Abstract
Although the perfusion-weighted imaging/diffusion-weighted imaging (PWI/DWI) mismatch model has been proposed to identify acute stroke patients who benefit from reperfusion therapy, the optimal definition of a mismatch is uncertain. We evaluated the odds ratio for a favorable clinical response in mismatch patients with reperfusion compared with no reperfusion for various mismatch ratio thresholds in patients enrolled in the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. A mismatch ratio of 2.6 provided the highest sensitivity (90%) and specificity (83%) for identifying patients in whom reperfusion was associated with a favorable response. Defining mismatch with a larger PWI/DWI ratio may provide greater power for detecting beneficial effects of reperfusion.
Keywords: mismatch, ischemic penumbra, thrombolysis, acute ischemic stroke, perfusion-weighted imaging, diffusion-weighted imaging
Introduction
It has been postulated that magnetic resonance imaging, specifically perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI), can be used to select acute stroke patients who are most likely to benefit from reperfusion therapy (Albers, 1999). This has been referred to as PWI/DWI mismatch hypothesis. The hypothesis predicts that PWI/DWI mismatch, defined as PWI lesion volume minus DWI lesion volume, provides an estimate of the ischemic penumbra and that the presence of a mismatch identifies patients who are most likely to benefit from reperfusion therapy. The results of the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study (Albers et al, 2006) support the validity of the mismatch hypothesis in patients treated with tissue plasminogen activator (tPA) therapy in the 3 to 6 h time window. The DEFUSE study showed that early reperfusion is associated with a more favorable clinical response in patients with the PWI/DWI mismatch profile, whereas patients without a mismatch did not appear to benefit from reperfusion.
Although the PWI/DWI mismatch concept is already being used for patient selection in clinical studies (Butcher et al, 2005; Hacke et al, 2005; Furlan et al, 2006), the criteria used to determine the presence of PWI/DWI mismatch have varied between the studies (Darby et al, 1999; Neumann-Haefelin et al, 1999; Schellinger et al, 2000; Parsons et al, 2002). In many studies, mismatch has been defined as PWI lesion volume of > 120% of DWI lesion, but this threshold was chosen arbitrarily. It is conceivable that the degree of PWI/DWI mismatch is an important factor in predicting the response to reperfusion therapy. Specifically, patients with a more extensive mismatch area may be more likely to benefit from reperfusion. The aim of our study was to determine if the criteria used to define a mismatch affect the likelihood that patients with a mismatch will benefit from early restoration of blood flow (reperfusion). Consequently, the results of this study may help to refine and optimize PWI/DWI mismatch selection criteria for future acute stroke studies.
Materials and methods
This is a substudy of the DEFUSE study. Methods and main results of the study have been reported (Albers et al, 2006). In this substudy, we included the patients who had a baseline PWI lesion ≥10 mL and technically adequate PWI and DWI data at both baseline and the 3 to 6 h follow-up scan. We looked for an association between the mismatch ratio and the presence of a favorable clinical response using logistic regression analysis. Subsequently, we investigated the association between early reperfusion and a favorable clinical response. We then assessed whether there was an interaction between early reperfusion, mismatch ratio, and favorable clinical response using logistic regression (Jaccard, 2001). Diffusion-weighted imaging was performed using a spin echo–echo-planar imaging sequence (field of view = 240 mm, repetition time = 5 secs, echo time = minimum allowed, slice thickness = 5 mm, number of slices = 19, slice gap = 1 mm, acquisition matrix 128 × 128) and lesion volumes determined using a semiautomated thresholding algorithm, which identified regions of high signal intensity that exceeded a region in the contralateral frontal lobe by more than 3 s.d. Dynamic susceptibility PWI was performed using gradient echo–echo-planar imaging (field of view = 240 mm, repetition time = 2 secs, echo time = 60 ms, slice thickness = 7 mm, number of slices = 12, slice gap = 0, acquisition matrix 128 × 128, dynamic scans = 40) and time to peak of the residue function (Tmax) maps were generated by deconvolution of the tissue concentration over time curve using an arterial input function from the contralateral middle cerebral artery. A 2-sec delay was used as the lower threshold indicating ischemic or hypoperfused tissue. Early reperfusion was defined as a ≥30% and ≥10 mL reduction in PWI lesion volume on the 3 to 6 h follow-up scan compared with the baseline (pretreatment scan). A ‘favorable clinical response’ was defined as National Institutes of Health Stroke Scale score of 0 to 1 or ≥8 point improvement at 30 days. Odds ratios for favorable clinical response for patients with PWI/DWI mismatch and early reperfusion were calculated for multiple possible PWI/DWI mismatch definitions. The optimal mismatch ratio for predicting a favorable clinical response in patients with early reperfusion was determined using receiver operating characteristic analysis between mismatch ratio and favorable clinical response. All statistical analyses were performed using SPSS statistical software (Chicago, IL, USA).
Results
Forty-five patients met the inclusion criteria for this substudy. There were no significant differences between patients who did or did not have early reperfusion in baseline characteristics, except for age (early reperfusion patients were older). There were no significant differences between patients with and without favorable clinical response (Table 1). The rate of early reperfusion in this cohort was 49% (22 of 45 patients). There was no significant relationship between mismatch ratio and the probability of reperfusion (P = 0.625 with Wilcoxon rank sum test). Neither mismatch ratio (OR: 0.99, 95% CI: 0.8 to 1.2, P = 0.87), nor reperfusion (OR: 1.6, 95% CI: 0.5 to 5.2, P = 0.47) was associated with favorable clinical response. However, the interaction term ‘mismatch ratio times reperfusion’ was significantly associated with favorable clinical response (P for interaction = 0.0004).
Table 1.
Comparisons of baseline characteristics
| Factors | Between patients with and without early reperfusion
|
Between patients with and without favorable clinical response
|
||||
|---|---|---|---|---|---|---|
| Patients with early reperfusion (n = 22) | Patients without early reperfusion (n = 23) | P-value | Patients with favorable clinical response (n = 18) | Patients without favorable clinical response (n = 27) | P-value | |
| Age, mean (s.d.) | 78.5 (8.4) | 66.5 (18.3) | 0.007 | 70.7 (17.1) | 73.5 (14.4) | 0.55 |
| White (%) | 91 | 83 | 0.41 | 83 | 89 | 0.59 |
| Female (%) | 55 | 61 | 0.67 | 61 | 56 | 0.71 |
| Baseline NIHSS, median (IQR) | 14 (9–16) | 13 (9–16) | 0.85 | 13.5 (10–16) | 13 (9–16) | 0.89 |
| Hypertension (%) | 64 | 57 | 0.63 | 44 | 70 | 0.08 |
| Cardiac disease (%) | 50 | 52 | 0.88 | 50 | 52 | 0.90 |
| Diabetes mellitus (%) | 36 | 22 | 0.28 | 33 | 26 | 0.59 |
| Cigarette smoking (current and previous) (%) | 36 | 48 | 0.44 | 44 | 41 | 0.81 |
| Hyperlipidemia (%) | 18 | 30 | 0.34 | 17 | 30 | 0.32 |
| Prior stroke/TIA (%) | 9 | 22 | 0.24 | 11 | 19 | 0.50 |
| DWI lesion volume baseline, median (IQR) (mL) | 19 (8–45) | 11 (5–26) | 0.26 | 14 (7–45) | 15 (5–30) | 0.89 |
| PWI lesion volume baseline, median (IQR) (mL) | 75 (44–127) | 66 (34–102) | 0.85 | 71 (49–97) | 65 (34–155) | 0.25 |
NIHSS, National Institutes of Health Stroke Scale; IQR, interquartile range.
Comparisons between two groups were performed with Student’s t-test (age), χ2-test (dichotomous variables), and Wilcoxon rank sum test (NIHSS, DWI lesion, PWI lesion).
In the presence of reperfusion, as shown in Figure 1, increasing mismatch ratio was associated with a higher response rate (OR: 1.7, 95% CI: 1.1 to 2.5). In the absence of reperfusion, increasing mismatch ratio was associated with a lower frequency of good clinical response (OR: 0.6, 95% CI: 0.3 to 0.9). Receiver operating characteristic analysis shows that a mismatch ratio of 2.6 gives the highest sensitivity (90%) and specificity (83%) for predicting a favorable clinical response in patients who achieve early reperfusion (odds ratio 31.5, 95% CI 3.7 to 265.4).
Figure 1.
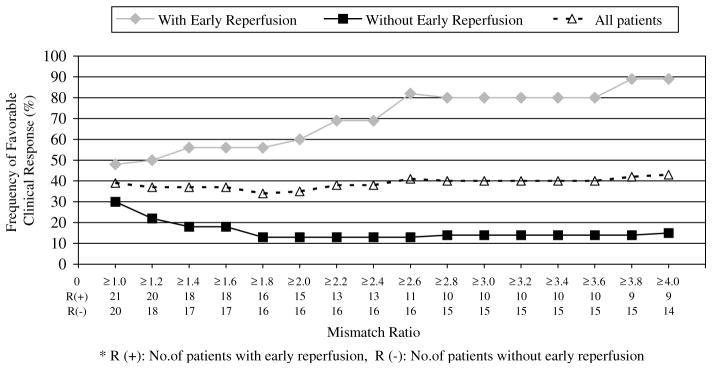
The frequency of favorable clinical response in patients with early reperfusion increases with increasing mismatch ratio. In contrast, larger mismatch ratios are associated with a decrease in favorable outcome for patients who did not have reperfusion. The overall rates of early reperfusion (approximately 50%) and favorable clinical response (about 40%) did not change significantly based on mismatch ratio. Four patients had mismatch ratios of < 1 and are not included in the figure. *R (+): number of patients with early reperfusion, R (−): number of patients without early reperfusion.
When the PWI/DWI ratio cutoff used to define mismatch was increased, the odds ratio for a favorable outcome in mismatch patients with early reperfusion versus mismatch patients without early reperfusion increased (R2 = 0.791; P = 0.001). The number of patients with a mismatch gradually decreased from 84% (38 patients) with a mismatch ratio definition of ≥1.2 to 51% (23 patients) with a ratio of ≥4 (Figure 1).
Discussion
The DEFUSE data show that for acute stroke patients with PWI/DWI mismatch, the favorable clinical response rate associated with early reperfusion is related to the degree of mismatch. There is a lack of consensus regarding the definition of PWI/DWI mismatch. For the DEFUSE study, the prespecified ratio chosen to define PWI/DWI mismatch was 1.2, and a minimum mismatch volume of 10 mL was required. Although a ratio of > 1.2 has been widely used in stroke trials (Butcher et al, 2005; Hacke et al, 2005; Furlan et al, 2006) as well as nonrandomized stroke studies (Schellinger et al, 2000; Parsons et al, 2002), others have defined PWI–DWI mismatch as a ratio > 1.0 (Darby et al, 1999; Neumann-Haefelin et al, 1999). To our knowledge, no previous study has attempted to determine the optimal definition for mismatch.
Our data suggest that a more stringent definition of mismatch, requiring a greater mismatch ratio, has the potential to identify patients who have a particularly robust clinical response to early reperfusion. These findings are consistent with the concept that the mismatch provides an estimate of the ischemic penumbra; patients estimated to have a large volume of penumbral tissue are most likely to benefit from reperfusion and most likely to have poor outcomes if reperfusion is not achieved.
The lower favorable clinical response rate in patients with reperfusion and small mismatch ratio has several possible explanations. It is likely that penumbral tissue is being recruited into the ischemic core during the delay between the baseline magnetic resonance imaging and the occurrence of reperfusion. This delay includes the ‘magnetic resonance imaging-to-needle’ time and the time from initiation of therapy to actual reperfusion. As a result, for patients with small mismatch ratios, little salvageable tissue may remain by the time reperfusion occurs. In addition, mismatch areas determined by PWI and DWI likely include regions of benign oligemia that will survive regardless of reperfusion, as well as critically hypoperfused areas at high risk of infarction (Kidwell et al, 1999). With smaller mismatch ratios, mismatch areas may primarily contain regions of benign oligemia.
A more stringent definition of PWI/DWI mismatch reduces the number of patients designated as having a mismatch. This would decrease the number of patients who are considered to be ‘eligible’ for a trial of a reperfusion therapy if ‘mismatch’ is used as an inclusion criterion for study entry. For example, requiring a mismatch ratio of ≥2.6 would have excluded 25% of the mismatch patients in DEFUSE. In retrospect, a more desirable choice might have been requiring a ratio of ≥1.8 because only about 15% of the patients would have been excluded; yet the odds ratio for achieving a favorable clinical response after early reperfusion would have more than doubled. While requiring a larger mismatch ratio would reduce the number of patients who are eligible for inclusion in a randomized trial of a reperfusion therapy, the anticipated improvement in the clinical response rate among patients with reperfusion (and low rate of favorable response in non-reperfusion patients) would reduce the total sample size estimate for the trial. This approach will require an automated PWI processing program that can rapidly identify the mismatch ratio. Several groups have developed programs that can accomplish this, but these tools have not yet been incorporated into the software packages offered by magnetic imaging imaging manufactures.
This study has some limitations; most importantly, the sample size was small and confirmation of these findings in a large data set is required. In addition, mismatch ratio varies based on the techniques used to analyze PWI data. In DEFUSE, perfusion maps were constructed using Tmax with a 2-sec delay as the lower threshold indicating hypoperfused brain tissue. Other Tmax thresholds or other PWI analysis techniques may yield different estimates of the optimal mismatch ratio. Tmax is the maximum of the tissue residue function characteristic to each voxel and reflects how much the tissue response lags behind the stimulus by an arterial input into the voxel. If the perfusion pressure decreases, the influx into a voxel after an arterial input stimulus is delayed and hence Tmax will increase. Tmax can be viewed as the bolus arrival time corrected for the confounding effects of bolus administration (e.g., injection rate and flow, cardiac output). While Tmax does not measure tissue microperfusion directly, studies (Shih et al, 2003; Thijs et al, 2004) have shown that Tmax is a sensitive surrogate marker that detects perfusion deficits and tissue that is likely destined for infarction if reperfusion does not occur.
It is possible that imbalances in baseline predictors of favorable outcome could confound our results. We believe that this is unlikely, as key baseline predictors of clinical outcomes did not differ between patients with high versus low mismatch ratios. In addition, a multivariate analysis that corrected for baseline imbalances in age, baseline National Institutes of Health Stroke Scale, time to treatment, PWI volume, and glucose was performed as part of the primary analysis of the DEFUSE results. The odds ratio for the association between reperfusion and favorable outcome in mismatch patients increased from 5.4 (95% CI, 1.1 to 25.8, P = 0.039) to 7.7 (95% CI, 1.3 to 44.8, P = 0.022) after correction for baseline imbalances.
A placebo-controlled trial is required to determine if tPA is an effective therapy beyond 3 h. If tPA is more effective than placebo for some patient subgroups beyond 3 h, our data argue that a larger mismatch ratio may identify tPA responders because large mismatch ratios are associated with a very high rate of good outcome if reperfusion occurs and a very low rate if reperfusion does not occur. However, it is uncertain whether tPA is associated with a higher reperfusion rate than placebo in patients with large mismatch ratios and whether the adverse effects of tPA differ based on mismatch ratio.
In conclusion, use of larger PWI/DWI mismatch ratios than have typically been chosen to define mismatch may provide greater power for detecting beneficial clinical effects of reperfusion in future clinical trials.
Acknowledgments
This article reports the results of a subanalysis of the DEFUSE study. The funding for the DEFUSE study was provided by NIH grants RO1 NS3 9325 and NS0 44848 to principal investigator, Gregory W Albers. The tissue plasminogen activator used in the study was provided by Genentech at no charge. Some of the authors have consulting relationships with Genentech or have received honoraria for lectures sponsored by Genentech, but none have any financial relationship with Genentech in excess of $10,000 per year. Vincent Thijs is supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen and the Flanders Institute of Biotechnology.
References
- Albers GW. Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute MRI for patient selection. Stroke. 1999;30:2230–7. doi: 10.1161/01.str.30.10.2230. [DOI] [PubMed] [Google Scholar]
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, Tress B, Donnan G, Davis S EPITHET Investigators. Refining the perfusion–diffusion mismatch hypothesis. Stroke. 2005;36:1153–9. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, Li T, Tress BM, Davis SM. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–52. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- Furlan AJ, Eyding D, Albers GW, Al-Rami Y, Lees KR, Rowley HA, Sachara C, Soehngen M, Warach S, Hacke W DEDAS Investigators. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–31. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fisher M, Furlan A, Kaste M, Lees KR, Soehngen M, Warach S DIAS Study Group. The desmoteplase in acute ischemic stroke trial (DIAS): a phase 2 MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- Jaccard J. Interaction effects in logistic regression: Sage University papers on quantitative applications in the social sciences. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 1999;34:2729–35. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Modder U, Freund HJ. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–7. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA, Davis SM. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Jansen O, Fiebach JB, Heiland S, Steiner T, Schwab S, Pohlers O, Ryssel H, Sartor K, Hacke W. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke. 2000;31:1318–28. doi: 10.1161/01.str.31.6.1318. [DOI] [PubMed] [Google Scholar]
- Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Villablanca JP, Vespa PM, Kidwell CS. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34:1425–30. doi: 10.1161/01.STR.0000072998.70087.E9. [DOI] [PubMed] [Google Scholar]
- Thijs VN, Somford DM, Bammer R, Robberecht W, Moseley ME, Albers GW. Influence of arterial input function on hypoperfusion volumes measured with perfusion-weighted imaging. Stroke. 2004;35:94–8. doi: 10.1161/01.STR.0000106136.15163.73. [DOI] [PubMed] [Google Scholar]


