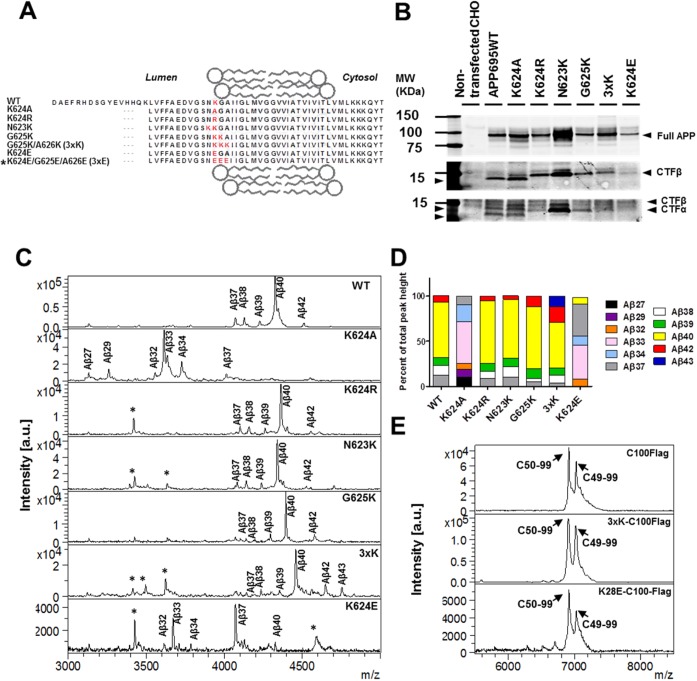
Figure 1.
Effects of point mutations in APP and CTF on the production of Aβ and AICD, respectively. (A) The WT APP and mutant APP sequences examined in this study are highlighted. The 3xE construct has been tested only in the in vitro assay (marked with an asterisk). (Attempts to produce a stable cell line of the K624E/G625E/A626E construct were not successful.) (B) The WT APP and mutant APP were stably expressed in CHO cells and detected via Western blotting using a 6E10 monoclonal antibody.74 (C) Aβ spectra obtained by MALDI-TOF analysis of conditioned media from CHO cells overexpressing WT and mutant forms of APP. Aβ isoforms are identified on the profiles with nonspecific peaks denoted with an asterisk. (D) Stacked bar graphs indicating the percent of each Aβ isoform derived from WT and mutant APP. These analyses were based on two to four experiments with two to five replicates in each experiment (the maximal SEM = ±2.5). (E) AICD spectra of WT CTFβ tagged with a Flag peptide (C100Flag), 3xK CTFβ tagged with Flag (3xK-C100Flag), and K28E CTFβ tagged with Flag (K28E-C100Flag) after immunoprecipitation and MALDI-TOF analysis. The positions of the two major products produced by γ-secretase cleavage at the ε-site, AICD50–99 and AICD49–99, are indicated. GSI treatment served as a control to select specific AICD peaks (data not shown).