Abstract
Folate-mediated one-carbon metabolism is essential for DNA synthesis, repair, and methylation. Perturbations in one-carbon metabolism have been implicated in increased risk of some cancers and may also affect inflammatory processes. We investigated these interrelated pathways to understand their relation. The objective was to explore associations between inflammation and biomarkers of nutritional status and one-carbon metabolism. In a cross-sectional study in 1976 women selected from the Women’s Health Initiative Observational Study, plasma vitamin B-6 [pyridoxal-5′-phosphate (PLP)], plasma vitamin B-12, plasma folate, and RBC folate were measured as nutritional biomarkers; serum C-reactive protein (CRP) and serum amyloid A (SAA) were measured as biomarkers of inflammation; and homocysteine and cysteine were measured as integrated biomarkers of one-carbon metabolism. Student’s t, chi-square, and Spearman rank correlations, along with multiple linear regressions, were used to explore relations between biomarkers; additionally, we tested stratification by folic acid fortification period and multivitamin use. With the use of univariate analysis, plasma PLP was the only nutritional biomarker that was modestly significantly correlated with serum CRP and SAA (ρ = −0.22 and −0.12, respectively; P < 0.0001). Homocysteine (μmol/L) showed significant inverse correlations with all nutritional biomarkers (ranging from ρ = −0.30 to ρ = −0.46; all P < 0.0001). With the use of multiple linear regression, plasma PLP, RBC folate, homocysteine, and cysteine were identified as independent predictors of CRP; and PLP, vitamin B-12, RBC folate, and homocysteine were identified as predictors of SAA. When stratified by folic acid fortification period, nutrition-homocysteine correlations were generally weaker in the postfortification period, whereas associations between plasma PLP and serum CRP increased. Biomarkers of inflammation are associated with PLP, RBC folate, and homocysteine in women. The connection between the pathways needs to be further investigated and causality established. The trial is registered at clinicaltrials.gov as NCT00000611.
Introduction
The importance of folate-mediated one-carbon metabolism in colorectal carcinogenesis has been shown in numerous epidemiologic and animal studies (1–3). Folate functions as a donor of one-carbon units and is essential for methylation reactions, including DNA methylation, as well as nucleotide and DNA synthesis, stability, and repair (4, 5). The universal and essential methyl donor, S-adenosylmethionine, is produced in the folate-dependent homocysteine cycle, which includes the conversion from homocysteine to methionine by demethylation of 5′methyltetrahydrofolate.
Several B vitamins, including folate, vitamin B-12, and vitamin B-6, play important roles as substrates or cofactors in one-carbon metabolism. Deficiencies of these B vitamins disturb the pathway leading to the following: 1) inhibited DNA synthesis and repair, 2) decreased DNA stability, 3) altered methylation patterns, and 4) hyperhomocysteinemia. Such disturbances in one-carbon metabolism are thought to contribute to cancer, particularly colorectal cancer (6), as well as cardiovascular disease and other metabolic and psychiatric disorders such as depression, dementia, and Alzheimer disease (7–11).
In recent years, there has been growing recognition that chronic inflammation is an important pathogenic factor underlying degenerative diseases, including cancer and cardiovascular disease (12). Moreover, there are increasing numbers of reports of associations between B vitamins, homocysteine, and inflammatory biomarkers, suggesting important interrelations between perturbations in one-carbon metabolism and inflammatory reactions. For example, patients with rheumatoid arthritis, diabetes, or stroke, conditions in which inflammation plays a crucial role, showed inverse associations between plasma pyridoxal-5′-phosphate (PLP)13 and the inflammatory marker C-reactive protein (CRP) in serum (13–18). Increased concentrations of the acute inflammatory marker serum amyloid A (SAA) can be found in patients with acute and chronic inflammation (19). Inflammation can result in cell damage and proliferation, and has been directly implicated in risk of carcinogenesis (e.g., through reactive oxygen species), causing damage to macromolecules, including DNA (20, 21).
To further define the interaction between the 2 biological pathways, one-carbon metabolism and inflammation, we investigated the association between the following: 1) nutritional biomarkers plasma PLP, vitamin B-12, plasma folate, and RBC folate; 2) biomarkers of inflammation (serum CRP and SAA); and 3) one-carbon metabolism (homocysteine and cysteine concentrations) in the Women’s Health Initiative Observational Study (WHI-OS) (22). A concurrent investigation of the interrelated pathways may yield new information regarding the relation. Depending on the status of folic acid fortification, the strength of the association may differ, but this has not yet been investigated previously.
The study presented here is largely exploratory, with only a limited number of set hypotheses. Specifically, we expected low plasma PLP status to be associated with high serum CRP and high SAA concentrations and high folate concentrations to be associated with decreased serum CRP concentrations. Furthermore, we hypothesized that high homocysteine and low cysteine concentrations would be associated with high serum CRP.
Participants and Methods
Study population
A cross-sectional study design was used to explore correlations between biomarkers of nutrition, inflammation, and one-carbon metabolism in 1976 women selected from the 93,676 participants in the WHI-OS, as part of a nested case-control study (23). All biomarkers were measured at baseline in participants without clinical disease. The WHI-OS enrolled participants at 40 U.S. clinical institutions between 1993 and 1998. The study design and characteristics of the cohort have been described elsewhere (22, 24). Eligibility requirements included postmenopausal status, age between 50 and 79 y, and low likelihood of loss to follow-up within 3 y due to relocation or death resulting from a preexisting medical condition. We subdivided participants into 3 groups reflecting time of recruitment in relation to folic acid fortification in the United States, i.e., prefortification (before 1996), perifortification (1996–1997), and postfortification (1998 and later) phases.
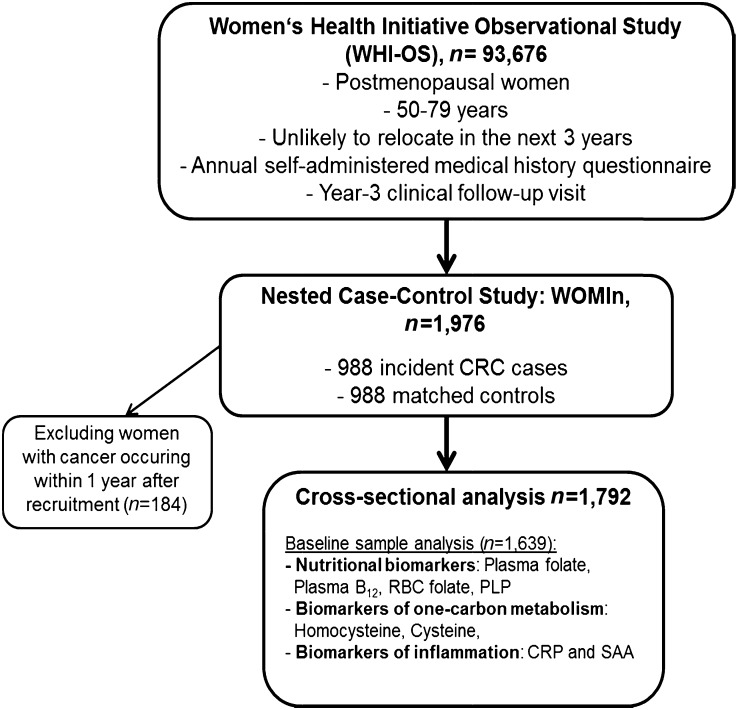
All biomarkers, including plasma PLP, plasma vitamin B-12, plasma folate, RBC folate, serum CRP, SAA, plasma homocysteine, and plasma cysteine, were measured by using fasting blood samples collected at recruitment into the cohort. Of the 1976 participants, 988 were selected because they later developed colorectal cancer, and988 were controls matched by age (±3 y), race/ethnicity, clinical center, date of blood draw (±6 mo for baseline and year 3 blood draws), and baseline hysterectomy status. For the analysis, women were excluded if they had preexisting intestinal disease, including history of colorectal cancer (n = 959), carcinoma in situ (n = 46), ulcerative colitis, Crohn disease, or if they were extremely under- or overweight as indicated by a BMI ≤15 or ≥50 kg/m2, respectively (n = 502). We excluded women with cancer occurring within 1 y after recruitment (n = 184) (Fig. 1). To further address the potential that some of the women had preclinical disease, in our analyses we excluded cases diagnosed within a year of baseline. The study was approved by the human subjects review boards at the WHI-OS Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center, at all 40 clinical centers at which participants were recruited for the WHI-OS study, and at the University of California, Davis, and by the ethics commission of the University of Heidelberg. Written informed consent was obtained from all participants.
FIGURE 1.
Flow chart of the study populations. CRC, colorectal cancer; CRP, C-reactive protein; PLP, pyridoxal-5′-phosphate; SAA, serum amyloid A; WOMIn, Women, One-Carbon Metabolism and Inflammation Study.
Demographic and health data collection
Demographic characteristics, including age, race, and education, were collected by using standardized instruments. Height and weight were measured at baseline by trained staff with the use of a common protocol, and BMI was calculated by using the equation BMI = weight (kg)/height (m2).
Sample processing and analysis
For the purpose of measuring the concentrations of the study-related biomarkers, a baseline blood sample was collected. Participants were instructed to fast at least 12 h before phlebotomy. Blood samples were kept at 4°C for up to 1 h before centrifugation. Plasma and serum were collected and stored at −70°C in a central biorepository until analysis.
Nutritional biomarkers.
Plasma folate and plasma vitamin B-12 were determined by radioassay (SimulTRAC B12/ FOLATE-SNB 57_Co/125I; MP Biomedicals) at the Biomarker Laboratory, Fred Hutchinson Cancer Research Center (X.S.), RBC folate was measured by radioassay (SimulTRAC; MP Biomedicals), and plasma PLP was measured by HPLC with fluorescence detection at the University of California, Davis (J.W.M.) (25). Interassay CVs for WHI-OS blind duplicate samples were as follows: RBC folate, 10.2%; plasma folate, 4.8%; vitamin B-12, 6.2% and plasma PLP, 4.8% (25).
One-carbon status markers.
Total plasma homocysteine and plasma cysteine were determined by HPLC with postcolumn fluorescence detection (26). Interassay CVs for the assays were as follows: homocysteine, 6.5%; cysteine, 7.1% (measured at the University of California, Davis; J.W.M.).
Inflammation markers.
Serum CRP and SAA were quantified at the Clinical Immunology Laboratory (University of Washington) by latex-enhanced nephelometry (BNII; Siemens) (M.H.W.). CVs for WHI-OS blind duplicate samples were 4.9% for SAA and 4.1% for CRP. Because renal function is a strong determinant of homocysteine and cysteine concentrations, all correlations including these biomarkers were adjusted for creatinine. Plasma creatinine was determined by the Jaffe rate reaction method (DxC Instrument; Beckman Coulter), with a CV of 4.1%.
Statistical analyses
Descriptive statistics were assessed for baseline characteristics of all participants. Means and SDs for selected biomarkers were calculated. All analyzed biomarkers were evaluated by using scatter plots and distribution tables. Unadjusted and adjusted Spearman rank correlation coefficients and corresponding P values for the study population were calculated. Pathway analyses were considered to visualize the strength of associations between a pair of biomarkers while taking into account the impact of other biomarkers in the pathway. The adjusted correlations were controlled for baseline BMI, age, and plasma creatinine. Additionally, plasma homocysteine and plasma cysteine were adjusted for plasma vitamin B-12, plasma folate, and the combination of both, due to the dependency on these nutrients in the homocysteine metabolic pathway. Correlation analyses were also stratified by BMI (<25, 25–30, >30–35, >35 kg/m2), age (<67, ≥67 y), fortification period [prefortification (1994–1995), perifortification (1996–1997), and postfortification (1998)], and multivitamin use (“currently taking” multivitamins with minerals: yes or no) (27). Univariate and multiple linear regression analyses were used to determine relations between the nutritional and one-carbon biomarkers with biomarkers of inflammation. Nutritional biomarkers included plasma vitamin B-6, plasma vitamin B-12, and RBC folate; one-carbon biomarkers included homocysteine and cysteine. All linear models were controlled for baseline BMI and age. In the multivariate analyses, all factors were included in the model and adjusted for age and BMI. Log transformations were performed on the inflammatory biomarkers of interest (serum CRP and SAA) to improve the normality of the distributions and to meet the required assumptions of the model building. Significance was defined as P < 0.05, and all statistical tests were 2-sided. Pathway analyses were conducted with R package qgraph (R Foundation), and all other analyses were conducted with SAS 9.3 (SAS Institute) (28).
Results
Baseline characteristics for all study participants (n = 1792) are shown in Table 1. Mean age was 67 y, mean BMI was 28.0 kg/m2, and the majority of participants were Caucasian. Nearly 40% of participants took multivitamins, and 75% used nonsteroidal anti-inflammatory drugs at the time of recruitment.
TABLE 1.
Baseline characteristics of the study population1
| Characteristic | Value |
| Age, y | 67 ± 7 (1792) |
| BMI, kg/m2 | 28 ± 6 (1773) |
| Ethnicity (n = 1792), n (%) | |
| White | 1538 (86) |
| Black or African American | 144 (8) |
| Other (Hispanic, Asian or Pacific Islander, American Indian or Alaskan Native, other, missing) | 110 (6) |
| Residence location (U.S. region) (n = 1792), n (%) | |
| Northeast | 434 (24) |
| South | 430 (24) |
| Midwest | 410 (23) |
| West | 518 (29) |
| Education, n (%) | |
| ≤High school | 375 (21) |
| ≥College | 1403 (79) |
| Taking any multivitamin, n (%) | |
| Yes | 701 (39) |
| No | 1091 (61) |
| Taking multivitamin without minerals, n (%) | |
| Yes | 80 (4) |
| No | 1712 (96) |
| Taking multivitamin with minerals, n (%) | |
| Yes | 626 (35) |
| No | 1166 (65) |
| NSAID use, n (%) | 1343 (75) |
| Pack-years of smoking | 10.8 ± 19.6 (1730) |
| Alcohol use (12 drinks ever), n (%) | 1577 (89) |
| Biomarker | |
| Plasma PLP, nmol/L | 97.5 ± 96.7 |
| Plasma vitamin B-12, pg/mL | 535 ± 274 |
| RBC folate, μg/L | 600 ± 254 |
| Plasma folate, μg/L | 19.5 ± 14.0 |
| Serum CRP, g/L | 4.6 ± 6.2 |
| SAA, g/L | 9.7 ± 22.3 |
| Plasma homocysteine, μmol/L | 8.6 ± 3.1 |
| Plasma cysteine, μmol/L | 285 ± 39.5 |
Values are means ± SDs (n) unless otherwise indicated. CRP, C-reactive protein; NSAID, nonsteroidal anti-inflammatory drug; PLP, pyridoxal-5′-phosphate; SAA, serum amyloid A.
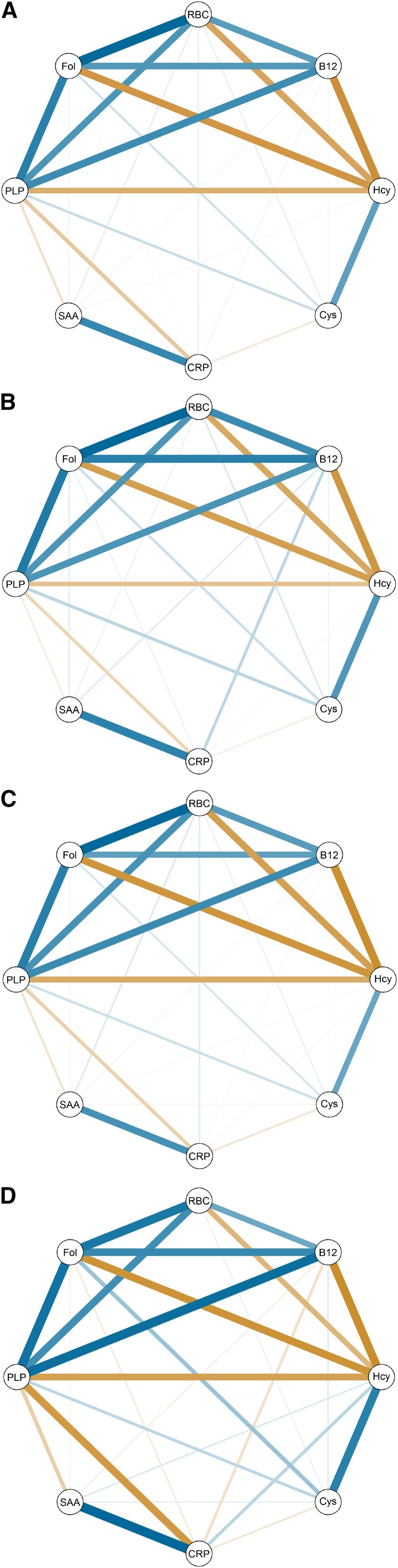
Spearman correlations between nutritional biomarkers, integrated markers of one-carbon status, and inflammatory biomarkers are presented in Table 2. A visual representation of the correlation matrices is given in Figure 2.
TABLE 2.
Spearman correlation matrix of nutritional, one-carbon status, and inflammation biomarkers in postmenopausal women, adjusted for urine creatinine, age, and BMI1
| Nutritional biomarkers | Integrated markers of one-carbon status | Inflammation biomarkers | |||||||||
| Plasma PLP | Plasma folate | Plasma folate2 | RBC folate | Plasma vitamin B-12 | Plasma Hcy | Plasma Hcy3 | Plasma Cys | Plasma Cys3 | Serum CRP | SAA | |
| Nutritional biomarkers | |||||||||||
| Plasma PLP | 0.49*** | 0.38*** | 0.41*** | 0.45*** | −0.34*** | −0.05 | 0.13*** | 0.10** | −0.22*** | −0.12*** | |
| Plasma folate | 0.59*** | 0.40*** | −0.43*** | N/A | 0.11*** | N/A | 0.01 | 0.03 | |||
| Plasma folate2 | 0.51*** | N/A | −0.30*** | N/A | 0.11*** | N/A | 0.001 | 0.02 | |||
| RBC folate | 0.38*** | −0.37*** | −0.10*** | 0.05 | −0.008 | 0.05 | 0.06 | ||||
| Plasma vitamin B-12 | −0.46*** | N/A | 0.03 | N/A | 0.03 | 0.03 | |||||
| Integrated markers of one-carbon status | |||||||||||
| Plasma Hcy | 0.38*** | 0.50*** | 0.003 | 0.03 | |||||||
| Plasma Hcy3 | 0.50*** | 0.50*** | 0.02 | 0.06 | |||||||
| Plasma Cys | −0.10*** | −0.02 | |||||||||
| Plasma Cys3 | −0.10*** | −0.02 | |||||||||
| Inflammation biomarkers | |||||||||||
| Serum CRP | 0.46*** | ||||||||||
**P < 0.01, ***P < 0.001. CRP, C-reactive protein; Cys, cysteine; Hcy, homocysteine; N/A, not applicable; PLP, pyridoxal-5′-phosphate; SAA, serum amyloid A.
Adjusted for plasma vitamin B-12.
Adjusted for plasma folate and plasma vitamin B-12.
FIGURE 2.
(A) Pathway analysis in the spring format for nutritional, inflammatory, and integrated biomarkers of one-carbon metabolism. All correlations are partial between each pair of nodes (adjusted for baseline BMI, age). Blue indicates a positive correlation and orange indicates a negative correlation. The weight of the line indicates the strength of the correlation. Pathway analyses for the prefortification (B), perifortification (C), and postfortification (D) periods. B12, plasma vitamin B-12; CRP, serum C-reactive protein; Cys, plasma cysteine; Fol, plasma folate; Hcy, plasma homocysteine; PLP, pyridoxal-5′-phosphate; SAA, serum amyloid A.
We evaluated the nutritional markers, and plasma PLP showed significant positive correlations with all of the other nutritional markers, i.e., with plasma folate (ρ = 0.49, P < 0.0001), RBC folate (ρ = 0.41, P < 0.0001), and vitamin B-12 (ρ = 0.45, P < 0.0001). Furthermore, plasma folate was strongly correlated with RBC folate (ρ = 0.59, P < 0.0001) and moderately with vitamin B-12 (ρ = 0.40, P < 0.0001); RBC folate was also correlated with vitamin B-12 (ρ = 0.38, P < 0.0001).
With regard to the markers of one-carbon metabolism and their association with nutritional biomarkers, homocysteine was inversely correlated with all nutritional biomarkers measured in this study [ranging in correlation coefficients from ρ = −0.46 (P < 0.0001) for vitamin B-12 to ρ = −0.30 (P < 0.0001) for plasma folate adjusted for plasma vitamin B-12]. Cysteine was moderately positively correlated with plasma PLP (ρ = 0.13, P < 0.0001) and plasma folate (ρ = 0.11, P = 0.002).
We investigated the inflammatory biomarkers serum CRP and SAA as outcomes in univariate models, and plasma PLP was the only nutritional one-carbon metabolism biomarker to show a modest inverse correlation with both serum CRP and SAA. Although homocysteine was not significantly correlated with serum CRP and SAA, cysteine showed significant inverse correlations with serum CRP (ρ = −0.10, P = 0.002), although not with SAA.
The stratified results by BMI and by multivitamin use showed no differences in correlation (Supplemental Table 1) When stratifying by fortification period (Table 3), the strengths of correlations between plasma PLP and homocysteine, cysteine, RBC folate, and plasma folate decreased from the prefortification to the postfortification period. We additionally stratified the results by serum CRP (≤, >3 mg/L) and homocysteine (≤, >12 μmol/L) (Supplemental Tables 2 and 3). The stratification by serum CRP showed differences in the correlation strength between plasma PLP and plasma vitamin B-12 (P = 0.06), with a stronger correlation in the serum CRP ≤3 mg/L category.
TABLE 3.
Spearman correlation matrix of biomarkers in postmenopausal women, stratified by pre-, peri-, and postfortification status and adjusted for urine creatinine, age, and BMI1
| Plasma Hcy | Plasma Cys | Serum CRP | SAA | Plasma vitamin B-12 | RBC folate | Plasma folate | |||||||||||||||
| Pre | Peri | Post | Pre | Peri | Post | Pre | Peri | Post | Pre | Peri | Post | Pre | Peri | Post | Pre | Peri | Post | Pre | Peri | Post | |
| Plasma PLP | −0.26*** | −0.37*** | −0.31*** | 0.16** | 0.13** | 0.12 | −0.17** | −0.20*** | −0.33***2 | −0.08 | −0.12** | −0.16*** | 0.42*** | 0.48*** | 0.43*** | 0.41*** | 0.45*** | 0.34*** | 0.51*** | 0.52*** | 0.42*** |
| Plasma folate | −0.41*** | −0.47*** | −0.35*** | 0.12* | 0.10* | 0.18*** | 0.05 | 0.007 | −0.06 | 0.06 | 0.03 | −0.04 | 0.49*** | 0.39*** | 0.35*** | 0.58*** | 0.62*** | 0.40*** | |||
| RBC folate | −0.37*** | −0.42*** | −0.24*** | 0.08 | 0.05 | 0.04 | 0.007 | 0.07 | 0.0001 | 0.05 | 0.08* | 0.002 | 0.45*** | 0.41*** | 0.26*** | ||||||
| Plasma vitamin B-12 | −0.44*** | −0.50*** | −0.37*** | 0.04 | 0.02 | 0.05 | 0.15* | 0.03 | −0.10 | 0.07 | 0.03 | −0.05 | |||||||||
| SAA | −0.006 | 0.02 | 0.06 | 0.005 | −0.04 | 0.04 | 0.48*** | 0.45*** | 0.45*** | ||||||||||||
| Serum CRP | −0.02 | −0.03 | 0.12 | −0.06 | −0.11** | −0.08 | |||||||||||||||
| Plasma Cys | 0.40*** | 0.36*** | 0.38*** | ||||||||||||||||||
Fortification periods: recruitment prefortification before 1996, perifortification between 1996 and 1997, and postfortification in 1998. *P ≤ 0.05, **P < 0.01, ***P < 0.001. CRP, C-reactive protein; Cys, cysteine; Hcy, homocysteine; peri, perifortification; PLP, pyridoxal-5′-phosphate; post, postfortificiation; pre, prefortification; SAA, serum amyloid A.
Two-tailed P-value between pre- and postfortification periods = 0.07.
However, the correlation between the nutritional biomarker plasma PLP and the inflammatory marker serum CRP increased in strength from ρ = −0.17 prefortification to ρ = −0.33 postfortification (prefortification × postfortification P-interaction = 0.07). Correlations were attenuated between 1) plasma and RBC folate, 2) plasma folate and vitamin B-12, and 3)plasma folate and homocysteine, across the periods with increasing folic acid fortification.
Linear regression analyses were performed, regressing nutritional and one-carbon metabolism biomarkers in 1 model predicting the inflammatory biomarkers serum CRP (mg/L) and SAA (mg/L), controlling jointly for age and BMI (Table 4). Significant results similar to the correlation analysis were observed when serum CRP (mg/L) and SAA (mg/L) were regressed on plasma PLP (nmol/L) (adjusted regression coefficient β = −1.46 and −1.04, respectively; P < 0.0001), after adjusting for all biomarkers. Furthermore, there were significant increases in log CRP by 0.32 mg/L (P < 0.0001) and log SAA by 0.25 mg/L (P = 0.001) for every 1-g/L increase in RBC folate. Also, vitamin B-12 (pg/mL) was inversely significantly associated with SAA (β: 0.23, P = 0.002), but not with CRP, in these multivariate models. Last, homocysteine (μmol/L) was significantly associated with both serum CRP (β: 21.04, P = 0.002) and SAA (β: 26.61, P < 0.0001). Cysteine (μmol/L) was significantly associated with serum CRP but not SAA.
TABLE 4.
Linear regression models predicting inflammatory biomarkers in postmenopausal women, adjusted for age and BMI1
| Serum CRP (mg/L) | SAA (mg/L) | |||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| β | P value | β | P value | β | P value | β | P value | |
| Nutritional biomarker | ||||||||
| Plasma PLP (nmol/L) | −1.47 | <0.0001 | −1.46 | <0.0001 | −0.91 | <0.0001 | −1.04 | <0.0001 |
| Plasma vitamin B-12 (pg/mL) | 0.05 | 0.49 | 0.10 | 0.19 | 0.14 | 0.04 | 0.23 | 0.002 |
| RBC folate (μg/L) | 0.25 | 0.0004 | 0.32 | <0.0001 | 0.17 | 0.02 | 0.25 | 0.001 |
| Plasma folate (μg/L) | 1.44 | 0.28 | 2.50 | 0.08 | 0.46 | 0.72 | 1.64 | 0.25 |
| Integrated marker of one-carbon status | ||||||||
| Plasma homocysteine (μmol/L) | 14.32 | 0.02 | 21.04 | 0.002 | 16.81 | 0.004 | 26.61 | <0.0001 |
| Plasma cysteine (μmol/L) | −2.19 | <0.0001 | −2.07 | <0.0001 | −0.70 | 0.14 | −0.87 | 0.08 |
n = 1639. CRP, C-reactive protein; PLP, pyridoxal-5′-phosphate; SAA, serum amyloid A.
Discussion
In this cross-sectional study we explored the associations between nutritional biomarkers, integrated markers of one-carbon metabolism, and biomarkers of inflammation among women enrolled in the WHI-OS.
As expected, all nutritional markers interrcorrelated positively. Furthermore, as expected from previous studies, homocysteine was inversely correlated with all nutritional biomarkers measured, because the compound is an integral part of the transmethylation and transsulfuration pathways, which are dependent on B vitamins.
An interesting finding is that plasma PLP was the only nutritional biomarker to show significant inverse correlations with both serum CRP and SAA in the univariate analysis; and importantly, this association remained consistent when stratifying by multivitamin use and grew stronger in the postfortification period. Furthermore, the multivariate analysis showed significant correlations for plasma PLP and the inflammatory markers. SAA and CRP are nonspecific hepatic inflammatory markers produced in response to infection, trauma, and other inflammatory states. In an acute phase, serum concentrations increase and slowly return to normal values within days, whereas persistent elevation occurs with chronic inflammatory states, including obesity (29, 30). In other work within this study population, increased concentrations of CRP and SAA were associated with increased risk of developing colorectal cancer (23). Low plasma PLP concentrations could lead to an early inflammatory response, characterized by increased serum CRP and SAA concentrations. Interestingly, SAA concentrations predicted overall survival (more strongly than CRP) in a prospective study in 807 breast cancer patients (31). To our knowledge, no previous studies have investigated correlations between plasma PLP and SAA.
The inverse association between plasma PLP and serum CRP reported here has been noted in previous studies in healthy populations and in connection with disease conditions. For example, the Framingham Heart Study showed that low plasma PLP was associated with higher CRP concentrations when 891 participants were divided into normal and high baseline CRP concentrations (32). Higher PLP quartiles were significantly associated with lower CRP concentrations (P-trend < 0.0001) in the Boston Puerto Rican Health Study, with a strong dose-response relation (33). The 2003–2004 NHANES reported that, among 2686 participants, plasma PLP was inversely related to serum CRP, independent of dietary PLP intake (P < 0.001) (34). An additional population-based study in 1320 participants by Gori et al. (35) showed a similar association for serum CRP and plasma PLP in both men and women. Some other groups did not report an inverse relation between PLP and CRP. For example, a study on B-vitamin status and inflammatory markers recorded no associations between plasma PLP and CRP concentrations in 519 healthy middle-aged men and women (36). In summary, there is evidence for an inverse relation between PLP and CRP, but it is not entirely reproducible in all study populations. Our work extends this further to a novel association with SAA, and to stronger correlations in the post- vs. prefortification period, as discussed below.
Actual folate intake of the cohort has been published previously. In this population, the mean dietary folate intake was 234 μg/d and the mean supplemental folic acid intake was 204 μg/d. Dietary folate intake was associated with an increased colorectal cancer risk among women who had experienced the initiation of folic acid fortification (P-interaction < 0.01) (27).
Correlations between plasma folate, RBC folate, and plasma vitamin B-12 and the inflammatory markers were not significant in the univariate analysis. In the multivariate model, however, RBC folate showed a significantly positive association with CRP and SAA, although less robust than the findings for PLP. This finding is surprising and may not have been observed previously, because other studies investigated folate biomarkers without adjusting for other biomarkers. Folic acid intake has increased in the United States since fortification started in 1996. Several studies have been conducted to clarify a potential link between folic acid supplementation and disease. Meta-analyses evaluating the effects of folic acid supplementation on cancer incidence and cardiovascular disease showed no significant association, with the exception of a slight beneficial effect of folic acid in stroke prevention (37, 38). Not all of the studies in the meta-analysis concurred; some of them accounted for folic acid fortification in some of the populations, and some did not.
Although substantial efforts have been undertaken to identify a connection between folic acid fortification and disease, it seems that no studies have been published regarding associations between higher folate status and elevated inflammatory markers (CRP and SAA). It is a caveat, however, that the observed expected inverse associations between RBC folate and homocysteine do not fit with the observed positive associations of RBC folate with serum CRP and SAA. Overall, it seems to be an appropriate next step to analyze these general correlations further and evaluate biologic mechanisms. Along these lines, we note that the antifolate drug, methotrexate, is used to treat rheumatoid arthritis, suggesting the anti-inflammatory effect of the drug is triggered by the inhibition of dihydrofolate reductase and consequent diminishing of de novo synthesis of purines and pyrimidines (39). This is consistent with our finding that folate and inflammation are directly correlated.
There is continued debate over folic acid supplementation and health effects (40). We observed differences in associations depending on fortification period. The inverse correlations between plasma PLP and the inflammatory markers serum CRP and SAA were stronger postfortification compared with the prefortification period. A similar pattern was observed for plasma PLP and SAA. This is a novel finding, which led us to the hypothesis that with a higher availability of folate, more plasma PLP is necessary for reactions in one-carbon metabolism that are linked to inflammatory processes.
Although serum CRP has been described as a general inflammatory marker, homocysteine is known as a modest predictor for coronary heart disease, a condition known to be associated with a chronic inflammatory process (41). As expected, we observed a positive association between homocysteine and serum CRP in our study population in the multivariate analyses. The univariate analysis correlations were not significant. Homocysteine showed similar results with SAA; the univariate analysis was nonsignificant, whereas the multivariate analysis presented a strong correlation. Furthermore, the significant inverse association between cysteine and CRP in the univariate and the multivariate analysis is an interesting finding, because cysteine, a partially essential amino acid, is synthesized in the transsulfuration pathway from homocysteine and serine and is required for glutathione synthesis. Glutathione plays a potential role in the etiology of multiple diseases including cancer, because it is an important antioxidant that mitigates oxidative stress (42, 43). In our analyses, cysteine also correlated with plasma PLP. This correlation was not surprising because PLP is a coenzyme for both cystathionine β-synthase and cystathionine γ-lyase, which are essential for the de novo synthesis of cysteine (44).
A key strength of this study is the large sample size. In addition, we investigated a fairly comprehensive set of nutritional and one-carbon biomarkers, and for the first time in this context the inflammatory marker SAA. Because of the design of the WHI-OS, we were able to investigate trends in associations across the various stages of folic acid fortification. A limitation may be the lack of generalizability of the results presented; the work is representative for women but not for men or the population at large. Potentially, some of the women in the study had preclinical disease at the time of recruitment, and biomarker measurements were only available at 1 time point. Additionally, indicators of kidney function were not assessed, which can be determinants of homocysteine and cysteine metabolism in women. Furthermore, the cross-sectional design of the study is a limitation; reverse causation cannot be ruled out.
In conclusion, biomarkers of inflammation were associated with the nutritional markers plasma PLP and RBC folate as well as with integrated indicators of one-carbon status (homocysteine and cysteine). These results suggest that the identification of biomarker patterns may provide a step forward in understanding links between inflammatory processes, nutritional status, and certain diseases characterized by chronic inflammatory reactions, such as cancer. Mechanistic studies analyzing the pathways behind the presented correlations are needed to further describe mechanisms and to establish causal relations.
Supplementary Material
Acknowledgments
The authors thank key investigators contributing to the WHI-OS, specifically—Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD): Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA): Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC), Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA), Rebecca Jackson (The Ohio State University, Columbus, OH), Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ), Jean Wactawski-Wende (University at Buffalo, Buffalo, NY), Marian Limacher (University of Florida, Gainesville/Jacksonville, FL), Robert Wallace (University of Iowa, Iowa City/Davenport, IA), Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); and Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); Women’s Health Initiative Memory Study (Wake Forest University School of Medicine, Winston-Salem, NC): Sally Shumaker. The authors also thank Katherine Howes for her contribution to measurements of homocysteine, cysteine, PLP, and RBC folate, Pamela Yang for measurements of plasma folate and vitamin B-12, and Rachel Galbraith for research assistance with the WOMIn Study. J.W.M., S.A.A.B., R.G., M.L.N., X.S., E.C.B., T.-Y.D.C., M.H.W., Y.Z., L.V.H., A.T.T., K.W.M., L.B.B., D.R.M., J.E.M., and C.M.U. designed and conducted the research; E.C.B., R.G., and Y.Z. analyzed data, and performed statistical analysis; C.A. wrote the draft; and C.M.U. and C.A. had primary responsibility for the final content. All authors critically reviewed, read, and approved the final version of the manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; PLP, pyridoxal-5′-phosphate; SAA, serum amyloid A; WHI-OS, Women’s Health Initiative Observational Study.
Literature Cited
- 1.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132 Suppl:2350S–5S. [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44:10–25. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich CM, Reed MC, Nijhout HF. Modeling folate, one-carbon metabolism, and DNA methylation. Nutr Rev. 2008;66 Suppl 1:S27–30. [DOI] [PubMed] [Google Scholar]
- 4.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomark Prev. 2004;13:511–9. [PubMed] [Google Scholar]
- 5.Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr. 2000;72:998–1003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XH, Ma J, Smith-Warner SA, Lee JE, Giovannucci E. Vitamin B6 and colorectal cancer: current evidence and future directions. World J Gastroenterol. 2013;19:1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22. [DOI] [PubMed] [Google Scholar]
- 8.Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, Sijben J, Groenendijk M, Stijnen T. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimers Dement. 2013 Oct 18. [DOI] [PubMed] [Google Scholar]
- 9.Hooshmand B, Polvikoski T, Kivipelto M, Tanskanen M, Myllykangas L, Erkinjuntti T, Makela M, Oinas M, Paetau A, Scheltens P, et al. Plasma homocysteine, Alzheimer and cerebrovascular pathology: a population-based autopsy study. Brain. 2013. Sep;136:2707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JM, Kim SW, Shin IS, Yang SJ, Park WY, Kim SJ, Shin HY, Yoon JS. Folate, vitamin B(12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry Investig. 2008;5:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr. 2002;75:908–13. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–94. [DOI] [PubMed] [Google Scholar]
- 14.Chiang EP, Bagley PJ, Roubenoff R, Nadeau M, Selhub J. Plasma pyridoxal 5′-phosphate concentration is correlated with functional vitamin B-6 indices in patients with rheumatoid arthritis and marginal vitamin B-6 status. J Nutr. 2003;133:1056–9. [DOI] [PubMed] [Google Scholar]
- 15.Chiang EP, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283–7. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Roubenoff RA, Selhub J, Nadeau MR, Cannon JG, Freeman LM, Dinarello CA, Rosenberg IH. Abnormal vitamin B6 status in rheumatoid cachexia: association with spontaneous tumor necrosis factor alpha production and markers of inflammation. Arthritis Rheum. 1995;38:105–9. [DOI] [PubMed] [Google Scholar]
- 17.Schernthaner GH, Plank C, Minar E, Bieglmayer C, Koppensteiner R, Schernthaner G. No effect of homocysteine-lowering therapy on vascular inflammation and haemostasis in peripheral arterial occlusive disease. Eur J Clin Invest. 2006;36:333–9. [DOI] [PubMed] [Google Scholar]
- 18.Koutroubakis IE, Dilaveraki E, Vlachonikolis IG, Vardas E, Vrentzos G, Ganotakis E, Mouzas IA, Gravanis A, Emmanouel D, Kouroumalis EA. Hyperhomocysteinemia in Greek patients with inflammatory bowel disease. Dig Dis Sci. 2000;45:2347–51. [DOI] [PubMed] [Google Scholar]
- 19.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000;7:64–9. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33. [DOI] [PubMed] [Google Scholar]
- 21.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–6. [DOI] [PubMed] [Google Scholar]
- 22.Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998:61–109. [DOI] [PubMed] [Google Scholar]
- 23.Toriola AT, Cheng TY, Neuhouser ML, Wener MH, Zheng Y, Brown E, Miller JW, Song X, Beresford SA, Gunter MJ, et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int J Cancer. 2013;132:2648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. [DOI] [PubMed] [Google Scholar]
- 25.Talwar D, Quasim T, McMillan DC, Kinsella J, Williamson C, O'Reilly DS. Optimisation and validation of a sensitive high-performance liquid chromatography assay for routine measurement of pyridoxal 5-phosphate in human plasma and red cells using pre-column semicarbazide derivatisation. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;792:333–43. [DOI] [PubMed] [Google Scholar]
- 26.Gilfix BM, Blank DW, Rosenblatt DS. Novel reductant for determination of total plasma homocysteine. Clin Chem. 1997;43:687–8. [PubMed] [Google Scholar]
- 27.Zschäbitz S, Cheng TY, Neuhouser ML, Zheng Y, Ray RM, Miller JW, Song X, Maneval DR, Beresford SA, Lane D, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women's Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epskamp S, Lourens J, Waldorp VD, Schmittmann DB. qgraph: Network visualizations of relationships in psychometric data. J Stat Softw. 2012;48:1–18. [Google Scholar]
- 29.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 30.MacGregor AJ, Gallimore JR, Spector TD, Pepys MB. Genetic effects on baseline values of C-reactive protein and serum amyloid a protein: a comparison of monozygotic and dizygotic twins. Clin Chem. 2004;50:130–4. [DOI] [PubMed] [Google Scholar]
- 31.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation. 2001;103:2788–91. [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gori AM, Sofi F, Corsi AM, Gazzini A, Sestini I, Lauretani F, Bandinelli S, Gensini GF, Ferrucci L, Abbate R. Predictors of vitamin B6 and folate concentrations in older persons: the InCHIANTI study. Clin Chem. 2006;52:1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folsom AR, Desvarieux M, Nieto FJ, Boland LL, Ballantyne CM, Chambless LE. B vitamin status and inflammatory markers. Atherosclerosis. 2003;169:169–74. [DOI] [PubMed] [Google Scholar]
- 37.Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J, Manson JE, Hankey GJ, Spence JD, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang HT, Lee M, Hong KS, Ovbiagele B, Saver JL. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. Eur J Intern Med. 2012;23:745–54. [DOI] [PubMed] [Google Scholar]
- 39.Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, Garner RC, Steele RJ, Wolf CR. Mutations in APC, Kirsten-ras, and p53—alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci USA. 2002;99:9433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JW, Ulrich CM. Folic acid and cancer—where are we today? Lancet. 2013;381:974–6. [DOI] [PubMed] [Google Scholar]
- 41.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 42.Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–11. [DOI] [PubMed] [Google Scholar]
- 43.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. [DOI] [PubMed] [Google Scholar]
- 44.Bailey LB. Folate in health and disease. 2nd ed. Boca Raton (FL): Taylor and Francis Group; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.