Abstract
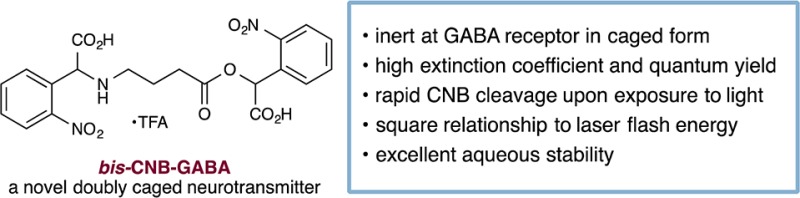
Photoactivatable “caged” neurotransmitters allow optical control of neural tissue with high spatial and temporal precision. However, the development of caged versions of the chief vertebrate inhibitory neurotransmitter, γ-amino butyric acid (GABA), has been limited by the propensity of caged GABAs to interact with GABA receptors. We describe herein the synthesis and application of a practically useful doubly caged GABA analog, termed bis-α-carboxy-2-nitrobenzyl-GABA (bis-CNB-GABA). Uncaging of bis-CNB-GABA evokes inward GABAergic currents in cerebellar molecular layer interneurons with rise times of 2 ms, comparable to flash duration. Response amplitudes depend on the square of flash intensity, as expected for a chemical two-photon uncaging effect. Importantly, prior to uncaging, bis-CNB-GABA is inactive at the GABAA receptor, evoking no changes in holding current in voltage-clamped neurons and showing an IC50 of at least 2.5 mM as measured using spontaneous GABAergic synaptic currents. Bis-CNB-GABA is stable in solution, with an estimated half-life of 98 days in the light. We expect that bis-CNB-GABA will prove to be an effective tool for high-resolution chemical control of brain circuits.
Introduction
Over the past two decades, caged neurotransmitters have emerged as a useful tool for the high-resolution, electrode-free chemical stimulation of single neurons or neural circuits. These probe compounds are prepared via covalent appendage of a light-sensitive protecting group—the cage—to a signaling molecule. With the cage in place, the signaling molecule is unable to activate its receptor. Upon delivery of a pulse of light, the cage is rapidly cleaved to reveal the active signaling molecule (Figure 1, top). When introduced into sliced or intact living brain tissue, caged neurotransmitters may activate neurotransmitter pathways at defined locations with micrometer and millisecond precision. Because they act one level upstream from intracellular voltage and second messenger signaling, caged neurotransmitters allow for a remarkable degree of specificity in chemical modulation of neural activity. The use of caged neurotransmitters offers important advantages over other established methods. Notably, it is possible to use patterned photostimulation techniques to achieve stimulation at many arbitrary locations in parallel;1,2 microelectrode-based methods are not amenable to this type of task. Moreover, neurotransmitter uncaging offers a useful alternative to optogenetic approaches3 because uncaging does not require gene delivery, is neurotransmitter-specific, and uses different wavelengths of light than those employed in optogenetics.
Figure 1.
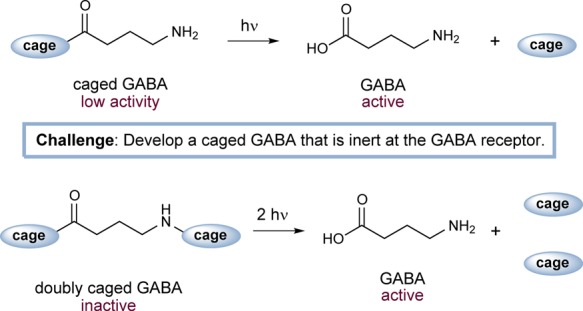
Top, conventional uncaging scheme for GABA. Bottom, chemical two-photon uncaging of double-caged GABA.
GABA (γ-amino butyric acid) is the chief vertebrate inhibitory neurotransmitter and is therefore an important target for caging. The ideal caged GABA neurotransmitter should exhibit a number of properties, including: (1) inertness at the receptor; (2) high combined extinction coefficient and quantum yield; (3) ability to undergo rapid cleavage to unveil the active neurotransmitter with few side products; and (4) excellent chemical stability in aqueous solution. To date, a number of caged GABA-based compounds have been developed that satisfy many of these criteria: α-carboxy-2-nitrobenzyl (CNB)-, 4-carboxymethoxy-5,7-dinitroindolinyl (CDNI)-, 1,3-bis(dihydroxyphosphoryloxy)propan-2-yloxy]-7-nitroindoline (DPNI)-, 4-methoxy-5,7-dinitroindolinyl (MDNI)-, and ruthenium-bipyridine-triphenylphosphine- (RuBi-GABA) have high combined extinction coefficient and quantum yield4,5 and cleave rapidly to generate neurotransmitters with few side products. All of these caged GABA compounds are chemically stable in aqueous solution on time scales of weeks or longer.6−8 However, in their caged form, they are not inactive. CNB-, CDNI-, DPNI-, and MDNI-caged GABA compounds are antagonists of GABAA receptors,6−8 as are RuBi-GABA,9 the related compound RuBi-glutamate,10 and 4-methoxy-7-nitroindolinyl (MNI)-glutamate.8 The practical limit at which these compounds can be used without interfering with neural circuit function is so low (<200 μM) that they cannot be used to attain the near-millimolar concentrations that occur locally during synaptic transmission.11,12 Presumably, these caged GABA compounds present residual receptor-binding epitopes such as amine7,8,13 or carboxylate.10,14 In specific cases such as RuBi, a phosphine moiety is also suspected to cause antagonistic effects.9
We sought to address the problem of receptor antagonism by adopting a “double-caging” strategy (Figure 1, bottom). Incorporation of two cages at different positions on a neurotransmitter has been shown to offer several advantages.15,16 First, release of the caged substrate is proportional to the square of the flash energy, creating a nonlinear effect resembling two-photon excitation17 and therefore improving spatial resolution. Moreover, a transmitter molecule modified at two locations is less likely to interact with its receptor than a single-caged analog, due to its reduced structural resemblance to the original transmitter.16 We envisioned modifying GABA at both the acid and amine positions with CNB, a readily synthesized cage with good water solubility.13 Caging at the N position would be achieved via direct modification of GABA,14 rather than through the use of a carbamate linker, which leads to slow uncaging kinetics.18 We describe herein the synthesis and evaluation of a doubly caged GABA analog, bis-CNB-GABA, 4. This compound is anticipated to emerge as a powerful tool for high-resolution control of brain circuits.
Results
Bis-CNB-GABA: Synthesis and Physical Properties
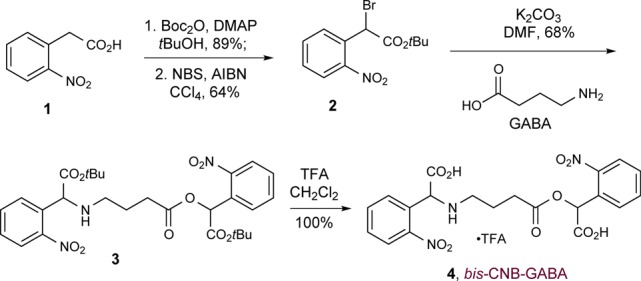
The synthesis of bis-CNB-GABA is outlined in Scheme 1. Nitrophenylacetic acid (1) was converted to its t-butyl ester, then brominated with NBS and AIBN to generate benzyl bromide 2. The latter was used to alkylate GABA simultaneously at both the amine and the acid positions (3); a final deprotection generated bis-CNB-GABA (4) as a tan powder. This material was shown by 1H NMR analysis to be >99% pure and to contain <0.2% residual GABA. A portion of the product was further purified by preparative HPLC. Notably, this synthetic route is comparable in ease to that of a single-caged GABA. Thus, bis-CNB-GABA is readily accessible from commercially available materials.
Scheme 1. Synthesis of Bis-CNB-GABA.
Bis-CNB-GABA exhibits maximal absorbance at 262 nm (ε = 7550 M–1 cm–1). Using mono-O-CNB-GABA as a reference standard to quantify conversion, the quantum yield (using 254 nm light) was 0.15 at the O-position and 0.032 at the N-position; these values are the same as the quantum yields determined at 308 nm excitation for the corresponding mono-CNB-GABA compounds.13,14 Finally, bis-CNB-GABA is soluble in pH 7.0 phosphate buffer solution up to 17 mM, at levels comparable to bis-CNB-glutamate.15
Effects of Uncaging to Evoke Currents in Molecular Layer Interneurons
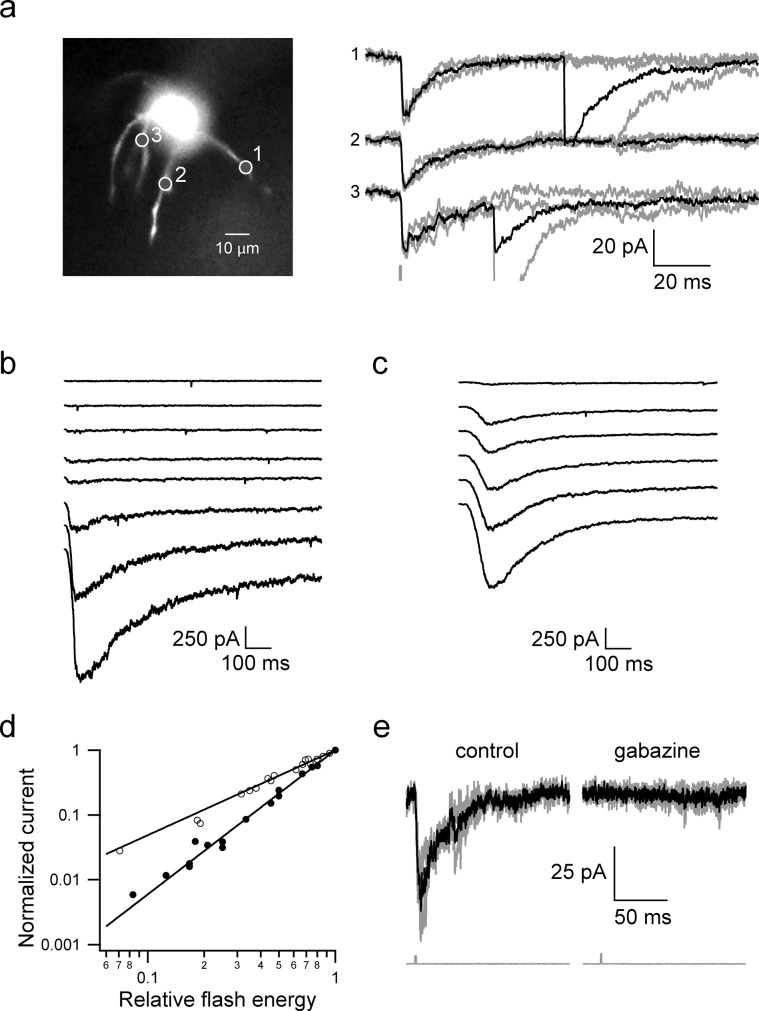
The biological properties of our synthetic bis-CNB-GABA were evaluated using whole-cell patch recording from cerebellar molecular layer interneurons. Molecular layer interneurons receive excitatory glutamatergic synapses as well as inhibitory GABAergic synapses that show a pronounced level of spontaneous activity (gray traces in Figure 2a). The effects of bis-CNB-GABA on these synaptic currents were tested. In the presence of bis-CNB-GABA, photolysis, using either a UV high-intensity LED focused to the back focal plane of the objective or a minimized 405 nm laser spot, produced currents (Figure 2b–d) that were reversibly eliminated in the presence of 3 μM of the GABAA receptor antagonist, gabazine (Figure 2e).
Figure 2.
Physiological responses to photolysis of bis-CNB-GABA. (a) A cerebellar molecular layer interneuron visualized using Alexa 488 in the patch recording electrode solution. Bis-CNB-GABA (0.6 mM) was photolyzed with a 405 nm laser spot in 3 different locations (indicated by 1–3). Laser-evoked GABAergic currents are shown on the right panel. Gray traces show individual sweeps. Black traces are averages. The gray sweep at bottom indicates the laser flash (1 ms duration, intensity 5 mW) as recorded using a photodiode. (b) Bis-CNB-GABA (1.4 mM) was photolyzed with a 365 nm LED at progressively higher flash energies (0.25–1.1 mW, 5–50 ms, 1.25–55 μJ). (c) Same experiment as (b) with mono-CNB-GABA (50 μM). (d) Normalized current as a function of relative LED flash energies plotted on a log–log scale. (e) Laser-evoked whole-cell current recorded in the absence and presence of 3 μM gabazine, a GABAA receptor antagonist. Note that a larger flash energy was used in the presence of gabazine.
As anticipated,15 the current amplitude evoked by photolysis of bis-CNB-GABA was related to laser flash energy by a square relationship (Figure 2d; log–log slope = 2.2 ± 0.1, n = 3 cell bodies). By contrast, the relationship for mono-O-CNB-GABA was close to linear (log–log slope = 1.3 ± 0.1, n = 3). For these measurements, evoked currents were normalized to the maximum current observed in the same neuron. Moreover, the integrated evoked current over time was proportional to the square of the laser energy. These power laws are consistent with a process in which each molecule of bis-CNB-GABA must cumulatively undergo two uncaging reactions in order to release an active GABAA agonist. This result is predicted by localized, nonlinear release of GABA at the laser’s focus spot and is in line with the localized release and spatial resolution seen in previous applications of chemical two-photon uncaging.15,16
Laser-Evoked GABA Responses
To test whether bis-CNB-GABA-evoked responses resemble physiological events in their kinetics, we measured the kinetic properties of flash-evoked responses. For responses comparable in size with spontaneous inhibitory postsynaptic currents (IPSCs), the 10–90% rise time was 2.2 ± 0.6 ms (n = 10); somewhat longer than the flash duration of 1.0 ms. This rise time is consistent with the dark-reaction time (1.5 ms) for the slower cage, N-mono-CNB-GABA.14 The falling t1/2 was 24.2 ± 8.2 ms (n = 10). These rates approach those of spontaneous events and are among the fastest described for other GABA cages. In some recordings (for example, Figure 2a responses at sites 1 and 3), the kinetics of laser-evoked events were nearly indistinguishable from those of spontaneous IPSCs, perhaps because of short electrotonic distances between the uncaging site and the recording electrode. In summary, photouncaging of bis-CNB-GABA was sufficiently rapid to mimic synaptic events.
Effects of Bis-CNB-GABA on Endogenous Synaptic Communication
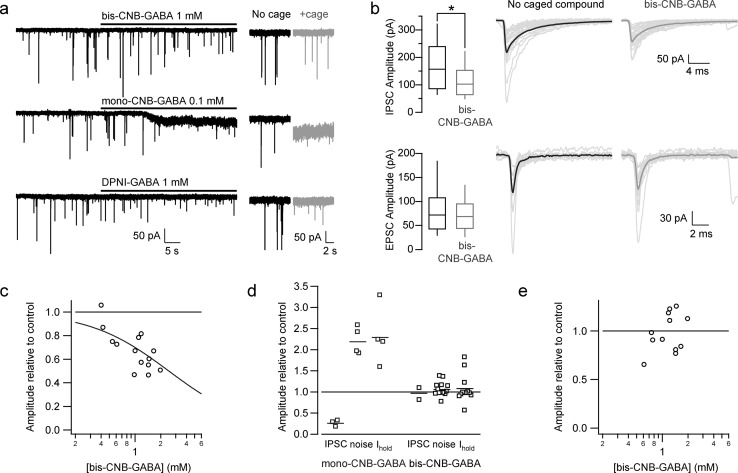
In order to evaluate the viability of bis-CNB-GABA as a probe compound, we made three measures of the potential undesirable effects of both mono- and bis-caged GABA analogs. First, we monitored changes in whole-cell holding current under voltage clamp, while caged GABA compounds were applied by bath application or by local perfusion (Figure 3).19 Under these conditions, mono-O-CNB-GABA (0.1 mM) led to increases of 66 ± 47 pA (mean ± SD, n = 6) in inward holding current at −60 mV (Ihold; Figure 3a,d). This inward current presumably arises either via indirectly evoked increases in excitation20 or from direct activation of GABAA receptors (by residual free GABA in the caged compound solution or by mono-O-CNB-GABA, which may itself have partial agonist activity). Similar, though less pronounced, results have been observed with DPNI-GABA (see Figure 3a and ref (7)). In contrast, application of bis-CNB-GABA (raw product, 0.4–2.0 mM) evoked no detectable change in the holding current (Figure 3d; ratio of holding current drug/control = 1.0 ± 0.3, n = 14, p = 0.7, Mann–Whitney test) or in its standard deviation, which is a measure of steady-state channel noise (Figure 3d, from 3.0 ± 0.5 to 3.2 ± 0.8 pA, n = 14, p = 0.3) measured during periods of no spontaneous currents. No difference in induced holding current was seen between raw product and HPLC-purified product.
Figure 3.
Quantification of unwanted effects of caged GABA. (a) Voltage clamp recordings from cerebellar interneurons exposed to caged GABA. Upper trace, 1 mM bis-CNB-GABA; middle trace, 0.1 mM mono-O-CNB-GABA; bottom trace, 1 mM DPNI-GABA. Right, expanded traces illustrating the detailed effects on steady-state holding current and fluctuations in holding current. (b) Effects of caged GABA on spontaneous IPSCs and excitatory postsynaptic currents (EPSCs). IPSCs and EPSCs were identified and separated based on kinetic criteria. Left, box plots of spontaneous postsynaptic current amplitudes in control conditions and in the presence of 1 mM bis-CNB-GABA. Boxes show interquartile range and whiskers show full range of values. Right, individual traces (gray) and average (block) of detected spontaneous IPSCs and EPSCs. (c) Dependence of spontaneous IPSC amplitude on bis-CNB-GABA concentration. The curve indicates a fit with KD = 2.5 ± 0.2 mM, nH = 0.93 ± 0.09. (d) Comparison of effects of mono-O-CNB-GABA (0.1 mM) and bis-CNB-GABA (1.0 mM, except for 0.4 mM for IPSCs) on spontaneous IPSC amplitude, standard deviation of holding current (noise), and holding current (Ihold). (e) Spontaneous EPSC size was unaffected by bis-CNB-GABA at all concentrations tested.
We next measured the effects of bis-CNB-GABA on spontaneous IPSCs (Figure 3b). At 1 mM, mono-O-CNB-GABA (IC50 = 28 μM; ref (6)) triggered a dramatic decrease in the rate of spontaneous IPSCs (not shown), presumably due to inhibition of GABAA receptors in the recorded neuron and/or of activity in presynaptically connected MLIs. In contrast, addition of bis-CNB-GABA served to reduce GABA current amplitudes by approximately one-third (Figure 3b, top; 0.4–2.0 mM; ratio of amplitudes during drug/control: 0.68 ± 0.17, n = 14). The concentration dependence of the reduction yielded an estimated IC50 of 2.5 mM (Figure 3c). This IC50 is, in fact, a lower bound; the material tested was crude product, which may contain minute amounts of mono-CNB-GABA. Bis-CNB-GABA therefore exhibits at least 100-fold lower affinity compared to its mono-caged analog. Moreover, the 10–90% rise time of IPSCs was unaffected by bis-CNB-GABA application (control, 0.44 ± 0.13 ms, vs bis-CNB-GABA, 0.44 ± 0.11 ms), in contrast with DPNI-GABA, which prolongs rise times.7 Taken together, these findings are consistent with the hypothesis that bis-CNB-GABA shows virtually no antagonist activity at submillimolar concentrations.
As a third and final test of the synaptic effects of bis-CNB-GABA, we measured its impact on spontaneous glutamatergic postsynaptic currents (EPSCs) recorded from MLIs. With this configuration, no change in the amplitude of the EPSCs was observed (Figure 3b, bottom) even with concentrations of bis-CNB-GABA up to 2 mM (Figure 3e, p = 0.4, Spearman rank order correlation test). In summary, at concentrations of 1 mM, our doubly caged bis-CNB-GABA was found to have minimal or no effects on holding current, IPSCs, or EPSCs.
Spontaneous Rate of Hydrolysis of Bis-CNB-GABA vs Mono-O-CNB-GABA
We next sought to study the hydrolytic stability of bis-CNB-GABA under normal handling conditions. Accordingly, we prepared samples of bis-CNB-GABA and mono-O-CNB-GABA in aqueous buffer (23 °C, 12 mM, pH = 7.4), stored the samples under fluorescent room lights, and determined accumulation of deprotected GABA at 1 day intervals.
The mechanism of photodecaging of the CNB group is an active area of study; several pathways are available to caged amines and caged carboxylate derivatives (for discussion see ref (21)). The dominant final photoproduct of bis-CNB-GABA was GABA, as identified using 1H NMR (Figure 4a). The combined amount of monodecaging (N-CNB-GABA) and double-decaging (GABA) photoproduct was quantified using the integrated multiplet at 2.15 ppm, with dimethoxyethane (singlet peaks at 3.20 and 3.45 ppm) as a standard. Over the first 8 days, we found that bis-CNB-GABA produced photoproduct spontaneously at an approximately linear rate (Figure 4b), 1.5 ± 0.1%/day (n = 4 runs), for an extrapolated half-life of t1/2 = 98 days (95% CI, 92 to 105 days). Mono-O-CNB-GABA produced GABA at a similar rate of 1.0 ± 0.2%/day (n = 2 runs) or t1/2 = 138 days (95% CI, 116–169 days), which is consistent with a prior report of <1% conversion in the dark at 24 h,13 but not consistent with another report.8
Figure 4.
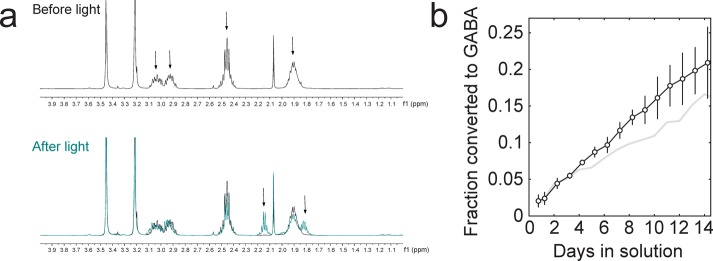
Accumulation of GABA in ambient room light. (a) 1H NMR data used to quantify GABA accumulation. Top, bis-CNB-GABA before light exposure. Arrows denote methylene peaks of bis-CNB-GABA. Peaks at ∼3.45 and ∼3.2 ppm in each spectrum correspond to dimethoxyethane, used as an internal standard. Bottom, the teal overlay indicates the postphotolysis spectrum of bis-CNB-GABA after 17 days of exposure to ambient fluorescent light. Arrows denote the two visible peaks corresponding to a combination of GABA and mono-N-CNB-GABA photoproducts. Note the lack of extraneous peaks in the 0–4 ppm range after light exposure. (b) In aqueous solutions in the light, accumulation of photoproducts from bis-CNB-GABA (black) and GABA from mono-CNB-GABA (gray). Error bars indicate SD.
Discussion
As described above, we have identified a novel, double-caged bis-CNB-GABA that is highly resistant to pre-uncaging interactions with GABAA receptors. Importantly, of a wide range of structurally diverse caged GABA analogs, bis-CNB-GABA exhibits the highest half-maximal concentration (IC50) of GABAA antagonistic activity (Table 1).
Table 1. Comparative properties of caged GABA and glutamate compounds in blocking synaptic GABAA currents.
It has been suggested that some cage groups may themselves antagonize GABAA receptors;9 if this were the case, then a double-caged-GABA analog might be expected to show an increased ability to block GABAA receptors. Our findings demonstrate the opposite and support the view that when CNB is used as the cage group, an exposed carboxyl or amine is a key factor in residual receptor interaction.
Although it is useful to compare the relative inertness of bis-CNB-GABA as a receptor antagonist with caged compounds already in use, such as DPNI-GABA or CDNI-GABA, a more appropriate comparison from a structure–function standpoint is with the mono-O-CNB-GABA analog. Such a comparison clearly reveals the advantages of adding a second cage of similar structure to the first. In this context, we have shown that adding a second cage to mono-CNB-GABA dramatically reduces receptor antagonism, by a factor of 100. We predict that other forms of N,O-bis-caged GABA compounds would exhibit comparable reduction of antagonism compared to their O-caged analogs. Because DPNI-GABA and CDNI-GABA incorporate carboxyl-modifying groups, preparation of bis-caged analogs of these compounds would require modification of the N-position with CNB or another cage. Such a “hybrid” caged GABA should similarly exhibit minimal receptor activity.
As described above, modification of GABA at the amino position by direct attachment of CNB affords uncaging responses consistent with a dark reaction time of 1.5 ms.14 A previous approach had made use of a carbamate linker, which generates neurotransmitter in ∼7 ms via a carbamate intermediate,18 out of concern that direct attachment would yield unwanted non-GABA side products. Our results demonstrate that, in fact, direct attachment can lead to efficient GABA production, as measured by NMR, and rapid photolysis as measured by the time course of photolyzed currents.
Importantly, the high speed of uncaging obtained with bis-CNB-GABA allows for a more highly focused chemical two-photon effect and, accordingly, micrometer-to-submicrometer localization in biological experiments. For a dark reaction longer than ∼0.2 ms, spatial resolution of uncaging for a diffraction-focused beam is limited by the distance that a caged compound diffuses before it produces agonist. For bis-CNB-GABA, dark reaction times of 28 μs13 and 1.5 ms and a diffusion constant of D = 0.3 μm2/ms would predict a root-mean-square spread of <x>1/2 = √(6·D·t)=0.2 and 1.6 μm, respectively. As a beam passes through brain tissue and becomes less focused due to scattering, diffraction- and diffusion-based limits might not be reached.
Caged compounds in solution are usually handled in room light, during which both spontaneous degradation and photolysis can occur. Under these conditions the rate of O-position degradation was similar for mono-O-CNB-GABA and bis-CNB-GABA. These findings are consistent with good stability at the carboxylate position, as previously reported,13 and with higher stability at the amino position. However, these results are not consistent with a claim of t1/2 = 17 h for mono-CNB-GABA in a study that did not report methods.8 During a week in the light, we estimate the production of N-CNB-GABA to be 10%; the accumulation of GABA during that period should therefore be <1%. Due to the possibility of accumulation of mono-CNB-GABA isomers in solution, bis-CNB-GABA should be kept dry before use, for instance through aliquoting of solutions in distilled water followed by lyophilization. It is of note that purification steps involving aqueous solution, such as preparative HPLC, might require a trade-off in the form of accumulated mono-CNB-GABA or GABA. We found the use of crude product, without HPLC purification, to be effective in biological experiments; accordingly, crude-product level purity may be acceptable for many biological experiments.
Conclusion
Several forms of caged GABAs have been synthesized over the past two decades. More recently, two-photon uncaging has introduced the possibility of nonlinear release of substrate and improved localization of GABA upon photolysis. Though several caged compounds have been designed that make use of this development in the synthesis of two-photon sensitive single-caged GABA,7−9 such compounds exhibit antagonistic activity at GABAA receptors, which limits the concentrations that can be employed. We describe herein the synthesis and evaluation of bis-CNB-GABA, the first caged GABA that takes advantage of chemical two-photon uncaging, achieving nonlinear localized release of GABA and a significant decrease in GABAA receptor antagonism prior to photolysis. Bis-CNB-GABA is a powerful advanced optical probe that may be used to study GABAergic inhibitory effects with a degree of resolution that permits the probing of single-synapse communication and neuronal integration.
Acknowledgments
This work was supported by NIH R01 NS045193. F.F.T. is supported by a Chaire d’Excellence from the University Paris Descartes and the CNRS. We thank Laura Miller, Jeffrey Garber, and David Ogden for help and comments.
Supporting Information Available
Experimental procedures and spectral data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
† 107 Avenue Louis Pasteur, Box 102, Boston, MA 02115.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Katz L. C.; Dalva M. B. J. Neurosci. Methods 1994, 54, 205–218. [DOI] [PubMed] [Google Scholar]
- Shoham S.; O’Connor D. H.; Sarkisov D. V.; Wang S. S.-H. Nat. Methods 2005, 2, 837–843. [DOI] [PubMed] [Google Scholar]
- Packer A. M.; Roska B.; Häusser M. Nat. Neurosci. 2013, 16, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A.; Nerbonne J. M. Annu. Rev. Biophys. Bioeng. 1982, 11, 151–175. [DOI] [PubMed] [Google Scholar]
- Adams S. R.; Tsien R. Y. Annu. Rev. Physiol. 1993, 55, 755–784. [DOI] [PubMed] [Google Scholar]
- Molnár P.; Nadler J. V. Eur. J. Pharmacol. 2000, 391, 255–262. [DOI] [PubMed] [Google Scholar]
- Trigo F. F.; Papageorgiou G.; Corrie J. E. T.; Ogden D. J. Neurosci. Methods 2009, 181, 159–169. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M.; Hayama T.; Kasai H.; Ellis-Davies G. C. R.. Nat. Chem. Biol. 2010, 6, 255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde E. M.; Zayat L.; Etchenique R.; Yuste R. Front. Neural Circuits 2008, 2, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E.; Araya R.; Peterka D. S.; Salierno M.; Etchenique R.; Yuste R. Front. Neural Circuits 2009, 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D.; Ropert N. J. Neurosci. 1999, 19, 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M.; Nusser Z. Nat. Rev. Neurosci. 2005, 6, 215–229. [DOI] [PubMed] [Google Scholar]
- Gee K. R.; Wieboldt R.; Hess G. P. J. Am. Chem. Soc. 1994, 116, 8366–8367. [Google Scholar]
- Wieboldt R.; Ramesh D.; Carpenter B. K; Hess G. P. Biochemistry 1994, 33, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Pettit D. L.; Wang S. S-H.; Gee K. R.; Augustine G. J. Neuron. 1997, 19, 465–471. [DOI] [PubMed] [Google Scholar]
- Sarkisov D. V.; Gelber S. E.; Walker J. W.; Wang S. S.-H.. J. Biol. Chem. 2007, 282, 25517–25526. [DOI] [PubMed] [Google Scholar]
- Denk W.; Strickler J. H.; Webb W. Science 1990, 248, 73–76. [DOI] [PubMed] [Google Scholar]
- Corrie J. E. T.; DeSantis A.; Katayama Y.; Khodakhah K.; Messenger J. B.; Ogden D. C.; Trentham D. R. J. Physiol. 1993, 465, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civillico E. F.; Shoham S.; O’Connor D. H.; Sarkisov D. V.; Wang S. S.-H.. Cold Spring Harbor Protoc. 2012, 8, pii: pdb.prot070656. [DOI] [PubMed] [Google Scholar]
- Dellal S. S.; Luo R.; Otis T. S. J. Neurophysiol. 2012, 107, 2958–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrie J. E. T.; Munasinghe V. R. N.; Trentham D. R.; Barth A. Photochem. Photobiol. Sci. 2008, 7, 84–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.