Abstract
Birt-Hogg-Dubé syndrome (BHD) is a rare autosomal dominant inherited disease caused by a germline mutation in the folliculin gene mapped in the region of chromosome 17p11.2. BHD predisposes the patient to cutaneous fibrofolliculomas (FFs), pulmonary cysts (PCs), and renal cell carcinoma (RC). Here, we present two cases of BHD in Japanese patients. One patient was a 37-year-old female, and the other a 35-year-old male. Each of the patients was affected by all three symptoms of BHD. Both patients had unremarkable FFs, asymptomatic PCs, and asymptomatic RC. The presence of RC was revealed by abdominal ultrasonic examination. We also summarized the data from 62 Asian cases of BHD from the available literature and found that their FFs were unremarkable. In addition, the proportion of BHD patients with FF is smaller for Asian patients than it is for Caucasian patients. We also found that it is rare for BHD patients in Asia to show all three symptoms of BHD. Careful inspection of the skin as well as skin biopsies are important for the early detection of BHD cases in Asia.
Key words: Birt-Hogg-Dubé syndrome, Fibrofolliculoma, Pulmonary cyst, Renal cell carcinoma
Case Presentations
Case 1
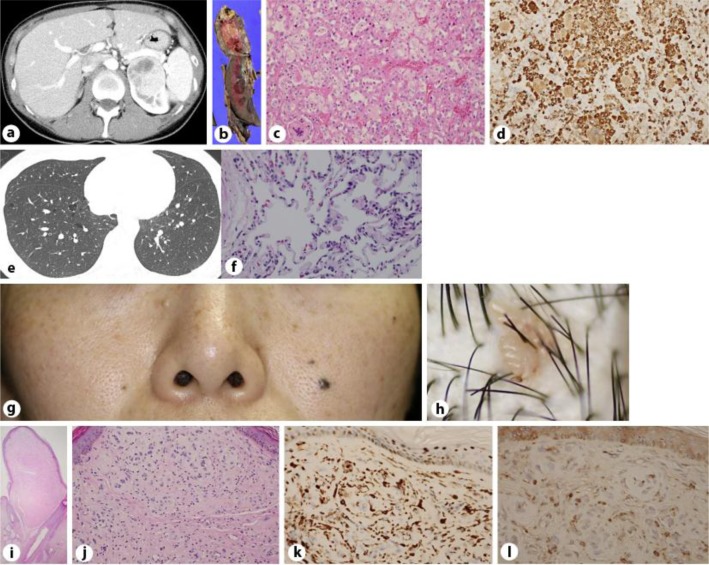
The first patient was a 37-year-old female who presented with an asymptomatic left renal tumor that was revealed by ultrasound and computed tomography (CT) examinations (fig. 1a). The CT examination also revealed the presence of asymptomatic bilateral multiple PCs (fig. 1e). Since the age of 35, a few firm papules had developed on her face and head (fig. 1g, h). The patient had no family history of pulmonary, renal, or cutaneous disease. A laparoscopic left nephrectomy was performed. On gross examination, the resected kidney was a yellowish, solid tumor with partial hemorrhage (fig. 1b). The tumor was identified as a clear cell renal carcinoma (fig. 1c) positive for vimentin stain (fig. 1d) and negative for CK-7. A skin biopsy of the papule on her head revealed it was a fibrofolliculoma (FF; fig. 1i, j) positive for factor 13a (fig. 1k) and c-kit (fig. 1l), and negative for CD34, α-smooth muscle actin (α-SMA), S100, and CD68. Because the findings for this patient met 1 major criterion and 2 minor criteria [1], she was diagnosed with Birt-Hogg-Dubé syndrome (BHD).
Fig. 1.
The abdominal CT revealed a low-density area on the left kidney of a 37-year-old female with BHD (a), which was histopathologically diagnosed as clear cell carcinoma. On gross examination, the resected kidney contained a partially hemorrhagic, yellowish, solid tumor (b). The tumor was composed of cells with clear to acidophilic cytoplasm (hematoxylin-eosin stain; c), which were positive for vimentin stain (d; 200× magnification). The thoracic CT revealed bilateral, multiple PCs in the basilar and mediastinal regions (e). Microscopic findings for the lung revealed that the septum of the cyst wall contained capillaries and was lined by pneumocytes on both surfaces (f; 200× magnification). Quiet, skin-colored papules were present on the patient's face (g). There were few pinkish-colored, verrucous papules on the scalp (h). Histopathological images (hematoxylin-eosin) of the FF. The stoma was rich in fibroblasts and was oriented in parallel bundles of root sheath-like fibers and neighboring enlarged hair follicles [20× magnification (i) and 200× magnification (j)] positive for factor 13a (k) and c-kit (l; 400× magnification).
Case 2
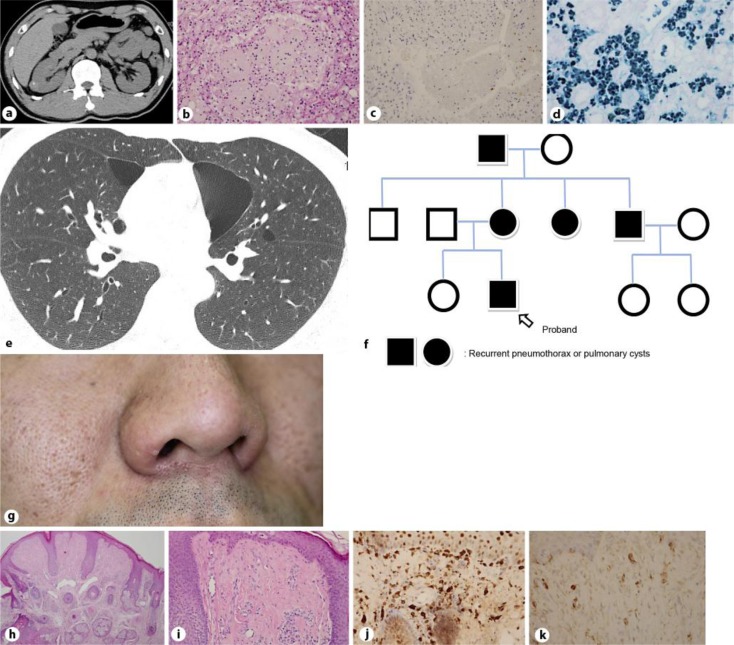
The second patient was a 35-year-old male who presented with an asymptomatic right renal tumor. The presence of the tumor was revealed by ultrasound and CT examinations (fig. 2a). A laparoscopic right partial nephrectomy was performed, and the tumor was diagnosed as a chromophobe renal cell carcinoma (RC; fig. 2b). The carcinoma was negative for CD10, vimentin, and c-kit, and was partially positive for CK-7 (fig. 2c) and colloidal iron (fig. 2d). When the patient was 40 years of age, a CT examination revealed the presence of asymptomatic bilateral multiple PCs (fig. 2e). His mother, aunt, uncle, and grandfather all had histories of recurrent pneumothorax (fig. 2f). A few firm papules were also present on the patient's face (fig. 2g). The results of the skin biopsies revealed that the papules were FFs (fig. 2h, i) positive for factor 13a (fig. 2j) and c-kit (fig. 2k), and negative for CD34, α-SMA, S100, and CD68. Because the findings for this patient met 1 major criterion and 2 minor criteria [1], he was diagnosed with BHD.
Fig. 2.
The abdominal CT revealed a low-density area on the right kidney of a 35-year-old male with BHD (a). The tumor was composed of cells with eosinophilic cytoplasm (hematoxylin-eosin; b), was partially positive for CK7 (c; 200× magnification) and colloidal iron (d; 400× magnification), and histopathologically diagnosed as chromophobe cell carcinoma. A thoracic CT revealed the presence of bilateral, basally located, multiple PCs (e). Pedigrees of the families affected with recurrent pneumothorax (f). Skin-colored papules were present on the nose (g). Histopathological images (hematoxylin-eosin) of the FFs [40× magnification (h) and 200× magnification (i)] positive for factor 13a (j) and c-kit (k; 400× magnification).
Discussion
BHD is a rare autosomal dominant inherited disease that is caused by a germline mutation in the folliculin gene mapped in the region of chromosome 17p11.2. This gene is involved in the signaling of the mammalian target of rapamycin [1]. In cases from Asia, the mutations are located on exons 5, 6, 9, 11, 12, 13, and 14, and on intron 5 (table 1) [2, 3]. The disease is characterized by the presence of cutaneous FFs, multiple PCs, and RC.
Table 1.
| Case | Year | First author | Age, years | Gender Skin histology | PC | Family history | RC | Mutation | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1990 | Ishiko [16] | 46 | M | ff, td, ac | – | – | – | UK |
| 2 | 2000 | Aoki [6] | 65 | M | ff, td | – | +s | – | UK |
| 3 | 2001 | Takahashi [17] | 53 | F | ff | – | +ff | + | UK |
| 4 | 2003 | Ishihara [18] | 52 | F | ff, td, ac | – | +s | UK | |
| 5 | 2004 | Kawasaki [7] | 26 | M | ff | – | +s | – | E11 |
| 6 | UK | M | NT | – | fath. of 5 | – | E11 | ||
| 7 | 2005 | Nagashima [19] | 51 | F | NF | – | – | + | NF |
| 8 | 2007 | Murakami T [14] | 55 | UK | NF | – | – | + | E11 |
| 9 | 2007 | Gunji [11] | 30 | F | NF | + | +p | – | E13 |
| 10 | 38 | F | NF | + | +p | – | E11 | ||
| 11 | 40 | F | NF | + | +p, r | – | E6 | ||
| 12 | 37 | F | NF | + | +p, r | – | I5 | ||
| 13 | 38 | F | NF | + | +p, r | – | E12 | ||
| 14 | 2007 | Saito [20] | 35 (Ch) | M | pff | – | – | – | UK |
| 15 | 2008 | Sadamasa [21] | 35 | M | ff, td | + | +s | – | UK |
| 16 | 2008 | Misago [22] | 67 | F | ff, td | – | +s | – | E13 |
| 17 | 38 | F | not ff, td | + | dau. of 16 | – | E13 | ||
| 18 | 35 | F | not ff, td | + | dau. of 16 | – | E13 | ||
| 19 | 2008 | Kim [23] | 31 (Ko) | F | pff, td | + | +p | – | E14 |
| 20 | 2009 | Imada [8] | 68 | M | ff | – | +s, r | + | E12 |
| 21 | 2009 | Miyasaka [24] | 34 | F | trunk, NT | + | +p | – | E12 |
| 22 | 2009 | Ando [25] | 58 | F | ff, ac? | + | +p | – | E12 |
| 23 | 2009 | Ishii [26] | 29 | F | NF | + | +p | – | UK |
| 24 | 40 | M | NF | + | bro. of 23 | – | E6 | ||
| 25 | 72 | M | NF | + | fath. of 23 | – | UK | ||
| 26 | 2009 | Koga [27] | 41 | F | NF | + | +ff | – | E12 |
| 27 | UK | M | ff | – | fath. of 26 | – | E12 | ||
| 28 | 2009 | So [28] | 57 (Ch) | F | ff | + | + p | – | E13? |
| 29 | 31 | F | NF | + | dau. of 28 | – | E13? | ||
| 30 | 28 | F | NF | + | dau. of 28 | – | E13? | ||
| 31 | 2010 | Hayashi [29] | 39 | F | NF | + | – | – | E13 |
| 32 | 2011 | Nakagawa [30] | 30’ | F | NF | + | +p, r | – | E12 |
| 33 | 30’ | M | NF | + | bro. of 32 | – | UK | ||
| 34 | 2011 | Sakairi [31] | 41 | F | NF | + | +ff | – | E12 |
| 35 | 55 | F | NF | + | +p | – | E12 | ||
| 36 | 2011 | Park [9] | 43 (Ko) | M | ff, td | – | +s, p | – | E11 |
| 37 | 2011 | Shin [10] | 46 (Ko) | F | NF | + | dau. of 37 | + | E5 |
| 38 | 69 | F | NF | + | +p, r | + | E5 | ||
| 39 | 2012 | Nagashima [32] | 69 | F | NF | + | – | + | E5 |
| 40 | 2012 | Ishizuka [33] | 45 | F | NT | + | +p | – | E11 |
| 41 | 2012 | Kim [12] | 40 (Ko) | F | not ff | + | +p, r | – | E6 |
| 42 | UK | M | UK | + | fath. of 41 | + | UK | ||
| 43 | UK | F | NF | + | sis. of 41 | – | E6 | ||
| 44 | UK | F | NF | + | sis. of 41 | – | E6 | ||
| 45 | 2012 | Kashiwada [34] | 60 | F | NT? | + | +s, p | – | E11 |
| 46 | 32 | M | NT? | + | son of 39 | – | E11? | ||
| 47 | 2012 | Tobino [35] | 43 | F | NF | + | +p | +* | E12 |
| 48 | UK | M | NF | + | son of 47 | – | UK | ||
| 49 | 2013 | Ema [36] | 53 | F | not ff | + | – | + | E6 |
| 50 | 2013 | Nishii [13] | 33 | F | not ff | + | +s, p | – | I5 |
| 51 | UK | M | extrm., NT | + | fath. of 50 | – | I5? | ||
| 52 | UK | M | UK | + | uncle of 50 | – | UK | ||
| 53 | UK | F | UK | + | G.M. of 50 | – | UK | ||
| 54 | 2013 | Haga [37] | 61 | F | NF | + | +p | – | E11 |
| 55 | 29 | M | NF | + | son of 54 | – | E11 | ||
| 56 | UK | M | NF | + | bro. of 54 | – | UK | ||
| 57 | This report | Murakami Y | 37 | F | ff | + | NF | + | UK |
| 58 | 35 | M | ff | + | +p | + | UK | ||
| 59 | UK | F | UK | + | moth. of 58 | – | UK | ||
| 60 | UK | F | UK | + | aunt of 58 | – | UK | ||
| 61 | UK | M | UK | + | uncle of 58 | – | UK | ||
| 62 | UK | M | UK | + | G.F. of 58 | – | UK | ||
Ch = Chinese; Ko = Korean; UK = unknown; ff = fibrofolliculoma; td = trichodiscoma; ac = acrochordon; NF = no cutaneous change could be found; NT = not tested; extrm. = extremity; s = skin lesion; p = pulmonary lesion; r = renal lesion; dau. = daughter; sis. = sister; bro. = brother; fath. = father; G.M. = grandmother; moth. = mother; G.F. = grandfather; E = exon; I = intron.
# Diagnosed as renal cyst by abdominal sonography without pathological examination.
Diagnosed as angiomyolipoma by CT images without pathological examination.
Toro et al. [4] reported that FFs are present in 90% of the families with BHD. The skin lesions usually appear as multiple, dome-shaped, whitish papules on the face after the age of 20 years. They are hamartomas that originate from the hair follicle, and are positive for CD34, factor 13a, c-kit, and CD68, and negative for α-SMA. The FFs in both of our cases were positive for factor 13a and c-kit, and were negative for α-SMA, CD34, and CD68.
Menko et al. [1] reported that PCs are present in more than 80% of the adult patients with BHD. These PCs are most often located as basal lung lesions. The median age at first occurrence is 38 years, and the pathological findings of the lung are not so specific. In case 1, there was a layer of alveolar epithelial cells on the inner wall of the PCs.
Pavlovich et al. [5] reported that renal tumors are present in 27% of the BHD patients at a mean age of 50.4 years. Histologically, they are hybrid oncocytic tumors in 67%, chromophobe RCs in 23%, and clear cell RCs in 7% of the BHD patients. Chromophobe RCs are positive for CK7, c-kit, and colloidal iron, partially positive for CD10, and negative for vimentin. Case 2 was diagnosed with chromophobe RC by hematoxylin-eosin stain, which was positive for CK7, partially positive for colloidal iron and negative for vimentin, CD10, and c-kit. Clear cell RC was positive for vimentin and partially positive for CK-7. Case 1 was diagnosed with clear cell RC by hematoxylin-eosin stain, which was positive for vimentin but negative for CK7.
More than 60 cases of BHD in Asia have been reported in the BHD literature. Kunogi et al. [2] described 30 BHD patients with pneumothorax and/or PCs and an FCLN mutation. Six (20%) cases had cutaneous lesions, only 1 (3%) of these was histologically diagnosed as FF, and only 1 patient (3%) had RC (table 2). Furuya and Nakatani [3] described 45 patients from 19 Asian families. Thirteen (29%) had FFs, 40 (89%) had PCs, and 9 (20%) had RCs. PCs, RCs, and FFs were present in only 2 of the patients (4%) (table 2).
Table 2.
Clinical findings of Asian cases compared with those in the USA or Europe
| Toro [4], Menko [1], Pavlovich [5] | Kunogi [2] | Furuya [3] | Murakami Y, this report | |
|---|---|---|---|---|
| Fibrofolliculoma | 90% | 020%* | 29% | 27% |
| Pulmonary cyst | 80% | 100% | 89% | 79% |
| Renal cell carcinoma | 27% | 003% | 20% | 18% |
Only 3% of the patients had a pathological diagnosis.
We summarized 62 Asian case reports of BHD, not including those by Kunogi et al. [2] and Furuya and Nakatani [3] (table 1) [6, 7, 8, 9, 10, 11, 12, 13, 14]. Seventeen (27%) patients had FFs or perifollicular fibromas (PFFs) [6, 7, 8, 9, 10], 46 (79%) had PCs [10, 11, 12, 13], 11 (18%) had RCs (aged 43–69 years, except for our cases; table 2) [8, 12, 14], 5 (8%) had PCs and FFs or PFFs [10], 5 (8%) had PCs and RC [12], and 2 (3%) had RCs and FFs or PFFs [8]. Only 2 cases had all three symptoms of PCs, RCs, and FFs or PFFs [3]. Therefore, our cases are only the third and fourth reported Asian BHD cases affected by all three symptoms. Compared with the other cases, our patients were younger (37 and 35 years) and had quiet FFs on the face and scalp lesions that were unremarkable. Shin et al. [10] described a Korean case with quiet FFs that appeared to be sebaceous hyperplasia. Kim et al. [12] also described a Korean BHD patient with quiet papular lesions that were 0.5–5 mm in diameter, but they were not diagnosed by biopsy.
Tsai et al. [15] reported that the Chinese scalp contains 0.72 follicular units (FUs) and the Caucasian scalp contains 1 FU/mm2 surface area. The average hair density is 1.37 mm2 in Chinese and 2 mm2 in Caucasians. Total hair count and the number of FUs in Asians are smaller than in Caucasians. This difference may account for the few and unremarkable FFs present on the faces and scalps of Chinese, Japanese, and Korean patients.
Careful inspection of the skin and skin biopsies are important for the early detection of Asian BHD. Early detection will lead to the early diagnosis of RC and reduce BHD mortality.
Acknowledgments
This work was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and by a grant from the Japanese Ministry of Health, Labor, and Welfare.
References
- 1.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, Nordenskjold M, Hansen TV, Solly J, Maher ER, European BHDC Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 2.Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, Gunji Y, Kikkawa M, Iwakami S, Hino O, Takahashi K, Seyama K. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 2010;47:281–287. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuya M, Nakatani Y. Birt-Hogg-Dube syndrome: clinicopathological features of the lung. J Clin Pathol. 2013;66:178–186. doi: 10.1136/jclinpath-2012-201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, Turner M, Choyke P, Merino MJ, Pinto PA, Steinberg SM, Schmidt LS, Linehan WM. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlovich CP, Grubb RL, 3rd, Hurley K, Glenn GM, Toro J, Schmidt LS, Torres-Cabala C, Merino MJ, Zbar B, Choyke P, Walther MM, Linehan WM. Evaluation and management of renal tumors in the Birt-Hogg-Dubé syndrome. J Urol. 2005;173:1482–1486. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 6.Aoki M, Kawana S. Guess what! Diagnosis and comments: Birt-Hogg-Dubé syndrome. Eur J Dermatol. 2000;10:407–409. [PubMed] [Google Scholar]
- 7.Kawasaki H, Sawamura D, Nakazawa H, Hattori N, Goto M, Sato-Matsumura KC, Akiyama M, Shimizu H. Detection of 1733insC mutations in an Asian family with Birt-Hogg-Dubé syndrome. Br J Dermatol. 2005;152:142–145. doi: 10.1111/j.1365-2133.2004.06283.x. [DOI] [PubMed] [Google Scholar]
- 8.Imada K, Dainichi T, Yokomizo A, Tsunoda T, Song YH, Nagasaki A, Sawamura D, Nishie W, Shimizu H, Fukagawa S, Urabe K, Furue M, Hashimoto T, Naito S. Birt-Hogg-Dubé syndrome with clear-cell and oncocytic renal tumour and trichoblastoma associated with a novel FLCN mutation. Br J Dermatol. 2009;160:1350–1353. doi: 10.1111/j.1365-2133.2009.09134.x. [DOI] [PubMed] [Google Scholar]
- 9.Park G, Kim HR, Na CH, Choi KC, Shin BS. Genetic study in a case of Birt-Hogg-Dubé syndrome. Ann Dermatol. 2011;23:S188–S192. doi: 10.5021/ad.2011.23.S2.S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin WW, Baek YS, Oh TS, Heo YS, Son SB, Oh CH, Song HJ. Birt-Hogg-Dubé syndrome, a rare case in Korea confirmed by genetic analysis. Ann Dermatol. 2011;23:S193–S196. doi: 10.5021/ad.2011.23.S2.S193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunji Y, Akiyoshi T, Sato T, Kurihara M, Tominaga S, Takahashi K, Seyama K. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet. 2007;44:588–593. doi: 10.1136/jmg.2007.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Yoo JH, Kang DY, Cho NJ, Lee KA. Novel in-frame deletion mutation in FLCN gene in a Korean family with recurrent primary spontaneous pneumothorax. Gene. 2012;499:339–342. doi: 10.1016/j.gene.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Nishii T, Tanabe M, Tanaka R, Matsuzawa T, Okudela K, Nozawa A, Nakatani Y, Furuya M. Unique mutation, accelerated mTOR signaling and angiogenesis in the pulmonary cysts of Birt-Hogg-Dubé syndrome. Pathol Int. 2013;63:45–55. doi: 10.1111/pin.12028. [DOI] [PubMed] [Google Scholar]
- 14.Murakami T, Sano F, Huang Y, Komiya A, Baba M, Osada Y, Nagashima Y, Kondo K, Nakaigawa N, Miura T, Kubota Y, Yao M, Kishida T. Identification and characterization of Birt-Hogg-Dubé associated renal carcinoma. J Pathol. 2007;211:524–531. doi: 10.1002/path.2139. [DOI] [PubMed] [Google Scholar]
- 15.Tsai RY, Lee SH, Chan HL. The distribution of follicular units in the Chinese scalp: implications for reconstruction of natural-appearing hairlines in Orientals. Dermatol Surg. 2002;28:500–503. doi: 10.1046/j.1524-4725.2002.01194.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishiko A, Konohana I, Ikutomi M, Araki Y. A case of multiple fibrofolliculomas (Birt-Hogg-Dubé syndrome) (in Japanese) Rinshouhifuka. 1990;44:1237–1243. [Google Scholar]
- 17.Takahashi A, Hayashi T, Yoshida O, Ueda K, Furukawa F, Shuin T. Renal cell carcinoma in the Birt-Hogg-Dubé syndrome: report of a case (in Japanese) Hinyokika Kiyou. 2001;47:719–721. [PubMed] [Google Scholar]
- 18.Ishihara S, Kimoto M, Konohana A, Yamaguchi J, Nagasaka T, Ishiko A. Birt-Hogg-Dubé syndrome (in Japanese) Hifubyoushinryou. 2003;25:1115–1118. [Google Scholar]
- 19.Nagashima Y, Mitsuya T, Shioi K I, Noguchi S, Kishida T, Hamano A, Tsuura Ohgo Y, Y, Ogawa T, Aoki I, Yao M. Renal oncocytosis. Pathol Int. 2005;55:210–215. doi: 10.1111/j.1440-1827.2005.01813.x. [DOI] [PubMed] [Google Scholar]
- 20.Saito R, Sawada M, Ito H, Ishizaki S, Harada T, Nakayama S. A case of Multiple perifollicular fibroma (in Japanese) Hifu Rinshou. 2007;49:205–209. [Google Scholar]
- 21.Sadamasa H, Ikegami N, Yasui M, Yaguchi H, Hiruma M, Ohara H, Kimura T. A case of Birt-Hogg-Dubé syndrome (in Japanese) Skin Research. 2008;7:189–193. [Google Scholar]
- 22.Misago N, Joh K, Yatsuki H, Soejima H, Narisawa Y. A BHD germline mutation identified in an Asian family with Birt-Hogg-Dubé syndrome. Acta Derm Venereol. 2008;88:423–425. doi: 10.2340/00015555-0439. [DOI] [PubMed] [Google Scholar]
- 23.Kim EH, Jeong SY, Kim HJ, Kim YC. A case of Birt-Hogg- Dubé syndrome. J Korean Med Sci. 2008;23:332–335. doi: 10.3346/jkms.2008.23.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyasaka Y, Sakuraba M, Oh S, Takamochi K, Miyamoto H, Suzuki K. A case of Birt-Hogg-Dubé syndrome with intrathoracic heterotopic endometriosis (in Japanese) Nihon Kokyuki Gakkai Zassi. 2009;23:641–646. [Google Scholar]
- 25.Ando K, U T, Omori T, Tajiri M, Ogura T. A case of Birt-Hogg-Dubé syndrome (in Japanese) Nihon Kokyuki Gakkai Zassi. 2009;23:807–811. [Google Scholar]
- 26.Ishii H, Oka H, Amemiya Y, Iwata A, Otani S, Kishi K, Shirai R, Tokimatsu I, Kawahara K, Kadota J. A Japanese family with multiple lung cysts and recurrent pneumothorax: a possibility of Birt-Hogg-Dubé syndrome. Intern Med. 2009;48:1413–1417. doi: 10.2169/internalmedicine.48.2144. [DOI] [PubMed] [Google Scholar]
- 27.Koga S, Furuya M, Takahashi Y, Tanaka R, Yamaguchi A, Yasufuku K, Hiroshima K, Kurihara M, Yoshino I, Aoki I, Nakatani Y. Lung cysts in Birt-Hogg-Dubé syndrome: histopathological characteristics and aberrant sequence repeats. Pathol Int. 2009;59:720–728. doi: 10.1111/j.1440-1827.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- 28.So SY. Spontaneous pneumothorax due to Birt-Hogg-Dubé syndrome in a Chinese family. Respirology. 2009;14:775–776. doi: 10.1111/j.1440-1843.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi M, Takayanagi N, Ishiguro T, Sugita Y, Kawabata Y, Fukuda Y. Birt-Hogg- Dubé syndrome with multiple cysts and recurrent pneumothorax: pathological findings. Intern Med. 2010;49:2137–2142. doi: 10.2169/internalmedicine.49.3670. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa K, Kitadate S, Saito M, Fujimoto Y, Kojima K, Oikawa T, Tsuchihara K, Iguchi M, Kou T, Osanai K, Toga H, Niida Y. Two cases of Birt-Hogg-Dubé syndrome in a family (in Japanese) Rynsho hoshasen. 2011;56:133–137. [Google Scholar]
- 31.Sakairi Y, Yoshino I, Okamoto T, Hoshino H, Yoshida S, Tanaka R, Koga S, Takahashi Y, Nakatani Y. Two surgical cases of pneumothorax with Birt-Hogg-Dubé syndrome; the hypothesis of cyst formation (in Japanese) JJSPCLD. 2011;11:25–28. [Google Scholar]
- 32.Nagashima Y, Furuya M, Gotohda H, Takagi S, Hes O, Michal M, Grossmann P, Tanaka R, Nakatani Y, Kuroda N. FLCN gene-mutated renal cell neoplasms: mother and daughter cases with a novel germline mutation. Int J Urol. 2012;19:468–470. doi: 10.1111/j.1442-2042.2011.02945.x. [DOI] [PubMed] [Google Scholar]
- 33.Ishizuka M, Miyazaki Y, Okamoto T, Komazaki Y, Tamaoka M, Seyama K, Inase N. A case of Birt-Hogg-Dubé syndrome with family history of pneumothorax and typical CT findings (in Japanese) Ochanomizu Igaku Zassi. 2012;60:113–117. [Google Scholar]
- 34.Kashiwada T, Shimizu H, Tamura K, Seyama K, Horie Y, Mizoo A. Birt-Hogg-Dubé syndrome and familial adenomatous polyposis: an association or a coincidence? Intern Med. 2012;51:1789–1792. doi: 10.2169/internalmedicine.51.7239. [DOI] [PubMed] [Google Scholar]
- 35.Tobino K, Seyama K. Birt-Hogg-Dubé syndrome with renal angiomyolipoma. Intern Med. 2012;51:1279–1280. doi: 10.2169/internalmedicine.51.7211. [DOI] [PubMed] [Google Scholar]
- 36.Ema R, Morioka S, Sakurai A, Miki Y, Tomita K, Nakamura H. A case of Birt-Hogg-Dubé syndrome with bilateral renal carcinoma and pneumothorax has a novel mutation in exon 6 of the folliculin gene (in Japanese) Nihon Kokyuki Gakkai Zassi. 2013;2:13–17. [Google Scholar]
- 37.Haga T, Fukuoka M, Morita M, Cho K, Kataoka H, Kurihara M. Birt-Hogg-Dubé syndrome cases of a mother and her son (in Japanese) Nihon Kyobu Rinsyo. 2013;72(6):678–682. [Google Scholar]