Abstract
Background and Purpose
Contrast medium extravasation (CE) in intracerebral hemorrhage (ICH) is a marker of ongoing bleeding and a predictor of hematoma expansion. The aims of the study were to establish an ICH model in which CE can be quantified, characterized in ICH during warfarin and dabigatran anticoagulation, and to evaluate effects of prothrombin complex concentrates on CE in warfarin-associated ICH.
Methods
CD 1-mice were pretreated orally with warfarin, dabigatran, or vehicle. Prothrombin complex concentrates were administered in a subgroup of warfarin-treated mice. ICH was induced by stereotactic injection of collagenase VIIs into the right striatum. Contrast agent (350 µL Isovue 370 mg/mL) was injected intravenously after ICH induction (2–3.5 hours). Thirty minutes later, mice were euthanized, and CE was measured by quantifying the iodine content in the hematoma using dual-energy computed tomography.
Results
The optimal time point for contrast injection was found to be 3 hours after ICH induction, allowing detection of both an increase and a decrease of CE using dual-energy computed tomography. CE was higher in the warfarin group compared with the controls (P=0.002). There was no significant difference in CE between dabigatran-treated mice and controls. CE was higher in the sham-treated warfarin group than in the prothrombin complex concentrates–treated warfarin group (P<0.001).
Conclusions
Dual-energy computed tomography allows quantifying CE, as a marker of ongoing bleeding, in a model of anticoagulation-associated ICH. Dabigatran induces less CE in ICH than warfarin and consequently reduces risks of hematoma expansion. This constitutes a potential safety advantage of dabigatran over warfarin. Nevertheless, in case of warfarin anticoagulation, prothrombin complex concentrates reduce this side effect.
Keywords: anticoagulation, contrast extravasation, dabigatran, dual-energy CT, hematoma expansion, intracerebral hemorrhage, PCC treatment, warfarin
Oral anticoagulation therapy plays an important role in the prevention of thrombotic and thromboembolic events. In patients with atrial fibrillation, vitamin K antagonists, such as warfarin, reduce the incidence of stroke by >60%.1 Although they are highly effective in this way, vitamin K antagonists are associated with increased rates of intracerebral hemorrhage (ICH), a severe complication with a short-term mortality rate of >50%.2,3 Recently new anticoagulants, such as the direct thrombin inhibitor, dabigatran, and the factor X inhibitors, rivaroxaban and apixaban, have been introduced into the market.4 In comparison with warfarin, these drugs show a similar effectiveness in reducing embolic events, but provide a better safety profile because they reduce the rate of intracerebral bleeding complications.5–8
Observational studies on ICH evolution while on warfarin anticoagulation revealed prolonged bleeding, enlarged hematoma volumes, and poorer functional outcomes compared with nonanticoagulant ICH.2,3,9,10 Although these data were not derived from randomized trials, the rapid reversal of anticoagulation, using concentrated coagulation factors (prothrombin complex concentrates [PCC]), is proclaimed as a treatment option to prevent massive hematoma expansion in cases of warfarin-ICH.2,3,11 Even less is known about the pathophysiology and potential therapeutic strategies in ICH associated with the administration of the new anticoagulants.
Recently, an animal model of anticoagulation-associated ICH has been developed.12 This model showed that warfarin anticoagulation increased hematoma volumes by 2- to 3-fold compared with nonanticoagulated controls.12 Furthermore, the rapid reversal of anticoagulation using hemostatic drugs (PCC, recombinant factor VII) revealed reduced ICH volumes as compared with mice whose anticoagulation was not reversed.13,14 Interestingly, dabigatran anticoagulation (in the therapeutic range) did not enlarge hematoma volume in experimental ICH as compared with nonanticoagulated controls.15 But increased ICH volumes were found in case of very high dabigatran concentrations.16
In humans, hematoma expansion occurs in the early phase of ICH and is associated with poor functional outcomes and high mortality rates.17–19 Several studies reported spot sign on computed tomography (CT) angiography and contrast extravasation (CE) in contrast-enhanced CT-images of patients with acute ICH as an aid to predict hematoma growth and poor neurological outcomes.20–26 More recently, CE, as evidence of ongoing bleeding, was shown to be a more sensitive predictor of ICH enlargement compared with the spot sign, which may also represent pseudoaneuryms.25,27
The aim of the present study was (1) to establish an experimental ICH model in which CE can be visualized and quantifed by dual-energy CT (DECT), (2) to characterize CE in ICH occurring during warfarin and dabigatran anticoagulation, and (3) to verify potential positive effects of concentrated coagulation factors (PCC) on ameliorating an exacerbated hematoma expansion in warfarin-associated ICH.
Material and Methods
Animals
All experiments were performed in accordance with the guide from the National Institutes of Health for the care and use of laboratory animals. Throughout this study, male CD1-mice aged 12 to 16 weeks were used. Mice were randomly and blindly assigned to 3 treatment groups: warfarin (W), dabigatran (D), and vehicle (tap water; C). The first set of animals (coagulation study; n=12) was used to measure coagulation parameters after W, D, and vehicle pretreatment, and after reversal of warfarin anticoagulation using PCC. A second set of animals (DECT study; n=44) was used to create a DECT-model of collagenase-induced ICH, in which CE can be visualized and quantified. A third set of animals (ICH-contrast injection study [ICH-CI]; n=45) was used to determine CE as the primary end point, as well as hematoma volume and neurological outcome as secondary end points in mice pretreated with W, D, or vehicle. In a fourth set of animals (PCC study; n=10), the effects of PCC or saline treatment on CE in warfarin-anticoagulated ICH mice were assessed.
Anticoagulation Therapy
Warfarin was administered according to a previously established protocol via drinking water.12,13 A 5-mg Coumadin tablet (crystalline warfarin sodium, Bristol Myers Squibb, NY) was dissolved in 375 mL tap water. Assuming a body weight of 40 g and daily water consumption of 15 mL per 100 g, each mouse received ≈0.1 mg (2.5 mg/kg) warfarin within a 30-hour feeding period.
Dabigatran was administered according to a recently established protocol.15 A 150 mg dabigatran tablet (150 mg, Pradaxa, Boehringer Ingelheim, Germany) was dissolved in 7.5 mL tap water, resulting in a dose of 20 mg/mL. Mice were orally fed by a gastric tube containing 0.15 mL of the respective solution (3× with 8-hour intervals). Assuming a body weight of 40 g, each mouse received ≈3 mg (75 mg/kg) of dabigatran.
Control mice were given 0.15 mL tap water by the same application modus in a similar manner (3× in 8-hour intervals).
Measurement of Coagulation Parameters
Coagulation parameters were measured in control animals (n=3) as well as in anticoagulated mice (at the end of the 30-hour warfarin feeding period [n=3] or 30 minutes after the last gavage of dabigatran [n=3]). In addition, coagulation parameters were determined in warfarin-treated mice 15 minutes after injecting PCC into the tail vein (n=3).
Blood was drawn through a cardiac puncture with a 3-mL syringe and a 19-gauge needle under deep anesthesia and was immediately transferred to plastic tubes containing 0.1 mL 3.2% citrate, resulting in a volume ratio of 9:1. International normalized ratio (INR) values, prothrombin time (PT), partial thromboplastin time, and diluted thrombin time (dTT) were quantifed on a coagulation analyzer (Trinity Biotech, Berkeley Heights, NJ).12,13,15
ICH Induction
Animals were anesthetized with isoflurane (1.5%–2%) in a 70% N2O- and 30% O2-mixture and placed in a stereotaxic frame. After opening the scalp, a small borehole was drilled, and a 32-gauge 0.5-µL injection needle (Hamilton 7000 series, Hamilton, Reno, NV) was slowly lowered into the right striatum (stereotaxic coordinates: 0 mm anterior, 2 mm lateral, 3.5 mm below the surface). Then, 0.5 µL of saline containing 0.2 U of collagenase VIIs (Sigma-Aldrich, St. Louis, MO) was injected during a 5-minute period. The needle was left in place for 10 minutes before it was slowly withdrawn during 5 minutes. Afterward, the borehole was sealed with bone wax, and the scalp was sutured. To preserve the body temperature, a heat lamp was used.12,13,15
Outcome Assessment
Neurological deficits were rated in a blinded fashion 2 or 3 hours after ICH induction using a 5-point scale (0, no apparent deficit; 1, slight deficit without circling motion; 2, circling to the right with gaining some distance; 3, heavy circling to the right without gaining distance or no movement at all; 4, deceased).12,13,15
CT Imaging and Contrast Medium Injection
To determine the appearance of the CT-images of our experimental ICH model, we imaged the brains of 2 sets of 4 animals. In the first set, the images were acquired 2.5 hours after ICH induction, without administration of contrast medium. In the second set, the animals received contrast medium 2 hours after ICH induction, and CT-images were acquired after 30-minute circulation time (ie, CT was performed 2.5 hours after ICH induction). We measured the Hounsfield (HU) attenuation differences between ipsilateral (within the subsequently histologically proven ICH area) and contralateral sites.
Then, we tried to determine a suitable time point for contrast injection (CI; ie, a time point when most of the hematoma has already developed but CE is still visible as a marker of ongoing bleeding). To do so, animals in each of the treatment groups (W, D, C) were reanesthetized after predefined intervals after ICH induction (2/2.5/3/3.5 hours; n=3 per treatment group and time point). Then, 350 µL of contrast agent (Isovue 370 mg/mL, Bracco Diagnostic Inc, Princeton, NJ) was slowly injected during 5 minutes into the left femoral vein using a polyethylene-10 tube. Animals were placed in their cages and were euthanized 30 minutes after the injection of contrast medium to stop the rapid vascular clearance of iopamidol.28 To prevent the decomposition of the brain tissue and the diffusion of the contrast agent, the corpses were immediately frozen at −80°.
After determining the optimal time point for CI after induction of ICH, we repeated the same procedures using 10 animals per treatment group in an experiment powered to analyze the differences in the primary end point, CE, among the anticoagulation regimes (W, D, C). A second time point, in which active bleeding (CE) was clearly present in anticoagulated animals and controls, served as an internal positive control (n=5 per treatment group).
DECT Scanning and Image Reconstruction
Frozen corpses were scanned on a dual-energy system (Siemens Definition Flash, Siemens Healthcare, Forchheim, Germany). The tube voltage and tube current settings were optimized independently for each X-ray source. Settings were chosen to split the overall dose equally between the 2 imaging chains. CT examinations were performed using the following protocol: Tube A, 80 kV; Tube B, 140 kV with tin (Sn) filter, 2200 mAs and 800 mAs, respectively, and a collimation of 32×0.6 mm.
The projection data acquired at 80 and 140 kV were reconstructed separately. Images were reconstructed at 0.6 mm slice thickness, with 0.3 mm increments using a dual-energy D50 image kernel. A mixed energy image set was calculated using a weighted sum of the 80 kV and the 140 kV images, in a 60% and 40% ratio, respectively, simulating a 120 kV image.
Semiquantitative and Computer-Aided Quantitative Measurement of CE
In the DECT study, CT-images were evaluated using a simple binary end point (ie, CE visible, CE not visible).
In the ICH-CI study, CE was assessed on a 5-point score, visually respecting and comparing the size and density of contrast enhancement (1: no CE, 2: probable CE, 3: visible CE, 4: bright CE, 5: bright CE with filling the right striatum area).
Furthermore, in the ICH-CI study, CT-images were processed using dedicated software (Syngo DE, Siemens Healthcare, Forchheim, Germany) to quantify CE. A 3-material decomposition algorithm based on water, brain parenchyma, and iodine was performed. From the original low and high kV data sets, a virtual noncontrast image and a functional and quantitative iodine image were derived. In the functional images, the presence of iodine is indicated by a special look-up table (orange color) and its intensity and attenuation value correspond to the relative concentration of iodine. The software allows for quantitative analysis of regions of interest (ROI). After selection on the iodine image, the software calculated the concentration of iodine in mg/mL and the ROI size in mm2. Because all CT-images were reconstructed at 0.6-mm slice thickness, we assessed the ROI volume for each slice by multiplying the ROI volume by 0.6 mm and determined the contrast amount by multiplying the ROI volume by the calculated iodine concentration. Afterward the values (contrast amount of each slice) were added up to get the total iodine content of lCH.
ICH Volume Assessment by Quantitative Hemoglobin Determination
After DECT imaging, the corpses were defrosted by a heating lamp for 1 hour. Brains were removed and placed in glass tubes containing 3 mL phosphate-buffered saline. Hematoma volume was determined via photometric hemoglobin measurements according to a standard protocol.12,13,15 Because the mice were not transcardially perfused before measuring hemoglobin, we determined the background level by measuring the mean blood volume in mouse brains under the same conditions, with the omission of ICH induction (20.1±2.6 µL). This background value was subtracted from each total quantified hematoma volume.
PCC Study
Forty-five minutes after ICH induction in warfarin-anticoagulated mice, either PCC (100 U/kg; Beriplex, CSL Behring, Marburg, Germany) or an equal amount of saline (140 µL) was injected into the tail vein (n=5 per group; W-PCC and W-S group).3,14 As described above, 350 µL of contrast agent was injected intravenously 3 hours after ICH induction. Thirty minutes later, mice were euthanized and scanned with DECT. CE was evaluated both semiquantitatively (visually) and quantitatively through computer-aided CE measurement. Hematoma volumes were measured by quantifying the hemoglobin content.
Statistical Analysis
SPSS version 21.0 was used for statistical analysis. Data are expressed as mean and standard error of mean (mean±SEM) or as median and interquartile range depending on scale levels and data distribution. Parametric data (quantitative CE measurements, hematoma volumes) were analyzed for between-group differences using a 1-way ANOVA with post hoc Bonferroni testing. Nonparametric data (semiquantitative CE measurements, functional outcomes) were analyzed for between-group differences using the Kruskall–Wallis test with post hoc testing (Mann–Whitney U test). A P≤0.05 was considered as statistically significant.
Results
Ex Vivo Measurement of Coagulation Parameters (Coagulation Study)
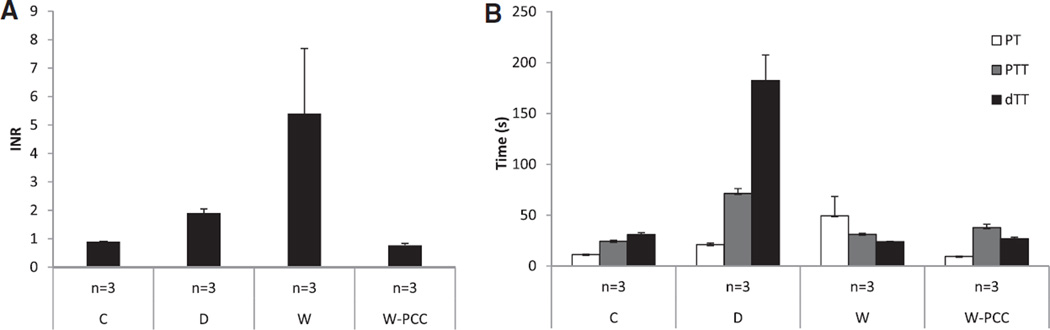
In nonanticoagulated (C) mice, INR values were 0.9±0.0, corresponding to a PT of 11.3±0.2 s. Warfarin anticoagulation increased INR values (5.4±2.3) and the PT (49.4±19.0 s). Partial thromboplastin time was 24.3±1.1 s in controls, but increased to 71.4±4.8 s in the D group. Similarly, dTT was normal in controls (31.0±1.9 s), but largely elevated in D mice (182.4±25.3 s). Fifteen minutes after PCC injection, the increased INR- and PT-values in W mice were normalized (INR=0.8±0.1, PT=9.4±0.2; Figure 1).
Figure 1.
Coagulation study: coagulation parameters in groups treated with different anticoagulants. A, International normalized ratio (INR; mean±SEM). B, Prothrombin time (PT), partial thromboplastin time (PTT), diluted thrombin time (dTT). C indicates controls; D, dabigatran; W, warfarin; and W-PCC, warfarin with subsequent anticoagulation reversal using prothrombin complex concentrates.
Determining the Time Point for CI (DECT Study)
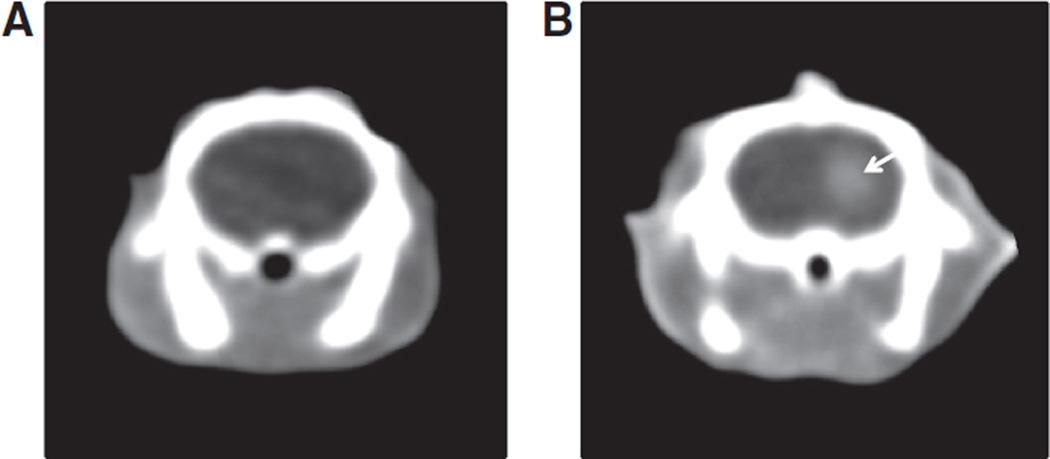
Brain hemorrhage was not visible on native CT-images performed 2.5 hours after ICH induction. In contrast-enhanced CT-images, however, we identified hyperdense signals (CE) in the right striatum (Figure 2). The HU attenuation difference between the ICH area and the contralateral brain parenchyma was low on native CT-images (−4≈+2HU), whereas a much higher difference was found in contrast-enhanced CT-images (+27≈+54 HU).
Figure 2.
Representative brain computed tomography (CT) images 2.5 hours after intracerebral hemorrhage (ICH) induction. A, Coronal contrast-naive CT-image. B, Coronal CT-image with contrast injection 2 hours after ICH induction (ie, 30 minutes before CT scanning). The contrast extravasation in the right striatum area (arrow) is clearly visible.
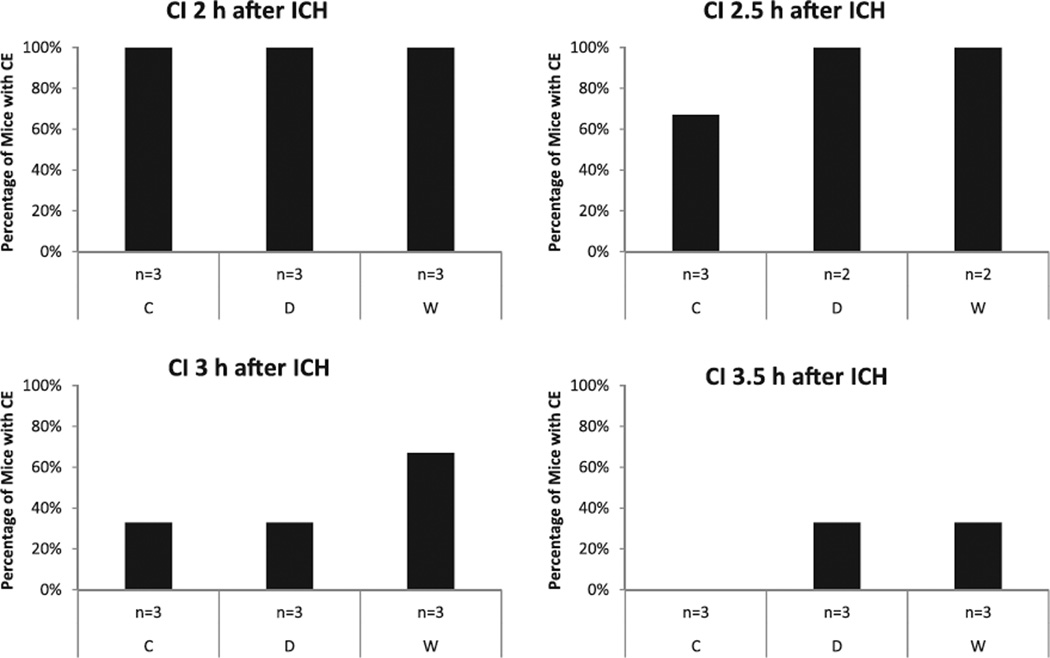
In the DECT study, we tried to identify a suitable time point for CI that subsequently allowed comparison of CE among different anticoagulation regimes. Visually, CE continuously decreased over time. In mice that received CI 2 hours after ICH induction, CE was apparent in 100% of all mice (W, D, C). In contrast, in mice that received CI 3.5 hours after ICH induction CE was apparent in only 33% of W and D mice, and in none of the C mice (Figure 3). We chose 3 hours as a suitable time point for CI when we performed the ICH-CI study, with the assumption that both an increase and a decrease of CE should be detectable, if present. Furthermore, we chose the 2-hour time point as an internal positive control, because at this time CE was visible in all 3 groups.
Figure 3.
Dual-energy computed tomography study: evaluation of contrast extravasation (CE) 30 minutes after contrast injection (CI) at different time points after intracerebral hemorrhage (ICH) induction: 2, 2.5, 3, and 3.5 hours. Presence of CE decreased with increasing time intervals from ICH induction to contrast injection. C indicates controls; D, dabigatran; and W, warfarin.
Semiquantitative and Computer-Aided Quantitative Measurement of CE (ICH-CI Study)
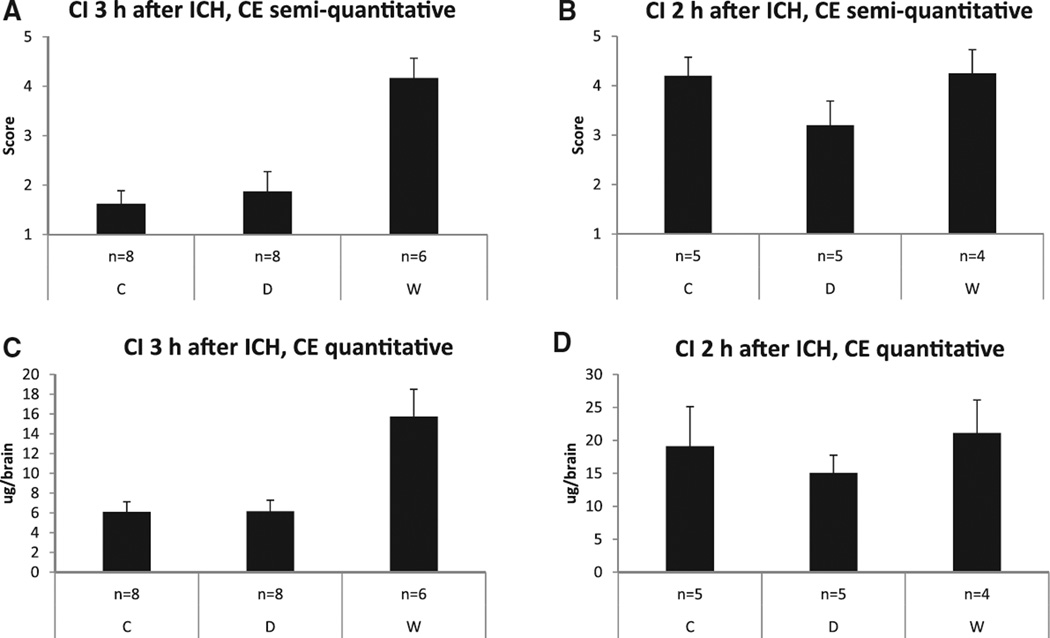
In mice that received CI 3 hours after ICH induction (ICH-CI3), the median visual evaluation score of CE was 4.5 (3.0–5.0) in the group pretreated with warfarin, 1.5 (1.0–2.8) in the dabigatran group, and 1.5 (1.0–2.0) in controls (P=0.004; post hoc W versus D, P=0.007; W versus C, P=0.002; D versus C, P=ns; Figure 4A). For the internal control group (ICH-CI2), the median evaluation score of CE was 4.5 (3.3–5.0) in the W group, 3.0 (2.5–4.0) in the D group, and 4.0 (3.5–5.0) in C mice (P=ns; Figure 4B).
Figure 4.
ICH-contrast injection study: semiquantitative and quantitative assessment of contrast extravasation (CE) 30 minutes after contrast injection (CI) at 3 hours (primary end point) and 2 hours (internal control) after intracerebral hemorrhage (ICH) induction. A and B, Semiquantitative evaluation of CE on a 5-point score (mean±SEM). C and D, Computer-aided quantitative iodine measurement derived from dual-energy computed tomography imaging. C indicates controls; D, dabigatran; and W, warfarin.
According to computer-aided quantitative iodine measurements, CE in W mice with CI 3 hours after ICH induction (ICH-CI3) was ≈2.5 fold higher than that in mice in groups D and C (W=15.7±0.8 µg; D=6.2±l.l µg; C=6.1±1.0 µg; P=0.001; post hoc W versus C, P=0.002; D versus C, P=ns; W versus D, P=0.002; Figure 4C). In the internal control group (ICH-CI2), mean CE was 21.1±5.0 µg in the W group, 15.1+2.7 µg in the D group, and 19.1+6.0 µg in C group (P=ns; Figure 4D).
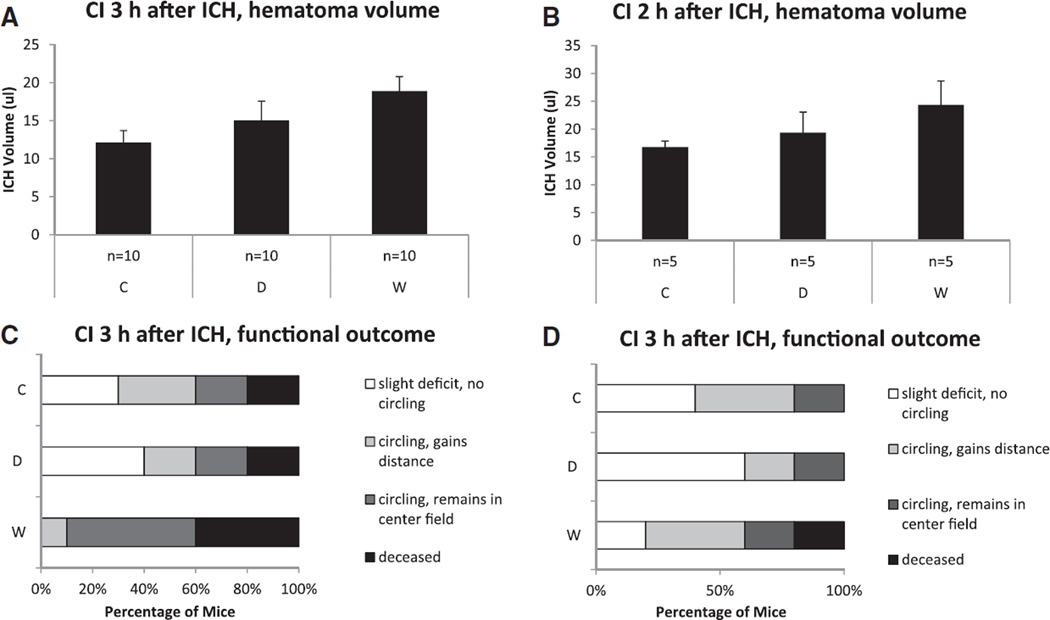
There was a trend toward increased hematoma volumes at 3.5 hours after ICH induction in the W group compared with controls (W: 18.9±2.6 µL; D: 15.0+1.9 µL; C: 12.1+1.6 µL; P=0.080; post hoc W versus C, P=0.079; D versus C, P=0.968; W versus D, P=0.574; Figure 5A). Considering functional outcome determined 3.5 hours after ICH induction, mice in the W group showed a trend toward worse median neuroscore than mice in both the D and control group (W: 3.0 [3.0–4.0]; D: 2.0 [1.0–3.3]; C: 2.0 [1.0–3.3]; P=0.063; post hoc W versus C, P=0.045; D versus C, P=0.814; W versus D, P=0.041; Figure 5C). Hematoma volume and functional outcome of the internal control group (ICH-CI2) are displayed in Figure 5B and 5D.
Figure 5.
ICH-contrast injection study: hematoma volume and functional outcome 30 minutes after contrast injection (CI) at 3 hours (primary end point) and 2 hours (internal control) after intracerebral hemorrhage (ICH) induction. A and B, Hematoma volume determined via photometric Hb-measurement (mean±SEM). C and D, Functional outcome assessed on an ordinal scale. C indicates controls; D, dabigatran; and W, warfarin.
PCC Study
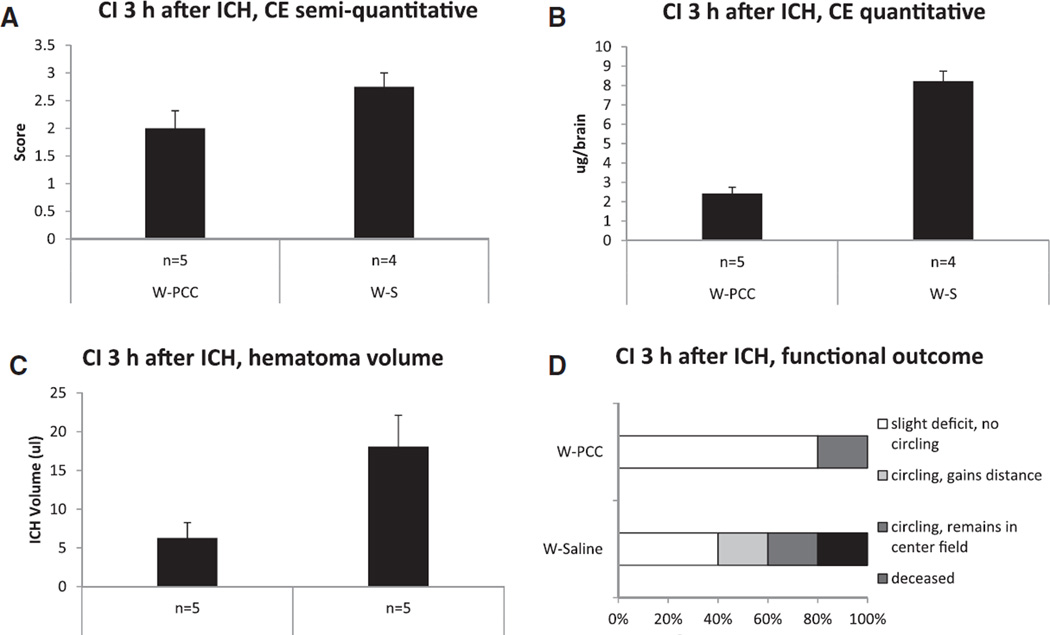
We observed the effects of PCC therapy on CE, hematoma volume, and functional outcome by either injecting PCC or saline in mice in group W. The median score of visual CE evaluation was 3.0 (2.3–3.0) in W-S group and 2.0 (1.5–2.5) in W-PCC group (P=ns). However, there was a significant difference in computer-aided quantitative CE measurement between W-S and W-PCC mice (W-S: 8.2±0.5 µg; W-PCC: 2.4±0.3 µg; P<0.001; Figure 6A and 6B). In addition, W-S mice had a 3-fold larger hematoma volume than the W-PCC group (W-S: 18.1+4.1 µL; W-PCC: 6.3±2.0 µL; P=0.031; Figure 6C). Considering the neurological outcome of W-S and W-PCC mice, there was no significant difference (W-S: 2.0 [1.0–3.5]; W-PCC: 1.0 [1.0–2.0]; P=ns; Figure 6D).
Figure 6.
Prothrombin complex concentrates (PCC) study: evaluation of contrast extravasation (CE), hematoma volume, and functional outcome 30 minutes after contrast injection (CI) at 3 hours after intracerebral hemorrhage (ICH) induction in warfarin-anticoagulated animals. Mice were randomized to either saline- (W-S) or PCC treatment (W-PCC) 45 minutes after ICH induction. A, Semiquantitative evaluation of CE on a 5-point score (mean±SEM). B, Computer-aided quantitative iodine measurement derived from dual-energy computed tomography imaging. C, Hematoma volume determined via photometric Hb-measurement. D, Functional outcome assessed on an ordinal scale. W indicates warfarin.
Discussion
We combined a mouse model of collagenase-induced ICH with advanced CT imaging technology. This allowed us to directly visualize and quantify CE, an indicator of ongoing bleeding, in anticoagulated mice and controls. Our study provides novel insights into the pathophysiology of anticoagulation-associated ICH. In contrast to ICH under warfarin anticoagulation that under a new anticoagulant, dabigatran, had less CE, suggesting an earlier termination of active bleeding. By showing that PCC treatment rapidly reverses warfarin anticoagulation as measured by reduced CE and hematoma expansion, we demonstrate a potential use of our model in testing future therapeutic strategies in anticoagulation-associated ICH.
Noncontrast CT is routinely used as a standard diagnostic method to identify ICH in patients with symptoms suspicious for acute stroke.29 More recently, clinical studies investigated the use of contrast-enhanced CT scanning in the acute phase of ICH. Both the spot sign and CE were reported to be associated with hematoma expansion, which is itself related to neurological deterioration and poor functional outcome.12,24,25 In a translational approach, we hypothesized that contrast medium injection would help us to depict active bleeding and, consequently, CE in an experimental model of ICH.
Seo et al28 have shown that very small volumes of blood that were surgically introduced in rat brains were visible through noncontrast CT, although major cerebral vessels and carotid arteries were only visible after injection of contrast agent. In our study, we were not able to depict ICH in noncontrast CT scans of mouse brains, because mice have a poor intrinsic x-ray attenuation contrast in the brain. However, after injecting the contrast agent, ICH was visualized because of a significant difference of HU attenuation between the bleeding area and the (healthy) brain parenchyma. The amount of contrast medium (Isovue, ≈130 mg for a 40 g mouse) that was injected might be considered as high. An ex vivo study described renal failure after injecting high dose of Isovue (1600 mg/kg) because of blood cell aggregation and decrease of blood flow.28,30 However, in other studies, Isovue injections of ≈105 mg in C57 mice (20–23 g) were well tolerated.31,32 In our experiments, all animals tolerated the given dose and survived after injection. Because we performed the CT scan on euthanized mice, we were not concerned about the radiation dose. Because the spatial resolution of the (human) CT scanner was limited and digital noise would impair the 3-material dual energy decomposition, we decided to maximize the dose that would give the highest possible signal-to-noise ratio. It remains unclear whether this DECT-model can be used with reasonable radiation exposure; however, a high resolution-small animal CT system may be an effective alternative.
By using 2 different voltages, DECT gets 2 simultaneous scans that can be used to derive virtual unenhanced and contrast-enhanced images. Additionally, because x-ray attenuation is energy-, material- or tissue dependent, the difference of material-specific attenuation can give more information about the chemical composition of the detected material. This allowed us to derive quantitative iodine images.
To develop our model, we first monitored the level of anticoagulation after warfarin, dabigatran, and vehicle pretreatment. The partial thromboplastin time and dTT values in 75 mg/kg dabigatran-pretreated animals were prolonged up to ≈3-fold and 5.8-fold of baseline, respectively. Administrating warfarin (2.5 mg/kg) for 30 hours via drinking water resulted in mean INR values of 5.4±2.3.12,15 In the next step, we characterized the kinetics of collagenase-induced ICH more in detail to identify a time point when most of the hematoma has already developed, but active bleeding is still present in a reasonable number of animals. Although CE (ie, active bleeding) was present in all animals in all treatment groups if contrast medium was injected 2 hours after ICH induction, only 2 out of 9 animals had CE if contrast medium was injected 3.5 hours after ICH induction. Thus, 3 hours seemed to be a time point where both an increase and a decrease in CE should be detectable.
In the subsequent study, powered to detect differences in CE between treatment groups, both the semiquantitative evaluation score and the iodine concentration derived from DECT imaging were significantly higher in warfarin-treated mice compared with controls and dabigatran-treated mice. This implies that, at the time point of CI, the bleeding was substantially active only in the warfarin-treated group and had presumably stopped in the control and dabigatran groups. These results provide an explanation for recently published data showing that dabigatran pretreatment does not enlarge hematoma volume in experimental ICH compared with controls, whereas warfarin pretreatment increases hematoma volume significantly (2–3-fold).12,15 Indeed, we found that during a very short observation window (a few hours after ICH induction), warfarin-treated mice showed a tendency toward increased hematoma volumes as compared with control mice and dabigatran-treated mice, whereas no such trend between control and dabigatran-treated mice was detected. Our results are also in agreement with a study demonstrating that hematoma expansion (assessed via repetitive MRI imaging) in warfarin-associated experimental ICH occurs mostly during the first 3 hours after ICH induction.33
The pathophysiological mechanisms by which dabigatran fails to enlarge hematoma volume, as happens with treatment with vitamin-K–based anticoagulants, are not yet fully understood. Previous studies have shown that a decrease of coagulation factor VII and X (as in warfarin therapy) delays clot-initiation time, resulting in an increased hematoma volume. In contrast, even reduced activity of factor II (as in dabigatran therapy) provides sufficient hemostasis comparable with control plasma.15,34 Another explanation might be that dabigatran interacts selectively and reversibly with the active site of the thrombin molecule and, as a result, does not interfere with the critical interaction between thrombin and thrombocytes.35,36
A potential treatment strategy for warfarin-associated ICH is the prevention of excessive hematoma expansion by reversing the decreased coagulation status as soon as possible.2,3 Clinical and experimental studies suggest that PCC rapidly reverses warfarin anticoagulation and also reduces the hematoma volume.2,13,37,38 However, clinical data from randomized trials are still missing. Our results confirm that INR values in anticoagulated mice were normalized within 15 minutes after PCC injection. Furthermore, hematoma volumes in sham-treated warfarin mice were 3-fold larger than the PCC-treated warfarin mice. CE was significantly higher in the sham-treated group compared with the PCC group, indicating that the active bleeding was rapidly terminated in the PCC group. In the future, it might be interesting to use this newly developed DECT model to investigate the duration of ICH in anticoagulated mice. This could lead to an expansion of the time window of hemostatic therapy and the ability to examine the effect of PCC at later time points. In addition, the time course of hematoma development and expansion, the dynamics of bleeding, and the effect of other hemostatic agents could be explored.39
Some limitations of the present study should be considered. First, imaging by DECT was initiated in ex vivo frozen corpses. Freezing the brain tissue may cause morphological changes, such as fractures, cellular shrinkage, and loss of staining.40,41 These effects could influence the morphology of contrast enhancement in ICH. However, after somatic death, autolytic processes, which depend on the postmortem interval, influence the ultrastructure of brain tissue. These changes affect diffusion properties, which in our study could alter the distribution of the contrast agent.42 Therefore, we decided to fix the tissue directly after death of the animals. Second, we quantified CE by measuring the size of the ROI and the iodine concentration from reconstructed slices with 0.6-mm thickness. Consequently, the calculated values could possibly be higher or lower than the actual values (partial volume effect), resulting in a loss or gain of apparent iodine concentration. However, because this effect would be apparent in each group, it should not influence relative differences between the groups. Third, hematoma expansion in the collagenase-induced ICH-mouse model may have a different timeline compared with hematoma expansion in humans. It has been reported that in humans, secondary bleeding into the hematoma, which leads to subsequent hematoma enlargement, is especially common in warfarin-induced ICH and worsens functional outcome and mortality.2,24,29 However, in this study, we were only able to visualize and quantify early hematoma expansion through measuring CE.
In conclusion, we provide an experimental ICH model combined with DECT imaging technology that allows the detection and quantification of CE in anticoagulated and non-anticoagulated mice. Furthermore, we applied a therapeutic paradigm to our model and proved that the rapid reversal of anticoagulation by means of PCC administration is effective in reducing hematoma expansion in warfarin-associated ICH. Future insights into the pathophysiology of hematoma expansion in ICH occurring during an altered coagulation status may be derived from this model.
Acknowledgments
Sources of Funding
This study was supported, in part, by the DARPA AXiS program, Grant Number: N66001-11–4204, P.R. Number: 1300217190, and by a research grant from Siemens Medical Solutions.
Footnotes
Disclosures
None.
References
- 1.Aguilar MI, Hart RG, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar MI, Hart RG, Kase CS, Freeman WD, Hoeben BJ, Gracia RC, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:81–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–262. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- 4.Deftereos S, Tsounis D, Giannopoulos G, Kossyvakis C, Panagopoulou V, Raisakis K, et al. Oral IIa and Xa inhibitors for prevention of stroke in atrial fibrillation: clinical studies and regulatory considerations. Curr Clin Pharmacol. 2012;7:166–174. doi: 10.2174/157488412800958749. [DOI] [PubMed] [Google Scholar]
- 5.Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Metaanalysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453–460. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 9.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 10.Neau JP, Couderq C, Ingrand P, Blanchon P, Gil R. VGP Study Group. Intracranial hemorrhage and oral anticoagulant treatment. Cerebrovasc Dis. 2001;11:195–200. doi: 10.1159/000047638. [DOI] [PubMed] [Google Scholar]
- 11.Hanger HC, Geddes JA, Wilkinson TJ, Lee M, Baker AE. Warfarin related intracerebral haemorrhage: better outcomes when reversal includes prothrombin complex concentrates. Intern Med J. 2012;43:308–316. doi: 10.1111/imj.12034. [DOI] [PubMed] [Google Scholar]
- 12.Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, et al. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39:3397–3404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foerch C, Arai K, Van Cott EM, van Leyen K, Lo EH. Rapid reversal of anticoagulation reduces hemorrhage volume in a mouse model of warfarin-associated intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:1015–1021. doi: 10.1038/jcbfm.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlunk F, Van Cott EM, Hayakawa K, Pfeilschifter W, Lo EH, Foerch C. Recombinant activated coagulation factor VII and prothrombin complex concentrates are equally effective in reducing hematoma volume in experimental warfarin-associated intracerebral hemorrhage. Stroke. 2012;43:246–249. doi: 10.1161/STROKEAHA.111.629360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauer A, Cianchetti FA, Van Cott EM, Schlunk F, Schulz E, Pfeilschifter W, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation. 2011;124:1654–1662. doi: 10.1161/CIRCULATIONAHA.111.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W, Schwarting S, Illanes S, Liesz A, Middelhoff M, Zorn M, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–3599. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 17.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Leira R, Dávalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Stroke Project. Cerebrovascular Diseases Group of the Spanish Neurological Society. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 19.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of Haematoma Growth and Outcome in Patients with Intracerebral Haemorrhage Using the CT-angiography Spot Sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 20.Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke. 2012;43:3427–3432. doi: 10.1161/STROKEAHA.112.664003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, Schwab K, et al. CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care. 2012;17:421–128. doi: 10.1007/s12028-012-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero JM, Heit JJ, Delgado Almandoz JE, Goldstein JN, Lu J, Halpern E, et al. Spot sign score predicts rapid bleeding in spontaneous intracerebral hemorrhage. Emerg Radiol. 2012;19:195–202. doi: 10.1007/s10140-012-1020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 25.Hallevi H, Abraham AT, Barreto AD, Grotta JC, Savitz SI. The spot sign in intracerebral hemorrhage: the importance of looking for contrast extravasation. Cerebrovasc Dis. 2010;29:217–220. doi: 10.1159/000267842. [DOI] [PubMed] [Google Scholar]
- 26.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Smith A, Hemphill JC, III, Smith WS, Lu Y, Dillon WP, et al. Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol. 2008;29:520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo Y, Hashimoto T, Nuki Y, Hasegawa BH. In vivo microCT imaging of rodent cerebral vasculature. Phys Med Biol. 2008;53:N99–N107. doi: 10.1088/0031-9155/53/7/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol. 2005;4:662–672. doi: 10.1016/S1474-4422(05)70195-2. [DOI] [PubMed] [Google Scholar]
- 30.Liss P, Nygren A, Olsson U, Ulfendahl HR, Erikson U. Effects of contrast media and mannitol on renal medullary blood flow and red cell aggregation in the rat kidney. Kidney Int. 1996;49:1268–1275. doi: 10.1038/ki.1996.181. [DOI] [PubMed] [Google Scholar]
- 31.Ulrich Kerl H, Isaza CT, Boll H, Schambach SJ, Nolte IS, Groden C, et al. Evaluation of a continuous-rotation, high-speed scanning protocol for micro-computed tomography. J Comput Assist Tomogr. 2011;35:517–523. doi: 10.1097/RCT.0b013e31821c662b. [DOI] [PubMed] [Google Scholar]
- 32.Schambach SJ, Bag S, Steil V, Isaza C, Schilling L, Groden C, et al. Ultrafast high-resolution in vivo volume-CTA of mice cerebral vessels. Stroke. 2009;40:1444–1450. doi: 10.1161/STROKEAHA.108.521740. [DOI] [PubMed] [Google Scholar]
- 33.Illanes S, Zhou W, Heiland S, Markus Z, Veltkamp R. Kinetics of hematoma expansion in murine warfarin-associated intracerebral hemorrhage. Brain Res. 2010;1320:135–142. doi: 10.1016/j.brainres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen VG, Cohen BM, Cohen E. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastog-raphy: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiol Scand. 2005;49:222–231. doi: 10.1111/j.1399-6576.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 35.Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. NEngl J Med. 2005;353:1028–1040. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 36.Mosesson MW, Hernandez I, Siebenlist KR. Evidence that catalytically-inactivated thrombin forms non-covalently linked dimers that bridge between fibrin/fibrinogen fibers and enhance fibrin polymerization. Biophys Chem. 2004;110:93–100. doi: 10.1016/j.bpc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Lorenz R, Kienast J, Otto U, Kiehl M, Schreiter D, Haertel S, et al. Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis. 2007;18:565–570. doi: 10.1097/MBC.0b013e3282010d7a. [DOI] [PubMed] [Google Scholar]
- 38.Quick JA, Bartels AN, Coughenour JP, Barnes SL. Experience with prothrombin complex for the emergent reversal of anticoagulation in rural geriatric trauma patients. Surgery. 2012;152:722–726. doi: 10.1016/j.surg.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Wartenberg KE, Mayer SA. Reducing the risk of ICH enlargement. J Neurol Sci. 2007;261:99–107. doi: 10.1016/j.jns.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Baraibar MA, Schoning P. Effects of freezing and frozen storage on histological characteristics of canine tissues. J Forensic Sci. 1985;30:439–147. [PubMed] [Google Scholar]
- 41.O’Donnell C, Bedford P, Burke M. Massive hemoperitoneum due to ruptured ectopic gestation: postmortem CT findings in a deeply frozen deceased person. Leg Med (Tokyo) 2011;13:245–249. doi: 10.1016/j.legalmed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 42.D’Arceuil H, de Crespigny A. The effects of brain tissue decomposition on diffusion tensor imaging and tractography. NeuroImage. 2007;36:64–68. doi: 10.1016/j.neuroimage.2007.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]