Abstract
Divergent disease triggers in neurodegeneration may induce convergent endogenous pathways in neuronal, glial and vascular elements as the central nervous system (CNS) attempts to compensate, remodel and recover. Dissecting these multicellular mechanisms and the integrative responses in cerebral blood flow and metabolism may allow us to understand the balance between injury and repair, validate new targets and define therapeutic time windows for neurodegeneration.
Remarkable advances have been made in the last decade in understanding the basic mechanisms of neurodegeneration. Progress has come on many fronts, including the molecular biology of cell death, animal models, genetics and neuroimaging of clinical disease. Many targets are being tested in a wide range of experimental studies and clinical trials. Along with the excitement, however, some caution may be warranted. Advances in our knowledge of excitotoxicity, oxidative stress, mitochondrial dysfunction and apoptosis similarly provided a rich repertoire of targets and drugs for ‘acute neurodegeneration’ after stroke and traumatic brain injury1,2. Yet, almost all clinical trials in stroke and brain trauma have failed thus far. Broadly speaking, there is still no clinically validated neuroprotectant. This article reports on one speculative hypothesis: triggers of disease in neurodegeneration may also act as stimuli that induce endogenous compensation and recovery. Hence, investigations into the pathophysiology of CNS disease should take into account not only the primary mechanisms of degeneration but also simultaneous processes of regeneration as the brain tries to repair itself.
Divergence in disease, convergence in repair?
The mammalian nervous system is highly complex. Many things can go wrong in many ways. Clinically, there are multiple ‘types’ of neurodegeneration, comprising major diseases such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Huntington’s disease and prion disease. This wide spectrum of neurodegenerative phenotypes reflects key differences in the proximal triggers and pathologic markers of disease: plaques and tangles in Alzheimer’s disease, Lewy bodies in Parkinson’s disease, motoneuron death in ALS and demyelination in multiple sclerosis. However, these seemingly divergent mechanisms also lead to multiple convergent pathways. Regardless of the initial triggers, many overlapping downstream and/ or secondary pathways are induced, including neuroinflammation3. An important challenge in designing therapies for neurodegeneration is to not only eliminate the initial disease triggers but also ameliorate the deleterious inflammatory responses within the CNS once the disease is under way.
Although the CNS was originally thought to be an immune-privileged organ, it is now known that inflammation can be triggered in injured brain tissue. For example, activation of microglia and responses in both innate and adaptive immunity accompany almost all types of neurodegeneration4,5. From an evolutionary perspective, inflammation can be interpreted as part of a highly conserved set of endogenous responses to injury and disease in any organ6. The same might be true in the context of neurodegeneration.
In spite of the highly divergent disease triggers in neurodegeneration, is it possible that these diseases also induce convergent downstream mechanisms of compensation, repair and remodeling (Fig. 1a)? This is obviously a speculative idea. But some evidence of endogenous recovery during neurodegeneration can indeed be detected in animal models and clinical studies.
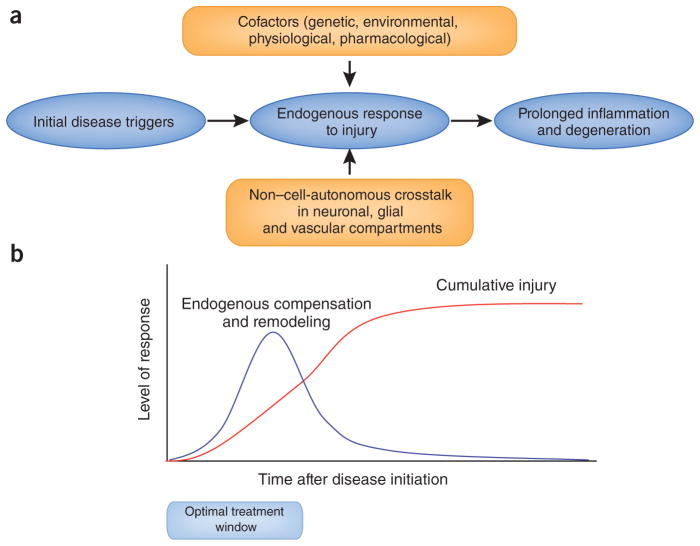
Figure 1.
Endogenous responses, cofactors and therapeutic time windows in neurodegeneration. The wide spectrum of pathology in neurodegeneration reflects a multitude of initial triggers involved in the induction of disease. But divergent upstream mechanisms also lead to convergent downstream pathways as the brain responds to disease and injury. Endogenous responses of the CNS may comprise both deleterious and potentially beneficial mechanisms of inflammation and neurovascular remodeling. Non–cell-autonomous crosstalk between neuronal, glial and vascular compartments form the basis for these phenomena, and many cofactors influence the signals and substrates of these endogenous responses over time. Ultimately, prolonged inflammation and injury lead to a cumulative disease burden that outstrips any endogenous attempts at compensation or remodeling. Understanding these transitions may help define therapeutic time windows where candidate treatments for neurodegeneration should be more effective.
It is well known that behavioral and motor adaptation allows for some individuals with Parkinson’s disease to compensate during the initial stages of disease. But beyond compensation, some degree of actual neuronal remodeling may also take place. Dendritic sprouting and reafferentation of damaged areas are known to occur in animal models of focal brain lesions. The most common animal models of Parkinson’s disease involve selective lesioning of dopaminergic neurons within the substantia nigra. Because the substantia nigra projects to the striatum, this depletes striatal dopamine and mimics clinical Parkinson’s disease. However, it is now recognized that local sources of dopamine may also exist in the mammalian striatum, deriving in part from intrinsic tyrosine hydroxylase–positive neurons7. After loss of dopaminergic input from the substantia nigra, local tyrosine hydroxylase–positive neurons seem to remodel and expand in rodent and primate models of Parkinson’s disease8,9. It has been proposed that similar substrates of neuroplasticity may also exist in individuals with Parkinson’s disease10. Whether these pathways truly restore clinically relevant dopamine function remains to be determined11.
Similar substrates of repair and remodeling may also be present in Alzheimer’s disease. Indeed, one of the early observations in Alzheimer’s disease was that hippocampal degeneration is often accompanied by a reorganization of acetylcholinesterase staining patterns, indicative of cholinergic sprouting from basal forebrain regions12. More recent studies now propose that increased expression of synaptic proteins, such as postsynaptic density protein-95, may underlie plasticity in prefrontal and frontal networks as the brain attempts to cognitively compensate for degenerating neurons13. Functional magnetic resonance imaging studies suggest that remapping of cortical networks may extend to motor systems as well14. Indeed, the degree of cognitive decline may be more closely related to an individual’s endogenous neuronal plasticity than to the cerebral abundance of amyloid or tangles per se15. Recent work is beginning to elucidate the underlying mechanisms. Perturbations in amyloid precursor protein processing lead to increased amyloid-β generation, assembly of toxic oligomers, plaque formation and Alzheimer’s disease pathology16. However, homeostatic forms and levels of amyloid-β may also act as functional regulators of transmitter release and synaptic function17,18. Ultimately, the balance between beneficial-adaptive and aberrant-maladaptive forms of synaptic and neuronal remodeling may significantly influence how Alzheimer’s disease pathology disrupts cerebral function as the disease progresses19.
Endogenous mechanisms of brain repair and recovery can be classified into several categories: behavioral compensation, activation of latent or parallel circuits, neuronal plasticity, remodeling of discrete networks and perhaps even neurogenesis. Traditionally, it was thought that no new neurons were generated after birth in mammalian brains. However, a wealth of studies have led to a paradigm shift. It is now generally accepted that pockets of ongoing neurogenesis persist in the adult brain, including active loci in the sub-ventricular zone lining the lateral ventricles and subgranular zone of the dentate gyrus. Emerging data suggest that neurogenesis is surprisingly plastic, and increased neurogenesis can be observed in models of CNS injury and disease20. Several suspected molecular mediators in Alzheimer’s disease, such as amyloid, presenilin 1, Notch and ErbB4, are known to participate in the molecular regulation of neurogenesis21. A survey of the animal model literature reveals that neurogenesis can sometimes be affected in Alzheimer’s disease, depending on the stage of disease and the underlying phenotypes of the mutant mice involved22. Whether alterations in neurogenesis actually occur in human neurodegeneration remains unclear and provides an exciting frontier for research.
An emerging concept in neuroscience suggests that neuronal responses are intricately linked to vascular signaling as part of an evolutionarily conserved phenomenon1,2,23. Hence, neuroplasticity may occur only within the context of brain microvessel remodeling24. New cells and networks need new blood supplies. If endogenous repair and neuronal recovery take place in neurodegeneration, is there a corresponding angiogenic response as well? Although the literature is sparse, there have been a few descriptions of augmented vascular growth in a wide spectrum of CNS diseases, including Alzheimer’s disease, Parkinson’s disease and multiple sclerosis25–29. Insofar as triggers of disease may conversely act as stimuli for repair, it might be interesting to ask whether mediators of neurodegeneration can promote vascular recovery. For example, amyloid peptides may augment angiogenesis by amplifying fibroblast growth factor signaling30. Furthermore, crosstalk between angiogenesis and neurogenesis may occur, as amyloid has been shown to increase rates of neuronal differentiation of bone marrow–derived endothelial precursor cells31. However, it is important to acknowledge that vascular remodeling is a crucial component of any inflammatory cascade. To some degree, then, angiogenesis in diseased brains may represent a nonspecific epiphenomenon associated with neurodegeneration.
Multiple mechanisms in multiple cells
The neurovascular unit is an increasingly accepted conceptual model in neuroscience, in which cell-to-cell signaling between neuronal, glial and vascular elements underlies both physiology and pathophysiology in the brain1,2. The term ‘neurodegeneration’ implies a purely neuronal phenomenon, but this is clearly not the case when one examines the signals and substrates of disease more closely. Disruptions in cell-cell signaling within the neurovascular unit may underlie non–cell-autonomous mechanisms during the response to injury and disease in the CNS32 (Fig. 1a).
For example, interactions between neurons and astrocytes are essential for the regulation of glutamate transmission. Without astrocytes, neurons become increasingly vulnerable to excitotoxicity33. Any disruption in astrocytic function can markedly promote neurodegeneration. Transgenic Huntington’s disease model mice that express mutant huntingtin only in cortical pyramidal neurons do not show neurodegeneration34, whereas mice that express huntingtin in astrocytes develop age-dependent neurological worsening35. In a mouse model of Niemann-Pick disease, expression of wild-type Niemann-Pick disease type C1 protein in astrocytes was sufficient to decrease the rate of neurodegeneration and increase life span36. Primary astrocyte and microglial cultures derived from the superoxide dismutase–mutant mouse model of ALS produce neurotoxic mediators in conditioned media that kill wild-type motoneurons37. In mouse models of Alzheimer’s disease, widespread perturbations of calcium signaling were detected in astrocyte networks long distances away from amyloid plaques38. Hence, protecting neurons alone may not be enough, and strategies to improve glial function may also be important for CNS disease therapies.
Signaling between endothelial cells and neurons may have especially crucial roles in neurodegeneration23,29,39. Endothelial dysfunction and perturbations in microvascular flow regulation precede the development of neuronal dysfunction in some mouse models of Alzheimer’s disease40. Subtle disruptions in the blood-CNS barrier may be detected before neuronal death in the superoxide dismutase–mutant mouse model of ALS41,42. Compromised blood flow in white-matter regions may precede lesion development in some individuals with multiple sclerosis43. Microvessels in the brain might not be simply an inert system for blood flow. Instead, the cerebral endothelium may also serve as an endocrine organ that actively exchanges signaling mediators and trophic factors with gray and white matter44–46. Disease triggers such as amyloid may disrupt these forms of trophic coupling between the vascular and neuronal compartments.
If non–cell-autonomous signaling between neurons, glia and blood vessels contributes to disease progression, is it possible that compensation and repair also involve coordinated remodeling in the entire neurovascular unit (Fig. 1a)? Again, examples can be drawn from findings in acute brain injury. Angiogenic and neurogenic plasticity are tightly coregulated after stroke and brain trauma47. This might not be surprising, as molecular mechanisms of neurogenesis and angiogenesis have been evolutionarily conserved so that similar mediators and pathways are involved in both phenomena48. After experimental stroke in rat models, newborn neurons are seen to migrate along ‘highways’ that seem to run in close proximity to remodeling blood vessels49. Promotion of neural plasticity enhances vascular regrowth; conversely, angiogenic stimulation enhances neural repair50,51. The pathophysiology of neurodegeneration will surely include analogous interactions between neuronal, glial and vascular cells.
Taken together, non–cell-autonomous mechanisms in neurodegeneration may provide a conceptual framework for asking further questions. How do these multicell pathways mediate the convergent mechanisms between degeneration and regeneration? Can differences in cell-to-cell signaling help explain the selective neuronal vulnerability that is the signature phenotype of so many CNS diseases? What are the mediators that regulate these processes? It has been proposed that brain injury and repair sometimes include biphasic mediators and mechanisms52. The same molecule or cell can behave differently at different phases of the disease. Overactivation of N-methyl-D-aspartic acid (NMDA) receptors induces excitotoxicity, but without NMDA signaling, neuronal plasticity cannot take place53. The stress-activated protein kinase c-Jun N-terminal kinase promotes neuronal apoptosis but is also required for axonal remodeling54. Activated microglia are key players in neuroinflammation, but certain forms of microglia can secrete beneficial neurotrophic factors55. Aggregated proteins may represent misfolded pathologic species or underlie protective endogenous attempts to sequester toxic molecules56. Understanding how these biphasic mechanisms are coordinated in multiple cell types will be essential for dissecting the precarious balance between neurodegeneration and ongoing compensation and repair.
Cofactors in risk and recovery
Advances in genetics have revealed a host of underlying mechanisms that increase risk of disease. In neurodegeneration, the power of genetics is clearly indicated in the tremendous number of sophisticated and targeted transgenic mouse models that are now available. However, it might still be useful to ask whether these mice represent models of disease or, more accurately, models of mechanisms. These discussions may not be purely semantic. The initial molecular triggers of neurodegeneration are cellular, but pathophysiology is expressed at the organ level, and clinical disease is usually manifested in patients with numerous other health problems requiring concurrent medications.
Would it be useful to consider the development of combination approaches whereby such cofactors are incorporated into the mouse models (Fig. 1a)? Because aging is a key cofactor in neurodegeneration, future studies should aim to test leading candidate therapies in aged transgenic mice. Concomitant vascular disease may be a crucial cofactor in dementia, with overlaps between vascular dementia and Alzheimer’s disease being especially important57. Various Alzheimer’s disease transgenic mice could be examined in the context of hypertension, diabetes and other forms of vascular dysfunction. Given a body of literature on the effects of statins on Alzheimer’s disease, these models could also be used to examine how cardiovascular medications such as statins and antihypertensive drugs interact with the various pathways targeted in neurodegeneration mouse models. Finally, as discussed above, differences in systemic inflammation may be crucial for disease pathogenesis. Almost all mouse models are developed and assessed in rigorously controlled housing conditions. But does this also mean that most of our transgenic mice have highly ‘clean’ inflammatory baselines, unlike those of a typical human with metabolic and vascular diseases? How do altered inflammatory baselines affect the balance between degeneration and regeneration?
Beyond promoting the risk of disease, these cofactors may also contribute to resistance to disease. Research efforts are largely focused on identifying factors and mediators that increase the rate of neurodegeneration. But individuals, even with similar loads of disease triggers, can have widely varying rates of neurological decline. Is it possible that differences in the ability to adapt, repair and remodel partly underlie this phenomenon? And, if so, might it be productive to screen for genes and cofactors that promote compensation and recovery and enhance resistance to neurodegeneration?
Time is brain
The mantra in stroke and trauma clinical trials is “time is brain.” After the initial ischemic, hemorrhagic or traumatic insult, brain cells inexorably begin to die. The longer one waits, the more cells are lost, and the less effective any putative neuroprotective therapy might be. Clinical trial design in stroke and trauma is highly focused on time of treatment, where only patients admitted within specific times after onset are enrolled. Is it possible that similar attention to treatment time windows is also essential for neurodegeneration (Fig. 1b)?
If one accepts the proposed hypothesis of ongoing degeneration and regeneration in CNS disease, then one would predict that initially, the disease burden would be light and neuronal dysfunction could be ameliorated by endogenous mechanisms of compensation, remodeling and repair. At this stage, patients might still be asymptomatic or relatively well. As the disease continued to progress beyond certain thresholds, the burden of disease would overcome the brain’s endogenous ability to cope. During this transition phase, neurodegeneration would accelerate, and saving the brain might become extremely difficult, no matter how potent the therapy may be. Hence, under some conditions, preventing disease progression might be more effective than trying to cure the brain after decades of neuronal dysfunction and death.
An example of this concept of ‘prevention’ versus ‘cure’ can be observed in the mouse model literature for ALS. Effective neuroprotection—that is, decreased motor impairment and increased life span—has been reported for a wide range of targets in superoxide dismutase–mutant mouse models of ALS58. Yet a closer look would reveal that the majority of these studies involved treatment of mice in the very early stages of disease. In contrast, most clinical trials in ALS recruit subjects that are sometimes quite far along in terms of disease progression and severity. This phenomenon is by no means unique to ALS. A recent study in the TG4510 tau-mutant mouse showed that, during the early stages of disease, many neurons were positive for active caspase but had not yet undergone apoptosis, suggesting that tau-bearing neurons are surprisingly long lived59. In an inducible cell model of tauopathy, turning off the gene encoding mutant tau allowed neurons to recover before degenerative cascades were too far gone60. Similarly, amyloid-β immunotherapy may work better at reducing amyloid load and deposition as a preventive measure rather than after pathology is established61. Hence, timing is crucial, and there might be windows of time in which Alzheimer’s disease therapies are more effective. Indeed, the majority of experimental treatments for Alzheimer’s disease have been tested in relatively young mutant mice, whereas clinical trials may typically involve a much more heterogeneous population of dementia patients62. However, it is important to recognize that translating time windows in mice into relevant times for therapy in humans may also be difficult.
Ultimately, early treatments for neurodegeneration should logically reap greater benefits. The sooner one tackles the disease, the better. But how would one find these patients? By definition, the individuals with earliest-stage disease may be asymptomatic. Neuroimaging or tissue biomarkers may be required63. If surrogate markers (imaging of amyloid, tau or neurotransmitter depletion) can be quantified and proven to rigorously correlate with clinical states, then one might envision clinical trials using biomarkers not only for subject selection and recruitment but also for the assessment of secondary outcome measures. No matter how potent one’s proposed target or drug, biological variation will always reveal responders and nonresponders. Defining the progressively worsening balance between degeneration and regeneration may ultimately allow us to find patients who still have a chance (Fig. 1b).
Collaborations and consortia
Complex problems require collaborative solutions. To begin with, the basic molecular mechanisms of neurodegeneration are highly multifactorial. If the proposed phenomenon of simultaneous compensation and repair is indeed ongoing, this would add yet another layer of complexity. Traditional approaches involving a single model, single pathway and single laboratory may not be enough. To dissect these underlying mechanisms of degeneration and regeneration in the entire neurovascular unit, multidisciplinary networks and consortia will be useful.
The richness of available models in neurodegeneration may be both an advantage and a disadvantage. The many cellular and animal models provide diverse and powerful opportunities for generating and testing hypotheses at the mechanistic level. However, in terms of serving as preclinical platforms for drug testing, they can also pose a challenge. Which models should one use for screening, development and optimization? Model systems span a wide phylogenetic range, comprising individual cells, Caenorhabditis elegans, Drosophila melanogaster, zebrafish, mice and perhaps even non-human primates. The workhorse in the field remains the transgenic mouse, but the majority of these highly targeted mice may represent models of mechanisms rather than standalone models of human disease. Without collaborations, the increasing heterogeneity of models and techniques may prove intractable.
A recent meta-analysis of the Alzheimer’s disease mouse model literature clearly showed that many of the models being tested involved presymptomatic mice62. Perhaps most important, many studies were underpowered and lacked proper randomization, blinding and statistical controls. It is notable that similar conclusions were reached in a meta-analysis of animal stroke models64. An emerging consensus in the stroke field suggests that consortia are needed to unravel these difficult differences between models and laboratories65. Would collaborative consortia also be productive in neurodegeneration? Early success in the Alzheimer Disease Neuroimaging Initiative suggests that broadly collaborative networks can indeed be built, where data are shared and findings can be rapidly disseminated and leveraged.
Questions and opportunities
Many questions remain, but these translational challenges also present us with new opportunities. Collaborative approaches should be useful for conducting rigorously powered, statistically controlled screening of candidate mechanisms, targets and drugs. Findings could be replicated in multiple models across multiple laboratories. The role of cofactors (age, gender, environment, baseline inflammation, metabolic and cardiovascular disease, and concurrent medications) could be more easily assessed. Different laboratories could also contribute expertise in dissecting non–cell-autonomous crosstalk between various neuronal, glial and vascular cell types in the mammalian CNS. Molecular mechanisms of selective neuronal death may not make sense unless interpreted in the context of integrated responses in cerebral blood flow and metabolism.
In spite of divergent disease triggers, it is likely that convergent downstream pathways of secondary response and remodeling will be activated during neurodegeneration. Dissecting the interplay between injury and repair may allow us to find therapies that maximize neuroprotection and neurorepair. Ultimately, understanding these transitions and mechanisms may help us find ways to identify patients with compensatory reserves who still have salvageable brains.
Acknowledgments
The author apologizes to colleagues whose important work could not be directly cited. Because of space limitations, mostly review articles were used as starting points for discussion. Many thanks to B. Bacskai, D. Selkoe, B. Hyman, M. Schwarzschild and M.M. Ning for helpful discussions; and F. Beal, D. Cleveland, E. Mandelkow, W. Robberecht and all participants in the Herrenhausen Symposium on Neurodegeneration for a wonderful educational experience.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Lo EH, Dalkara T, Moskowitz MA. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz MA, Lo EH, Iadecola C. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block ML, Hong JS. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Lucin KM, Wyss-Coray T. Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 7.Betarbet R, et al. J Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchard V, et al. J Neurochem. 1995;64:1669–1679. doi: 10.1046/j.1471-4159.1995.64041669.x. [DOI] [PubMed] [Google Scholar]
- 9.Tandé D, et al. Brain. 2006;129:1194–1200. doi: 10.1093/brain/awl041. [DOI] [PubMed] [Google Scholar]
- 10.Porritt MJ, et al. Lancet. 2000;356:44–45. doi: 10.1016/S0140-6736(00)02437-5. [DOI] [PubMed] [Google Scholar]
- 11.Huot P, Levesque M, Parent A. Brain. 2007;130:222–232. doi: 10.1093/brain/awl332. [DOI] [PubMed] [Google Scholar]
- 12.Hyman BT, Kromer LJ, Van Hoesen GW. Ann Neurol. 1987;21:259–267. doi: 10.1002/ana.410210307. [DOI] [PubMed] [Google Scholar]
- 13.Leuba G, et al. Neurobiol Dis. 2008;30:408–419. doi: 10.1016/j.nbd.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Agosta F, et al. Hum Brain Mapp. 2010;31:515–525. doi: 10.1002/hbm.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacono D, et al. Neurology. 2009;73:665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selkoe DJ. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramov E, et al. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 18.Pearson HA, Peers C. J Physiol (Lond) 2006;575:5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palop JJ, et al. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch P, Kokaia Z, Lindvall O, Brustle O. Lancet Neurol. 2009;8:819–829. doi: 10.1016/S1474-4422(09)70202-9. [DOI] [PubMed] [Google Scholar]
- 21.Lazarov O, Marr RA. Exp Neurol. 2010;223:267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marlatt MW, Lucassen PJ. Curr Alzheimer Res. 2010;7:113–125. doi: 10.2174/156720510790691362. [DOI] [PubMed] [Google Scholar]
- 23.Zacchigna S, Lambrechts D, Carmeliet P. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 24.Madri JA. J Physiol Pharmacol. 2009;60(Suppl 4):95–104. [PubMed] [Google Scholar]
- 25.Barcia C, Emborg ME, Hirsch EC, Herrero MT. Front Biosci. 2004;9:277–282. doi: 10.2741/1145. [DOI] [PubMed] [Google Scholar]
- 26.Desai BS, Schneider JA, Li JL, Carvey PM, Hendey B. J Neural Transm. 2009;116:587–597. doi: 10.1007/s00702-009-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holley JE, Newcombe J, Whatmore JL, Gutowski NJ. Neurosci Lett. 2010;470:65–70. doi: 10.1016/j.neulet.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 28.Vagnucci AH, Jr, Li WW. Lancet. 2003;361:605–608. doi: 10.1016/S0140-6736(03)12521-4. [DOI] [PubMed] [Google Scholar]
- 29.Zlokovic BV. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Cantara S, et al. FASEB J. 2004;18:1943–1945. doi: 10.1096/fj.04-2114fje. [DOI] [PubMed] [Google Scholar]
- 31.Jin HK, Bae JS, Furuya S, Carter JE. Cell Prolif. 2009;42:571–586. doi: 10.1111/j.1365-2184.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilieva H, Polymenidou M, Cleveland DW. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg PA, Aizenman E. Neurosci Lett. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 34.Gu X, et al. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Bradford J, et al. J Biol Chem. 2010;285:10653–10661. doi: 10.1074/jbc.M109.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, et al. J Neurosci Res. 2008;86:2848–2856. doi: 10.1002/jnr.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai M, et al. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iadecola C. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 40.Iadecola C. Cell Mol Neurobiol. 2003;23:681–689. doi: 10.1023/A:1025092617651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbuzova-Davis S, et al. PLoS One. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong Z, et al. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varga AW, et al. J Neurol Sci. 2009;282:28–33. doi: 10.1016/j.jns.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arai K, Lo EH. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dugas JC, et al. J Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo S, et al. Proc Natl Acad Sci USA. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arai K, Jin G, Navaratna D, Lo EH. FEBS J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmeliet P, Tessier-Lavigne M. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 49.Thored P, et al. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 50.Ohab JJ, Fleming S, Blesch A, Carmichael ST. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taguchi A, et al. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo EH. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 53.Ikonomidou C, Turski L. Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 54.Waetzig V, Zhao Y, Herdegen T. Prog Neurobiol. 2006;80:84–97. doi: 10.1016/j.pneurobio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Perry VH, Nicoll JA, Holmes C. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 56.Williams AJ, Paulson HL. Trends Neurosci. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fotuhi M, Hachinski V, Whitehouse PJ. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 58.Benatar M. Neurobiol Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 59.de Calignon A, et al. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Kruger U, Mandelkow E, Mandelkow EM. Neurodegener Dis. 2010;7:103–107. doi: 10.1159/000285516. [DOI] [PubMed] [Google Scholar]
- 61.Lemere CA, Masliah E. Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zahs KR, Ashe KH. Trends Neurosci. 2010;33:381–399. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Hampel H, et al. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 64.O’Collins VE, et al. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 65.Fisher M, et al. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]