Abstract
The neurovascular unit provides a conceptual framework for investigating the pathophysiology of how brain cells die after stroke, brain injury, and neurodegeneration. Emerging data now suggest that this concept can be further extended. Cell–cell signaling between neuronal, glial, and vascular elements in the brain not only mediates the mechanisms of acute injury. But integrated responses in these same elements may also be required for recovery as the entire neurovascular unit attempts to reorganize and remodel. Understanding the common signals and substrates of this transition between acute injury and delayed repair in the neurovascular unit may reveal useful paradigms for augmenting neuronal, glial, and vascular plasticity in damaged and diseased brain.
Keywords: Neuron, Astrocyte, Pericyte, Microglia, Endothelium, Plasticity, Stroke, Traumatic brain injury, Neurodegeneration
Introduction
The importance of cell–cell signaling between all elements of the neurovascular unit in stroke, brain injury, and neurodegeneration has been extensively discussed.1–4 Dysfunctional crosstalk between neurons, glia, and vascular compartments contributes to multiple aspects of acute pathophysiology in central nervous system (CNS) disease. Impaired glutamate release–reuptake mechanisms in neurons and astrocytes can amplify excitotoxicity.5 Perturbed signaling between cerebral endothelium, astrocytes, and pericytes can disrupt blood–brain barrier (BBB) integrity.6 Dysfunctional coupling between neuronal activation and vascular responses can promote deleterious spreading depression.7 And ultimately, disordered signaling between all neurovascular and gliovascular elements can underlie the evolution of neuroinflammation and cell death.8 By understanding how these complex multicellular events unfold, we may be able to move beyond a singular focus on ‘preventing neuron death’ towards a more integrative approach where we attempt to rescue function within and signaling between all cell types in the entire neurovascular unit.
To date, the neurovascular unit has been mostly applied as a conceptual tool to guide the investigation into acute mechanisms of injury. More recently, it is recognized that embedded within the acute pathophysiology of CNS disease, is the endogenous response of damaged brain itself.9 The evolution of brain injury and neurodegeneration comprises a dynamic balance and imbalance between initial triggers of injury and evolutionarily conserved responses of brain plasticity, remodeling, and compensation.10 The processes of acute injury and of long-term recovery are likely to involve analogous cell–cell signaling pathways, along with non-cell-autonomous mechanisms in the brain. In this short opinion piece, we briefly outline the principles of this idea and discuss recent data that may help us find common mechanisms of injury and repair in the neurovascular unit (Fig. 1).
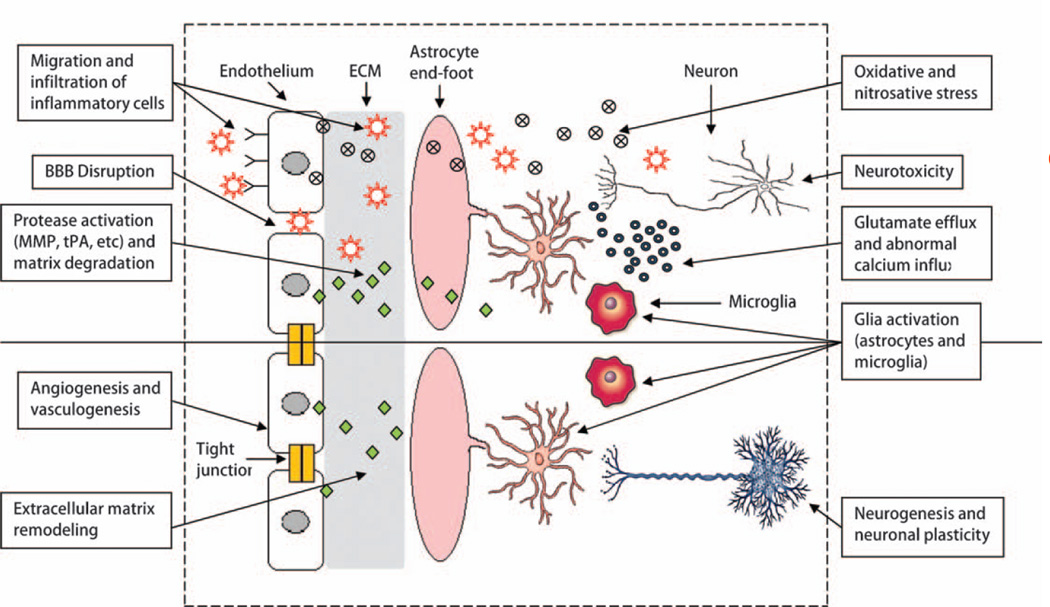
Figure 1.
Schematic of the multicellular interactions that mediate the transition from injury into repair in the neurovascular unit. During injury and disease, the BBB is leaky, inflammation is damaging, and neurotoxicity predominates. But during repair, endogenous mechanisms are activated that involve angiogenesis and neurogenesis, trophic glial reactions, and recruitment of beneficial aspects of inflammation and remodeling. In this simplified schematic, we only depict neurons, astrocytes, microglia and endothelium. Of course, recovery after CNS injury will also involve many other cell types including pericytes, smooth muscle cells, oligodendrocytes, infiltrating or resident immune cells as well as systemic responses in other organs. Ultimately, cell–cell signaling between all elements of the neurovascular unit is required to support neural plasticity and functional compensation and recovery.
Cell–Cell Interactions for Remodeling
One of the best early examples of cell–cell signaling in the neurovascular unit may be found in the original observations of the so-called neurovascular niche for neurogenesis. For decades, the standard model proclaimed that adult mammalian brains did not grow new neurons. But this paradigm was overturned when it was discovered that even in adult brains (at least in rodents), there existed pockets of ongoing neurogenesis, for example, in the subventricular zone next to the lateral ventricles and the dentate regions within the hippocampus. A closer examination of these neurogenic pockets revealed that neuroblasts always seemed to be closely associated with active microvessels, suggesting that endothelial–neuroblast crosstalk may exist.11 Indeed, it has now been shown that coculturing neuroblasts with brain endothelium significantly promoted neurogenesis.12 Of course, whether these primarily rodent phenomena persist in higher human brains remains to be determined.13,14
From an evolutionary perspective, the underlying molecular mediators of neurogenesis and angiogenesis overlap and are highly conserved.15 Hence, after stroke and trauma, neurogenesis and angiogenesis appear to be tightly coregulated, especially during the recovery phase post-injury. Migrating neuroblasts move along perivascular pathways.16 Promoting neurogenesis seems to augment angiogenesis and vice versa.17,18 Some of these interdependent mechanisms may involve growth factors such as brain-derived neurotrophic factor (BDNF).19 Since growth factors not only promote cell growth, but also cell survival, it becomes increasingly clear that the cerebral endothelium may not just comprise ‘empty pipes’ for blood flow. Instead, they may represent an intricate endocrine organ embedded within the brain itself, supporting neuronal function and defending the brain parenchyma against neurotoxicity.20,21
Another example of multicellular crosstalk in the neurovascular unit involves the interactions between the brain microvessel and surrounding astrocytes and pericytes. Developmentally, maturation of the BBB requires the coordinated development of adjacent glial cells.6 During brain injury or neurodegeneration, signaling between astrocytes, pericytes and endothelium become disrupted. Hence, repairing the leaking barrier entails restoring function in the entire gliovascular system including crosstalk between astrocytes and pericytes.22
Plastic crosstalk can also be found between neurons and astrocytes. Release–reuptake of neurotransmitters is essential for brain function, and loss of proper communication between neurons and astrocytes exacerbates excitotoxicity. During remodeling of damaged or diseased neuronal circuits, crosstalk mechanisms may play especially critical roles. Astrocytes are known to release thrombospondin-1 which is a major regulator of synaptic maturation.23 Reactive astrocytes also release tissue plasminogen activator, which may be required for recovering neurons to remodel their dendritic arbors.24 Indeed, several studies have suggested that the therapeutic benefits of stem cell therapies may depend in part on the ability of astrocytes to amplify the effects on neuronal remodeling.25 Mining the astrocyte secretome for novel mediators of neuroplasticity may be an important direction for future research.
Interactions between neurons, glia, and endothelium underlie the ability of the cortex to recover from injury and disease. However, for the large human brain, what happens in white matter may be even more important. Emerging data now suggest that besides a neurovascular niche, an analogous oligovascular niche may also exist. Astrocytes and cerebral endothelial cells secrete many trophic factors that support oligodendrocyte precursor cells.26,27 And after injury, vascular endothelial growth factor (VEGF)-mediated endothelial recovery is linked with the proliferation and migration of oligodendrocyte precursor cells.28,29 Oligodendrogenesis and angiogenesis are intricately linked.30 Without proper remodeling in white matter, the recovering brain cannot reconnect.
Finally, accumulating data is beginning to suggest that non-cell-autonomous mechanisms may extend beyond the narrow confines of the CNS itself. Interactions between the blood/immune system and CNS underlies not just stroke and trauma,8 but also neurodegeneration.31 After focal strokes, alterations in a broad range of genes are readily detected in circulating blood cells.32 Beyond the obvious implications for biomarkers, systemic blood responses may also have therapeutic implications. A recent study provided proof of principle — oral administration of drugs that modify tryptophan metabolism in blood significantly ameliorated synaptic loss and functional deterioration in mouse models of Alzheimer’s and Huntington’s disease.33 Hence, contrary to traditional assumptions, the CNS is not isolated from the rest of the body. Crosstalk between multiple cell types in the CNS and non-CNS are likely to be exceedingly complex. In the end, a systems biology approach may be required to truly dissect the mechanisms involved.34
Biphasic Mechanisms in Injury and Repair
The biphasic nature of many mediators in neurobiology is well known. For example, trophic factors such as nerve growth factor and BDNF promote cellular survival via their primary receptors TrkA and TrkB respectively. But in contrast to these neuroprotective effects, both factors can also be neurotoxic via overactivation of the p75NTR receptor.35 Within the context of the remodeling neurovascular unit, similar biphasic properties of many other factors and mediators may also emerge.
One of the most studied targets in neuroprotection may be the NMDA-type glutamate receptor. Over-activation of the NMDA receptor clearly induces acute excitotoxicity and neuronal death in many animal models of cerebral ischemia, brain trauma, and neurodegeneration.5 However, in the delayed phase after injury and disease, these same NMDA signals may be required for recovery. WithoutNMDA signaling, neuroplasticity and repair of neuronal circuitry cannot take place. Beneficial NMDA mechanisms may involve augmentation of protective CREB signaling in neurons.36 NMDA signaling may also promote the endogenous neurogenesis that occurs after brain injury.37 Indeed, this may be where crosstalk in the neurovascular unit is manifested. A recent study suggests that neuroblast migration along the rostral migratory stream is dependent on proper communication between glutamate released from neuroblasts and NMDA receptors present on guiding astrocytic sheaths.38
Another example of the biphasic nature of mediators in the neurovascular unit involves neurovascular proteases from the matrix metalloproteinase (MMP) family. Dysregulated MMPs are clearly detrimental. By degrading neurovascular matrix, MMPs damage the BBB and cause edema, hemorrhage, and neuronal death.39 Knockout of MMP genes or inhibition with selected drugs all prove significantly protective in animal models of stroke and brain trauma. Indeed, a large body of experimental data has led to the initiation of clinical stroke trials testing minocycline as an MMP inhibitor.40 Recently, however, a new aspect of MMP functioning has become apparent. Whereas MMPs disrupt neurovascular matrix and cause injury during acute stroke, they can promote neurovascular remodeling in peri-infarct cortex during the delayed stages of stroke recovery.41 MMPs also mediate the movement of neuroblasts during the endogenous neurogenic response that is triggered after brain injury.42 Whereas inhibition of MMPs during the first few hours after stroke reduces infarction, the same inhibitors worsen outcomes when applied several days later.43
The idea of biphasic mediators is not restricted to acute brain injury per se. Similar patterns may be observed in ‘slower’ diseases of neurodegeneration. A good example may perhaps be found in the pleiotropic actions of amyloid-beta. In Alzheimer’s disease, hippocampal degeneration is often accompanied by a reorganization of cholinergic networks from the basal forebrain.44 From a functional perspective, plasticity in frontal regions and remapping of associated cortical networks may help the brain cognitively compensate for degenerating neurons.45–47 But how does this happen? Of course, amyloid overload is a key step in plaque formation and generation of toxic oligomers.48 However, it has also been proposed that homeostatic levels of amyloid may help regulate transmitter release and synaptic function.49,50 Ultimately, the balance between beneficial-adaptive versus aberrant-maladaptive forms of synaptic and neuronal remodeling may significantly influence how disease evolves in Alzheimer brains.51
Reactive gliosis is a seminal feature of damaged or diseased brain. Traditionally, the glial scar is thought to be detrimental. Reactive glia secrete many inhibitory substrates that retard axonal and dendritic regrowth.52 Thus, antibody blockade of NOGO improved recovery in mouse models of focal stroke and brain injury.53 Protease-mediated digestion of chondroitin sulfate proteoglycans enhanced functional recovery after stroke and CNS trauma.54 Peptide inhibitors of semaphorins reconnected axons in a mouse model of spinal cord injury.55 More recently, however, a more nuanced view of the reactive astrocyte has emerged. Reactive astrocytes can also release many trophic factors, such as nerve growth factor, basic fibroblast growth factor, platelet-derived growth factor, brain-derived neurotrophic factor, ciliary neurotrophic factor, Neuropilin-1, vascular endothelial growth factor, and others.52,56,57 Many of these trophic factors may be beneficial by promoting neuronal survival and augmenting coordinated responses in synaptogenesis, neurogenesis, and angiogenesis.58,59 Proof of concept can be obtained by suppressing these reactive astrocytes. For example, preventing reactive astrocytes from releasing the damage-associated molecular pattern mediator called HMGB1 results in worsened recovery after focal cerebral ischemia in mice.60,61
The other major class of reactive glia in the CNS comprise the microglia. Microglia are resident immune cells of the CNS and serve as sensors and effectors in the normal and pathologic brain.62,63 Microglia have important homeostatic roles in normal brain. For example, microglia contribute to synapse remodeling and neurogenesis,64–67 and they can also be involved in blood vessel formation.68 But after injury or disease, the role of microglia become even more complex. After an ischemic lesion, resident microglia are activated and accumulate within minutes of ischemia onset.69 Post-ischemic microglial can be highly neurotoxic by producing damaging cytokines, reactive oxygen and nitrogen species, and extracellular proteases such as MMPs.70,71 However, it is now recognized that microglial activation is not a univalent state. Activated microglia can exhibit phenotypic and functional diversity. At least two activated phenotypes have been described: the classically activated M1 or the alternatively activated M2. Inflammatory M1 microglia release high deleterious levels of tumor-necrosis factor-alpha, IL-1beta, reactive oxygen species, and nitric oxide. In contrast, the M2 microglia produce much lower levels of nitric oxide, and higher levels of anti-inflammatory cytokines and neurotrophic factors, such as glial cell line-derived neurotrophic factor, BDNF, basic fibroblast growth factor, insulin-like growth factor 1, transforming growth factor-beta, and VEGF.65,72–75 These types of microglia can be neuroprotective, help clear toxic byproducts of tissue damage, and promote neurogenesis and neuroplasticity. Because microglia possess both beneficial and harmful properties, optimal timing of microglia-based interventions relative to disease onset and progression will be necessary for therapeutic gain.
In theory, if one can distinguish the differential signaling mechanisms of detrimental versus beneficial CNS responses, one may design combination therapies to protect and repair the neurovascular unit. One example can be found in biphasic endothelial reactions to injury in the brain. In order to restorative angiogenesis to take place, endothelial cells will have to disengage and move. But in the process of disengaging, BBB integrity may be perturbed. VEGF is a potent angiogenic factor that induces endothelial cell proliferation, survival, migration, and invasion during development and in pathological conditions. However, VEGF also increases vascular permeability, and can lead to edema and extensive tissue injury in ischemic tissue after myocardial infarction or stroke.76,77 In animal models of stroke, early administration of VEGF (within 1 hour of onset) worsens outcome by increasing brain edema, whereas later administration (48 hours after onset) is beneficial.78 Combination of angiopoietin-1 and VEGF or coexpression of angiopoietin-1 with VEGF increases BBB structural integrity and reduces edema and brain damage after ischemia, but does not affect the angiogenic effects of VEGF.79,80 Recent studies show that the Roundabout 4 (Robo4)–Slit2 signaling axis regulates vascular integrity by counteracting the effects of VEGF, and suggests that Slit2 can both positively and negatively regulate angiogenesis by binding to Robo1 and Robo4, respectively.81 Activation of Robo4 blocks VEGF signaling and VEGF-induced angiogenesis and vascular permeability. Further dissection of this pathway may lead to novel ways of optimizing neurovascular protection and repair within the VEGF domain.82 Ultimately, any attempt to develop targeted therapies in brain injury and neurodegeneration must take into account the biphasic nature of all mediators in the remodeling neurovascular unit.
Conclusions
The concept of the neurovascular unit emphasizes that function and dysfunction in the CNS is not based on the neuron alone. All elements in neuronal, glial, and vascular compartments contribute to physiology and pathophysiology. In this brief opinion piece, we have attempted to extend this concept. Multiphasic and multicellular interactions between all these different cell types should also play a central role as the brain attempts to reorganize, compensate, and recover after stroke, trauma, and neurodegeneration (Fig. 1). In order to discover and optimize new therapies for neuroprotection and neurorepair, we will have to rigorously understand how plasticity within the entire neurovascular unit mediates the graded transition from initial injury into delayed remodeling.
Acknowledgements
This work is supported by grants from the American Heart Association and NINDS.
Footnotes
Some concepts discussed here are based in part on data and ideas presented in previous reviews (Lok et al., Neurochem Res. 2007; Lo, Br J Pharmacol. 2008; Lo, Nat Med. 2008; Arai et al, FEBS J. 2009; Hayakawa et al., Ann New York Acad Sci. 2010; Lo, Nat Med. 2010; Moskowitz et al., Neuron. 2010).
References
- 1.del Zoppo GJ. Relationship of neurovascular elements to neuron injury during ischemia. Cerebrovasc Dis. 2009;27(Suppl 1):65–76. doi: 10.1159/000200442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 3.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 4.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, et al. Cell–cell signaling in the neurovascular unit. Neurochem Res. 2007;32(12):2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 5.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29(5):268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 8.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo EH. Degeneration and repair in central nervous system disease. Nat Med. 2010;16(11):1205–1209. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 13.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, et al. Human neuroblastsmigrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 14.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 16.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29(13):4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28(33):8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci USA. 2008;105(21):7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J, et al. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One. 2010;5(2):e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen LH, Li Y, Chopp M. Astrocytic endogenous glial cell derived neurotrophic factor production is enhanced by bone marrow stromal cell transplantation in the ischemic boundary zone after stroke in adult rats. Glia. 2010;58(9):1074–1081. doi: 10.1002/glia.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29(14):4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J Neurosci Res. 2010;88(4):758–763. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31(29):10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9(3):340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Q, Zhang ZG, Chopp M. MRI evaluation of white matter recovery after brain injury. Stroke. 2010;41(10 Suppl):S112–S113. doi: 10.1161/STROKEAHA.110.595629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 32.Sharp FR, Jickling GC, Stamova B, Tian Y, Zhan X, Liu D, et al. Molecular markers and mechanisms of stroke: RNA studies of blood in animals and humans. J Cereb Blood Flow Metab. 2011;31(7):1513–1531. doi: 10.1038/jcbfm.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning MM, Lo EH. Opportunities and challeneges in omics. Transl Stroke Res. 2010;1(4):233–237. doi: 10.1007/s12975-010-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blochl A, Blochl R. A cell-biological model of p75NTR signaling. J Neurochem. 2007;102(2):289–305. doi: 10.1111/j.1471-4159.2007.04496.x. [DOI] [PubMed] [Google Scholar]
- 36.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25(17):4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14(1):10–18. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- 38.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65(6):859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50(4):329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 40.Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, et al. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41(10):2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 42.Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26(13):3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38(2 Suppl):748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 44.Hyman BT, Kromer LJ, van Hoesen GW. Reinnervation of the hippocampal perforant pathway zone in Alzheimers disease. Ann Neurol. 1987;21:259–267. doi: 10.1002/ana.410210307. [DOI] [PubMed] [Google Scholar]
- 45.Leuba G, Walzer C, Vernay A, Carnal B, Kraftsik R, Piotton F, et al. Postsynaptic density protein PSD-95 expression in Alzheimer’s disease and okadaic acid induced neuritic retraction. Neurobiol Dis. 2008;30(3):408–419. doi: 10.1016/j.nbd.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Agosta F, Rocca MA, Pagani E, Absinta M, Magnani G, Marcone A, et al. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31(4):515–525. doi: 10.1002/hbm.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, et al. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73(9):665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12(12):1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 50.Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006;575(Pt 1):5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 53.Lee DH, Strittmatter SM, Sah DW. Targeting the Nogo receptor to treat central nervous system injuries. Nat Rev Drug Discov. 2003;2(11):872–878. doi: 10.1038/nrd1228. [DOI] [PubMed] [Google Scholar]
- 54.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 55.Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12(12):1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 56.Strauss S, Otten U, Joggerst B, Pluss K, Volk B. Increased levels of nerve growth factor (NGF) protein and mRNA and reactive gliosis following kainic acid injection into the rat striatum. Neurosci Lett. 1994;168(1–2):193–196. doi: 10.1016/0304-3940(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 57.Tower DB, Young OM. The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem. 1973;20(2):269–278. doi: 10.1111/j.1471-4159.1973.tb12126.x. [DOI] [PubMed] [Google Scholar]
- 58.Mocchetti I, Wrathall JR. Neurotrophic factors in central nervous system trauma. J Neurotrauma. 1995;12(5):853–870. doi: 10.1089/neu.1995.12.853. [DOI] [PubMed] [Google Scholar]
- 59.Tokita Y, Keino H, Matsui F, Aono S, Ishiguro H, Higashiyama S, et al. Regulation of neuregulin expression in the injured rat brain and cultured astrocytes. J Neurosci. 2001;21(4):1257–1264. doi: 10.1523/JNEUROSCI.21-04-01257.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayakawa K, Arai K, Lo EH. Role of ERK map kinase and CRM1 in IL-1beta-stimulated release of HMGB1 from cortical astrocytes. Glia. 2010;58(8):1007–1015. doi: 10.1002/glia.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30(4):871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 63.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 64.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158(3):1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 65.Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23(1):83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 66.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 67.Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57(8):835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 68.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47(8):3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- 69.Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7(1):111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 70.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 71.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 72.Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, et al. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5(7):e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merson TD, Binder MD, Kilpatrick TJ. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromolecular Med. 2010;12(2):99–132. doi: 10.1007/s12017-010-8112-z. [DOI] [PubMed] [Google Scholar]
- 74.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiefer R, Streit WJ, Toyka KV, Kreutzberg GW, Hartung HP. Transforming growth factor-beta 1: a lesion-associated cytokine of the nervous system. Int J Dev Neurosci. 1995;13(3–4):331–339. doi: 10.1016/0736-5748(94)00074-d. [DOI] [PubMed] [Google Scholar]
- 76.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200(6):581–597. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 78.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen F, Walker EJ, Jiang L, Degos V, Li J, Sun B, et al. Coexpression of angiopoietin-1 with VEGF increases the structural integrity of the blood–brain barrier and reduces atrophy volume. J Cereb Blood Flow Metab. 2011;31(12):2343–2351. doi: 10.1038/jcbfm.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25(11):1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 81.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14(4):448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acevedo LM, Weis SM, Cheresh DA. Robo4 counteracts VEGF signaling. Nat Med. 2008;14(4):372–373. doi: 10.1038/nm0408-372. [DOI] [PubMed] [Google Scholar]