Abstract
Background and Purpose
White matter injury caused by cerebral hypoperfusion may contribute to the pathophysiology of vascular dementia and stroke, but the underlying mechanisms remain to be fully defined. Here, we test the hypothesis that oxidative stress interferes with endogenous white matter repair by disrupting renewal processes mediated by oligodendrocyte precursor cells (OPCs).
Methods
In vitro, primary rat OPCs were exposed to sublethal CoCl2 for 7 days to induce prolonged chemical hypoxic stress. Then, OPC proliferation/differentiation was assessed. In vivo, prolonged cerebral hypoperfusion was induced by bilateral common carotid artery stenosis in mice. Then, reactive oxygen species production, myelin density, oligodendrocyte versus OPC counts, and cognitive function were evaluated. To block oxidative stress, OPCs and mice were treated with the radical scavenger edaravone.
Results
Prolonged chemical hypoxic stress suppressed OPC differentiation in vitro. Radical scavenging with edaravone ameliorated these effects. After 28 days of cerebral hypoperfusion in vivo, reactive oxygen species levels were increased in damaged white matter, along with the suppression of OPC-to-oligodendrocyte differentiation and loss of myelin staining. Concomitantly, mice showed functional deficits in working memory. Radical scavenging with edaravone rescued OPC differentiation, ameliorated myelin loss, and restored working memory function.
Conclusions
Our proof-of-concept study demonstrates that after prolonged cerebral hypoperfusion, oxidative stress interferes with white matter repair by disrupting OPC renewal mechanisms. Radical scavengers may provide a potential therapeutic approach for white matter injury in vascular dementia and stroke.
Keywords: mice, oligodendrocyte, reactive oxygen species, white matter diseases
Approximately 50% of the human brain is composed of white matter,1 and white matter damage is a critical component of stroke and vascular dementia. Why is white matter so vulnerable? Traditional models suggest that intrinsic anti-oxidant properties in white matter may be relatively low.2,3 Furthermore, lipid-rich myelin may provide a source and substrate for the generation of reactive oxygen species.4,5
White matter is not static, and homeostasis can be sustained by endogenous repair and renewal processes. Even after adolescence, myelin-forming mature oligodendrocytes in the white matter can be generated from oligodendrocyte precursor cells (OPCs).6,7 When white matter is damaged in stroke or other neurodegenerative diseases, residual OPCs rapidly proliferate, migrate to fill demyelinated areas,8,9 and differentiate into mature oligodendrocytes to restore myelin integrity.9,10 An emerging concept in neuroscience emphasizes that the mechanisms of neuronal disorders involve a balance between initial injury and endogenous repair.11
Is it possible that pathophysiology in white matter may also involve a disruption of endogenous OPC recovery mechanisms? A radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is clinically used in Japan for acute ischemic stroke.12 In vivo studies with rodent stroke models suggest that the neuroprotective effects of edaravone may take place in penumbral-like regions where oxygen radicals are typically generated.13,14 In addition, edaravone is demonstrated to show protective effects in in vitro cell culture systems.15–19 In this study, therefore, we use this radical scavenger to test the hypothesis that oxidative stress interferes with endogenous white matter repair by disrupting compensatory OPC-to-oligodendrocyte differentiation.
Methods
Cell Culture
Primary cortical OPCs were prepared and maintained according to our previous work.20 To differentiate OPCs into myelin basic protein–positive oligodendrocytes, the culture medium was switched to DMEM containing 1% penicillin/streptomycin, 10 ng/mL ciliary neurotrophic factor, 15 nmol/L triiodo-l-thyronine (T3), and 2% B27 supplement (DMEM medium). OPC cultures were treated with sublethal (1 µmol/L for 7 days) CoCl2 to induce prolonged chemical hypoxic conditions as described before.21,22 Hypoxic conditions were confirmed by an increase in hypoxia-inducible factor-1α (HIF-1α) expression. Edaravone (Mitsubishi Tanabe Pharma) was dissolved in dimethysulfoxide. The final concentration of dimethysulfoxide in the culture medium was <0.1%, which had no effects on OPC survival and function. In vitro experiments were performed in duplicate, repeated 3× to 6× independently (see online-only Data Supplement for detailed methods of in vitro cell culture experiments).
Cerebral Prolonged Hypoperfusion Model
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For inducing cerebral chronic hypoperfusion stress, a microcoil (0.18-mm diameter; Sawane Spring Co) was applied to bilateral common carotid arteries. Male C57/black 6 (C57BL/6) mice (sham: 22 mice, vehicle group: 37 mice, edaravone group: 38 mice, 10 weeks old, Charles River Institute) were anesthetized with 4.0% isoflurane and then maintained on 1.5% isoflurane in 70% N2O and 30% O2, maintaining the rectal temperature between 36.5°C and 37.5°C. The operation time was ≈20 minutes per mouse, and the interval between the 2 microcoils was 5 minutes. The cerebral blood flow was measured before/after the microcoil placement as described previously.21 The radical scavenger edaravone (3 mg/kg IP) or vehicle was treated twice per week starting at day 0 until the mice were euthanized. Animals were euthanized on days 0, 7, 14, and 28. All in vivo experiments and measurements were performed in a blinded and randomized manner. Animal numbers and experimental time points for each experiment are described in figure legends (see online-only Data Supplement for detailed methods of end point assessments).
Statistical Analysis
Power estimates were calculated based on α=0.05 and β=0.8 to obtain group sizes appropriate for detecting effect sizes in the range of 30% to 50% for in vivo models and 40% to 50% for cell cultures models. Statistical significance was evaluated using the unpaired t test to compare differences between the 2 groups and a 1-way ANOVA followed by the Tukey honestly significant difference test for multiple comparisons. Data are expressed as mean±SD. A P<0.05 was considered statistically significant.
Results
Oxidative Stress Disrupts OPC Differentiation In Vitro
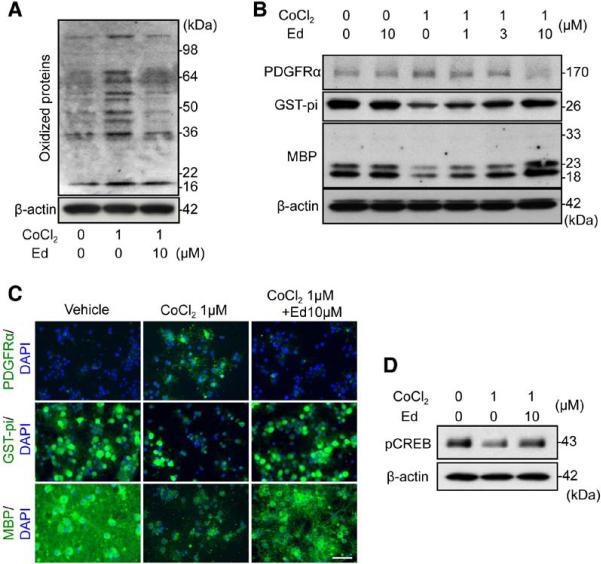
When OPCs were subjected to sublethal chemical hypoxia (1 μmol/L CoCl2 for 7 days) to induce prolonged hypoxic conditions,21 their in vitro differentiation into mature oligodendrocytes was significantly inhibited (Figure IA in the online-only Data Supplement). Dichlorofluorescein and oxyblot assays confirmed that CoCl2 treatment induced oxidative stress (Figure 1A; Figures IB and II in the online-only Data Supplement). Cotreatment with the radical scavenger edaravone rescued OPC differentiation (Figure 1B and 1C; Figure II in the online-only Data Supplement). cAMP response element-binding (CREB) signaling is known to mediate OPC differentiation.23 As expected, CoCl2 treatment decreased the phosphorylation level of CREB in OPC cultures, and edaravone successfully restored phospho-CREB levels (Figure 1D; Figure II in the online-only Data Supplement).
Figure 1.
Oxidative stress disturbed oligodendrocyte precursor cell (OPC) differentiation in vitro. A, Representative oxyblot image of 7-day CoCl2-treated OPCs with or without edaravone. B, Representative Western blot images of 7-day CoCl2-treated OPCs with or without edaravone. PDGF-R-α, OPC marker; GST-pi and MBP, oligodendrocyte marker. C, Representative images of PDGF-R-α, GST-pi, and MBP staining in cultured rat OPCs. PDGFRα, GST-pi, MBP: green, DAPI: blue. Scale bar, 25 μm. D, pCREB Western blot image of 7-day CoCl2-treated OPCs with or without edaravone (see Figure II in the online-only Data Supplement for quantitative results of Figure 1A, 1B, and 1D). DAPI indicates 4',6-diamidino-2-phenylindole; Ed, edaravone treatment; GST, glutathione S-transferase; MBP, myelin basic protein; pCREB, phospho-cAMP response element-binding; and PDGF-R-α, platelet-derived growth factor-receptor-α.
Prolonged Cerebral Hypoperfusion Induces Oxidative Stress and White Matter Dysfunction In Vivo
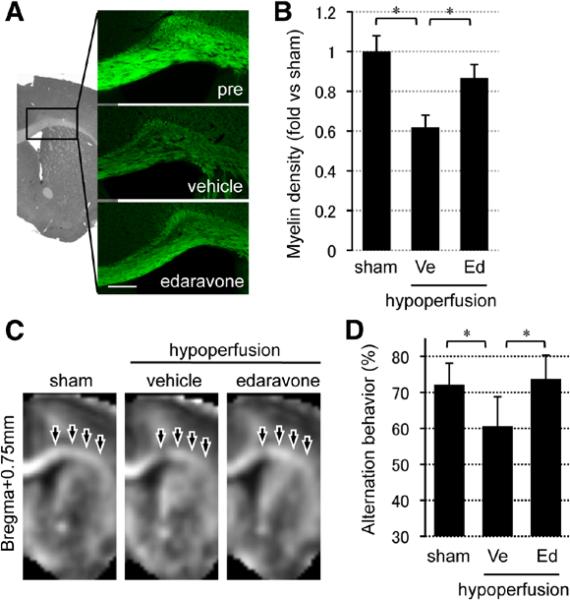
When mice were subjected to microcoil placement, their cerebral blood flows decreased (vehicle group: 55.0±12.6%, edaravone group: 55.2±11.5%) as reported previously.21,24 After 28 days of cerebral hypoperfusion, corpus callosum regions showed a reduction in myelin staining (Figure 2A and 2B), reduced the white matter index on MRI (Figure 2C), and decreased myelin basic protein expression in Western blots (Figure III in the online-only Data Supplement). Consistent with white matter damage, working memory function was also perturbed in hypoperfused mice, detected as a decrease in alternating rates in the Y-maze test without any changes in locomotor activity (Figure 2D; Figure IV in the online-only Data Supplement). Oxidative stress may be involved because levels of oxidized proteins were elevated in affected white matter (Figure V in the online-only Data Supplement), and treatment with the radical scavenger edaravone (3 mg/kg IP, twice per week for 4 weeks) significantly decreased oxidized protein levels (marker of oxidative stress), increased myelin staining, and rescued Y-maze performance (Figure 2A–2D; Figures III–V in the online-only Data Supplement).
Figure 2.
Prolonged cerebral hypoperfusion stress induced oxidative stress and white matter dysfunction. A, Representative fluoromyelin staining images of the white matter region (corpus callosum) at day 28. Scale bar, 100 μm. B, Relative intensity of fluoromyelin signals in corpus callosum. C, MR images (bregma +0.75 mm) of sham animals and vehicle- or edaravone-treated white matter injury groups at day 28. D, Alternation behavior (index of working/spatial memory) at day 28 in Y-maze test. Values are mean±SD. n=5 for A–C and n=10 for D. Ed indicates edaravone treatment group; and Ve, vehicle. *P<0.05.
Oxidative Stress Induces Cell Death in Oligodendrocytes and Oligodendrocyte Precursors In Vivo
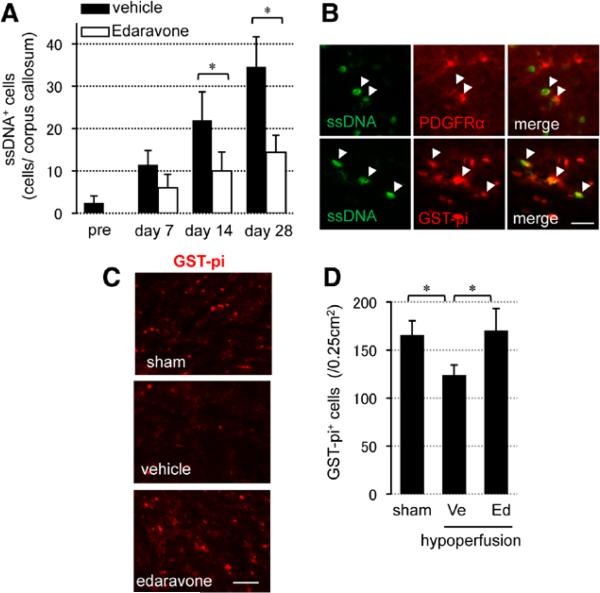
After cerebral hypoperfusion, the number of single-stranded DNA and terminal deoxynucleotidyl transferase dUTP nick end labeling–positive cells progressively increased with time after hypoxia in areas of white matter injury (Figure 3A; Figure VIA in the online-only Data Supplement). Both cell death markers colocalized with oligodendrocytes (labeled with glutathione S-transferase [GST]-pi) and OPCs (labeled with platelet-derived growth factor-receptor-α [PDGF-R-α]; Figure 3B; Figure VIB in the online-only Data Supplement). Treatment with the radical scavenger edaravone reduced the total number of dead cells (Figure 3A; Figure VIA in the online-only Data Supplement). In addition, by 28 days, the number of GST-pi–positive oligodendrocytes was restored by edaravone treatment (Figure 3C and 3D).
Figure 3.
Oxidative stress induced cell death in oligodendrocytes and oligodendrocyte precursors in prolonged cerebral hypoperfusion mice. A, Numbers of single-stranded DNA (ssDNA)–positive (ssDNA+) cells in corpus callosum. B, ssDNA staining with a oligodendrocyte precursor cell marker (PDGF-R-α) or a mature oligodendrocyte marker (GST-pi) in the vehicle-treated group at day 28. Scale bar, 10 μm. C, Representative images of GST-pi staining at day 28. Scale bar, 25 μm. D, Numbers of GST-pi–positive (GST-pi+) cells in the lateral side of corpus callosum at day 28. Values are mean±SD of n=5. Ed indicates edaravone treatment group; GST, glutathione S-transferase; PDGF-R-α, platelet-derived growth factor-receptor-α; and Ve, vehicle. *P<0.05.
Blockade of Oxidative Stress Restores the Compensatory Response in Oligodendrocyte Renewal
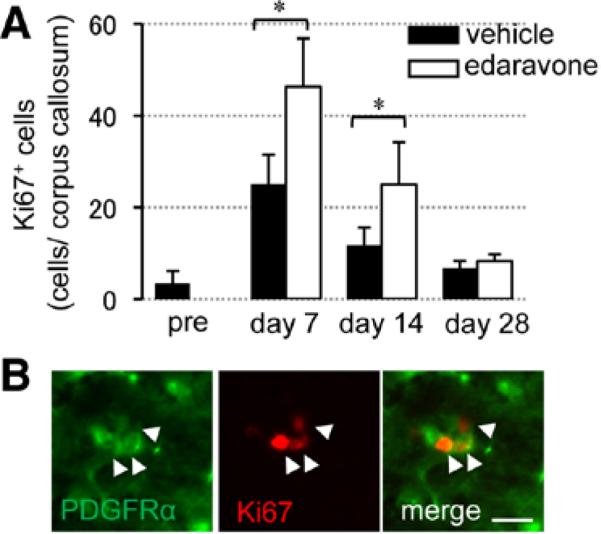
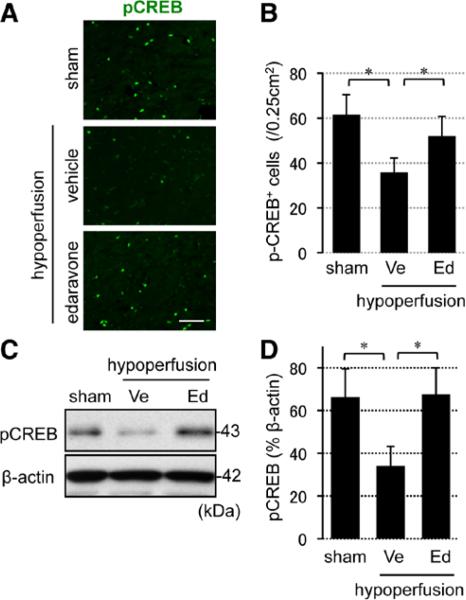
When hypoperfused mice were treated with edaravone, the number of Ki67-positive proliferating cells was increased at days 7 and 14 (Figure 4A), and most Ki67 signals colocalized with PDGF-R-α–positive OPCs (Figure 4B). To check whether OPCs can successfully differentiate into mature oligodendrocytes at later time points, mice were injected with 5-bromo-2'-deoxyuridine (BrdU) at day 14, and the brain sections were stained with anti-BrdU and anti–GST-pi antibodies at day 28 (Figure 5A). Edaravone treatment increased the number of BrdU-incorporated cells (Figure 5B), and importantly, the edaravone-treated group showed larger ratio of BrdU/GST-pi double staining (Figure 5C), suggesting that reactive oxygen species suppression promoted OPC-to-oligodendrocyte differentiation. Finally, prolonged hypoperfusion stress blunted CREB signaling in affected white matter, and edaravone treatment recovered the level of phospho-CREB (pCREB) in the white matter (Figure 6A–6C), consistent with the involvement of CREB in OPC-to-oligodendrocyte maturation.
Figure 4.
Reactive oxygen species suppression promoted compensative oligodendrocyte precursor cell proliferation. A, Numbers of Ki67-positive (Ki67+) cells in the white matter. B, Double staining of Ki67 with PDGF-R-α in the edaravone-treated group at day 7. Scale bar, 10 μm. Values are mean±SD of n=5. Ed indicates edaravone treatment group; PDGF-R-α, platelet-derived growth factor-receptor-α; and Ve, vehicle. *P<0.05.
Figure 5.
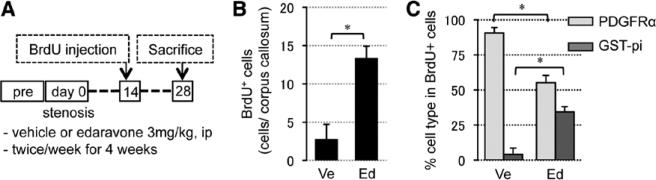
Reactive oxygen species suppression promoted in vivo oligodendrocyte precursor cell differentiation under hypoxic conditions. A, Experimental schedule of cell differentiation assay in vivo. B, Numbers of BrdU-positive (BrdU+) cells in corpus callosum at day 28 (BrdU was incorporated in proliferating cells at day 14). C, Ratio of BrdU/PDGF-R-α or BrdU/GST-pi double-labeled cells in total BrdU+ cells at day 28. Values are mean±SD of n=5. BrdU indicates 5-bromo-2'-deoxyuridine; Ed, edaravone treatment group; GST, glutathione S-transferase; PDGF-R-α, platelet-derived growth factor-receptor-α; and Ve, vehicle. *P<0.05.
Figure 6.
Reactive oxygen species suppression promoted CREB activation in white matter brains. A, Representative images of pCREB staining in the white matter at day 28. Scale bar, 25 μm. B, Numbers of pCREB-positive (pCREB+) cells in the lateral side of corpus callosum at day 28. C and D, pCREB Western blot images in the white matter at day 28. β-actin is an internal control. Values are mean±SD of n=5. CREB indicates cAMP response element-binding; Ed, edaravone treatment group; pCREB, phospho-CREB; and Ve, vehicle. *P<0.05.
Discussion
It has now been well accepted that adult brains retain some regenerative capacities under certain conditions. In gray matter, adult neurogenesis in the neurovascular niche may play an essential role in maintaining and repairing neuronal function.25,26 Similarly, in white matter, oligodendrogenesis can mediate plasticity under normal conditions and repair after injury.27 Past studies have demonstrated that oligodendrogenesis after white matter injury may mirror well-defined processes that normally occur in the developmental period.6,28 OPCs may respond to short and sublethal ischemic injury that causes myelin damage,29 especially in the margins of infarcts after focal ischemia, as well as within ischemic white matter lesions after chronic cerebral hypoperfusion.8 Hence, OPCs may potentially mediate a compensatory response that underlies white matter repair after damage and disease.10,29 In this study, we used a combination of in vitro culture and in vivo mouse models to demonstrate that after cerebral hypoperfusion, oxidative stress disrupts OPC-to-oligodendrocyte differentiation and interferes with endogenous repair mechanisms in damaged white matter.
For normal physiology, OPCs play vital roles to maintain white matter homeostasis, and this phenomenon may be essential for sustaining connectivity in the brain.30 Of course, these cell types are susceptible to cell death after oxidative stress.31 But in addition to outright cell death, renewal and repair mechanisms may also be affected. Our data suggest that oxidative stress retards the compensative responses in oligodendrocyte regeneration under white matter injury, in part by blocking the requisite CREB signaling that promotes recovery. The CREB signaling may be one of the most important factors for oligodendrocyte regeneration. The CREB pathway is essential for cell survival in a broad way. The CREB/cAMP response element transcriptional pathway regulates the expression of both Bcl-2 and brain-derived neurotrophic factor (BDNF),32,33 and cAMP response element–mediated gene expression enhances ischemic tolerance.34 Similarly, neurons with ischemic preconditioning stimulus show more resistance to the following devastating injuries in a CREB-dependent manner.35 Also, in the white matter, CREB signaling protects and regenerates mature oligodendrocytes against chronic ischemic injury via activating Bcl-2 and cyclooxygenase-2 (COX-2) transcription.23,36,37 Our findings are generally consistent with this beneficial pathway.
There are 2 important caveats in this study. First, in vivo functional outcomes were only assessed with a single test, that is, the Y-maze test. For preclinical development of stroke therapeutics, multiple behavioral tests are typically required. However, in the present study, our purpose was not to develop edaravone as a stroke drug. Instead, edaravone was only used as a chemical probe to scavenge radicals to test our pathophysiological hypothesis that oxidative stress mediates the observed responses in hypoperfused white matter. Furthermore, Maki et al38 reported that both the radial 8-arm maze test and the Y-maze test would show similar results in this model. Nevertheless, this is an important caveat, and future studies should use other cognitive tests to examine the effects of antioxidants on white matter remodeling after hypoperfusion injury. Second, although OPCs are quite sensitive to oxidative stress31,39 and antioxidants such as minocycline can protect OPCs,40 it must be recognized that the balance between injury and repair depends on dose, that is, the levels of radicals involved. Although excessive free radicals generated in neurodegeneration are cytotoxic, low levels of radicals may be essential for brain homeostasis and remodeling.41 For example, N-methyl-D-aspartate (NMDA) activation by extracellular glutamate promotes oligodendrocyte differentiation and myelination from an adult multipotent stem cell population via oxygen radical generation.42 In addition, oxygen radicals may also participate in OPC migration, which is a critical step for oligodendrogenesis after white matter injury.43 Hence, basal levels of oxygen free radicals may act as a second messenger for sustaining oligodendrocyte function. We demonstrate that radical suppression by the scavenger edaravone promotes OPC-to-oligodendrocyte regeneration, but ultimately, long-term effects of antioxidant drugs on white matter remodeling after injury should be carefully examined to determine the balance between beneficial and potentially detrimental effects.
In conclusion, this proof-of-concept study demonstrates that during prolonged cerebral hypoperfusion, oxidative stress disrupts compensatory mechanisms of OPC-to-oligodendrocyte renewal, and this process may interfere with endogenous repair and worsen white matter dysfunction. Insofar as hypoperfusion may damage white matter in stroke and vascular dementia, antioxidant radical scavengers may provide a broad therapeutic approach for cerebrovascular disorders.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported, in part, by the Deane Foundation, the Executive Committee on Research Fund for Medical Discovery of Massachusetts General Hospital, American Heart Association, National Institutes of Health, Research Abroad from the Uehara Memorial Foundation, National Research Foundation of Korea through the World Class University Program (R31-2008-000-10103-0), and the Global Research Laboratory Program (2011-0021874).
Footnotes
Disclosures
None.
References
- 1.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravindranath V, Shivakumar BR, Anandatheerthavarada HK. Low glutathione levels in brain regions of aged rats. Neurosci Lett. 1989;101:187–190. doi: 10.1016/0304-3940(89)90528-4. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T, Senda M. Evaluation of glutathione localization in brain using 99mTc meso-HMPAO. J Nucl Med. 1999;40:1056–1060. [PubMed] [Google Scholar]
- 4.Konat GW, Wiggins RC. Effect of reactive oxygen species on myelin membrane proteins. J Neurochem. 1985;45:1113–1118. doi: 10.1111/j.1471-4159.1985.tb05530.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, et al. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010;30:299–310. doi: 10.1038/jcbfm.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong VW. Prospects of repair in multiple sclerosis. J Neurol Sci. 2009;277(suppl 1):S16–S18. doi: 10.1016/S0022-510X(09)70006-1. [DOI] [PubMed] [Google Scholar]
- 10.Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Lo EH. Degeneration and repair in central nervous system disease. Nat Med. 2010;16:1205–1209. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edaravone Acute Infarction Study Group Effect of a novel free radical scavenger, edaravone (mci-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 13.Amemiya S, Kamiya T, Nito C, Inaba T, Kato K, Ueda M, et al. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005;516:125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito KI. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1997;281:921–927. [PubMed] [Google Scholar]
- 15.Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain Res. 2010;1307:22–27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan WJ, Yasuhara T, Shingo T, Muraoka K, Agari T, Kameda M, et al. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Wang S, Ding JH, Hu G. Edaravone protects against MPP+ -induced cytotoxicity in rat primary cultured astrocytes via inhibition of mitochondrial apoptotic pathway. J Neurochem. 2008;106:2345–2352. doi: 10.1111/j.1471-4159.2008.05573.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki T, Kitao T, Nakagawa K, Fujisaki H, Takegawa Y, Koda K, et al. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–1333. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- 20.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, et al. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke. 2013;44:2573–2578. doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegner M. Transcriptional control in myelinating glia: flavors and spices. Glia. 2000;31:1–14. doi: 10.1002/(sici)1098-1136(200007)31:1<1::aid-glia10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 25.Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(suppl 4):95–104. [PubMed] [Google Scholar]
- 26.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang RL, Chopp M, Roberts C, Jia L, Wei M, Lu M, et al. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab. 2011;31:614–625. doi: 10.1038/jcbfm.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. doi: 10.1002/(SICI)1097-4547(19971015)50:2<215::AID-JNR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 30.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126(pt 3):515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 31.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 32.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 33.Walton M, Sirimanne E, Williams C, Gluckman P, Dragunow M. The role of the cyclic AMP-responsive element binding protein (CREB) in hypoxicischemic brain damage and repair. Brain Res Mol Brain Res. 1996;43:21–29. doi: 10.1016/s0169-328x(96)00144-1. [DOI] [PubMed] [Google Scholar]
- 34.Hara T, Hamada J, Yano S, Morioka M, Kai Y, Ushio Y. CREB is required for acquisition of ischemic tolerance in gerbil hippocampal CA1 region. J Neurochem. 2003;86:805–814. doi: 10.1046/j.1471-4159.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- 35.Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z, et al. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21:9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006;37:1539–1545. doi: 10.1161/01.STR.0000221783.08037.a9. [DOI] [PubMed] [Google Scholar]
- 37.Saini HS, Gorse KM, Boxer LM, Sato-Bigbee C. Neurotrophin-3 and a CREB-mediated signaling pathway regulate Bcl-2 expression in oligodendrocyte progenitor cells. J Neurochem. 2004;89:951–961. doi: 10.1111/j.1471-4159.2004.02365.x. [DOI] [PubMed] [Google Scholar]
- 38.Maki T, Ihara M, Fujita Y, Nambu T, Miyashita K, Yamada M, et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42:1122–1128. doi: 10.1161/STROKEAHA.110.603399. [DOI] [PubMed] [Google Scholar]
- 39.French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–3087. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz T, Endesfelder S, Chew LJ, Zaak I, Bührer C. Minocycline protects oligodendroglial precursor cells against injury caused by oxygen-glucose deprivation. J Neurosci Res. 2012;90:933–944. doi: 10.1002/jnr.22824. [DOI] [PubMed] [Google Scholar]
- 41.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 42.Cavaliere F, Urra O, Alberdi E, Matute C. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 2012;3:e268. doi: 10.1038/cddis.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.