, untreated food, and environmental samples, without sample preparation or chromatographic separation. In addition to fast turnaround times and in situ analysis capabilities, disposable cartridges designed for ambient ionization source are used. Further study will be continued to improve the sample handling protocol and to develop a more advanced dual miniature ion trap system to optimize time and sample usage as well as improving software for quantitative POC MS analysis.
Acknowledgments
This work was supported by National Science Foundation (Projects CHE 0847205 and DBI 0852740), the National Center for Research Resources (5R21RR031246-03) and the National Institute of General Medical Sciences (8 R21 GM103454, 1R01GM106016) from the National Institutes of Health, and the Alfred Mann Institute at Purdue University. The authors thank John Hertig, Nick Manicke, and Ziqing Lin for helpful suggestions in development of instrumentation and analytical methods.
Supporting Information Available
Additional information as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kaiser R. E.; Cooks R. G.; Moss J.; Hemberger P. H. Rapid Commun. Mass Spectrom. 1989, 3, 50–53. [Google Scholar]
- Sinha M. P.; Tomassian A. D. Rev. Sci. Instrum. 1991, 62, 2618–2620. [Google Scholar]
- Bryden W. A.; Benson R. C.; Ecelberger S. A.; Phillips T. E.; Cotter R. J.; Fenselau C. Johns Hopkins APL Tech. Dig. 1995, 16, 296–310. [Google Scholar]
- Sinha M. P.; Gutnikov G. Anal. Chem. 1991, 63, 2012–2016. [Google Scholar]
- Syms R. R. A.; Tate T. J.; Ahmad M. M.; Taylor S. Electron. Lett. 1996, 32, 2094–2095. [Google Scholar]
- Grebner T. L.; Neusser H. J. Int. J. Mass Spectrom. Ion Processes 1994, 137, L1–L6. [Google Scholar]
- Austin D. E.; Wang M.; Tolley S. E.; Maas J. D.; Hawkins A. R.; Rockwood A. L.; Tolley H. D.; Lee E. D.; Lee M. L. Anal. Chem. 2007, 79, 2927–2932. [DOI] [PubMed] [Google Scholar]
- Ouyang Z.; Wu G. X.; Song Y. S.; Li H. Y.; Plass W. R.; Cooks R. G. Anal. Chem. 2004, 76, 4595–4605. [DOI] [PubMed] [Google Scholar]
- Badman E. R.; Johnson R. C.; Plass W. R.; Cooks R. G. Anal. Chem. 1998, 70, 4896–4901. [DOI] [PubMed] [Google Scholar]
- Gao L.; Song Q. Y.; Patterson G. E.; Cooks R. G.; Ouyang Z. Anal. Chem. 2006, 78, 5994–6002. [DOI] [PubMed] [Google Scholar]
- Patterson G.; Grossenbacher J.; Wells M.; Keil A.; Gregory M.. Portable MS Instruments. In The Proceedings of the 55th ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, June 3–7, 2007.
- Contreras J. A.; Murray J. A.; Tolley S. E.; Oliphant J. L.; Tolley H. D.; Lammert S. A.; Lee E. D.; Later D. W.; Lee M. L. J. Am. Soc. Mass Spectrom. 2008, 19, 1425–1434. [DOI] [PubMed] [Google Scholar]
- Rafferty D. In The Pittsburgh Conference on Analytical, Chemistry and Applied Spectroscopy: Philadelphia, PA, 2013. [DOI] [PubMed]
- Gao L.; Cooks R. G.; Ouyang Z. Anal. Chem. 2008, 80, 4026–4032. [DOI] [PubMed] [Google Scholar]
- Yamashita M.; Fenn J. B. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar]
- Carroll D. I.; Dzidic I.; Stillwell R. N.; Haegele K. D.; Horning E. C. Anal. Chem. 1975, 47, 2369–2373. [DOI] [PubMed] [Google Scholar]
- Sokol E.; Noll R. J.; Cooks R. G.; Beegle L. W.; Kim H. I.; Kanik I. Int. J. Mass Spectrom. 2011, 306, 187–195. [Google Scholar]
- Huang G. M.; Xu W.; Visbal-Onufrak M. A.; Ouyang Z.; Cooks R. G. Analyst 2010, 135, 705–711. [DOI] [PubMed] [Google Scholar]
- Sanders N. L.; Kothari S.; Huang G. M.; Salazar G.; Cooks R. G. Anal. Chem. 2010, 82, 5313–5316. [DOI] [PubMed] [Google Scholar]
- Lapack M. A.; Tou J. C.; Enke C. G. Anal. Chem. 1990, 62, 1265–1271. [Google Scholar]
- Cooks R. G.; Ouyang Z.; Takats Z.; Wiseman J. M. Science 2006, 311, 1566–1570. [DOI] [PubMed] [Google Scholar]
- Nemes P.; Vertes A. TrAC, Trends Anal. Chem. 2012, 34, 22–34. [Google Scholar]
- Cody R. B.; Laramee J. A.; Durst H. D. Anal. Chem. 2005, 77, 2297–2302. [DOI] [PubMed] [Google Scholar]
- Haapala M.; Pol J.; Saarela V.; Arvola V.; Kotiaho T.; Ketola R. A.; Franssila S.; Kauppila T. J.; Kostiainen R. Anal. Chem. 2007, 79, 7867–7872. [DOI] [PubMed] [Google Scholar]
- Wang H.; Liu J. J.; Cooks R. G.; Ouyang Z. Angew. Chem., Int. Ed. 2010, 49, 877–880. [DOI] [PubMed] [Google Scholar]
- Manicke N. E.; Abu-Rabie P.; Spooner N.; Ouyang Z.; Cooks R. G. J. Am. Soc. Mass Spectrom. 2011, 22, 1501–1507. [DOI] [PubMed] [Google Scholar]
- Zhang Z. P.; Xu W.; Manicke N. E.; Cooks R. G.; Ouyang Z. Anal. Chem. 2012, 84, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy R. D.; Manicke N. E.; Ouyang Z.; Cooks R. G. Analyst 2012, 137, 2344–2349. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Wang H.; Maas J. D.; Chappell W. J.; Manicke N. E.; Cooks R. G.; Ouyang Z. Int. J. Mass Spectrom. 2012, 312, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks P. I.; Dalgleish J. K.; Shelley J. T.; Kirleis M. A.; McNicholas M. T.; Li L.; Chen T.-C.; Chen C.-H.; Duncan J. S.; Boudreau F.; Noll R. J.; Denton J. P.; Roach T. A.; Ouyang Z.; Cooks R. G.. Anal. Chem., submitted. [DOI] [PubMed]
- Gao L.; Sugiarto A.; Harper J. D.; Cooks R. G.; Zheng O. Y. Anal. Chem. 2008, 80, 7198–7205. [DOI] [PubMed] [Google Scholar]
- Ouyang Z.; Noll R. J.; Cooks R. G. Anal. Chem. 2009, 81, 2421–2425. [DOI] [PubMed] [Google Scholar]
- Cooks R. G.; Manicke N. E.; Dill A. L.; Ifa D. R.; Eberlin L. S.; Costa A. B.; Wang H.; Huang G. M.; Zheng O. Y. Faraday Discuss. 2011, 149, 247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicke N. E.; Yang Q. A.; Wang H.; Oradu S.; Ouyang Z.; Cooks R. G. Int. J. Mass Spectrom. 2011, 300, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Cooks R. G.; Ouyang Z. Anal. Chem. 2013, 85, 5632–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S. H.; Marshall A. G. Int. J. Mass Spectrom. Ion Processes 1996, 157, 5–37. [Google Scholar]
- Ren Y.; Liu J.; Li L.; McLuckey M. N.; Ouyang Z. Anal. Methods 2013, 5, 6686–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. P.; Cooks R. G.; Ouyang Z. Analyst 2012, 137, 2556–2558. [DOI] [PubMed] [Google Scholar]
- Cooks R. G.; Busch K. L.; Glish G. L. Science 1983, 222, 273–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.