Abstract
Knowledge of stability during sample transportation and changes in biomarker concentrations within person over time are paramount for proper design and interpretation of epidemiologic studies based on a single measurement of biomarker status. Therefore, we investigated stability and intraindividual vs. interindividual variation in blood concentrations of biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway. Whole blood (EDTA and heparin, n = 12) was stored with an icepack for 24 or 48 h, and plasma concentrations of 38 biomarkers were determined. Stability was calculated as change per hour, intraclass correlation coefficient (ICC), and simple Spearman correlation. Within-person reproducibility of biomarkers was expressed as ICC in samples collected 1–2 y apart from 40 postmenopausal women and in samples collected up to 3 y apart from 551 patients with stable angina pectoris. Biomarker stability was similar in EDTA and heparin blood. Most biomarkers were essentially stable, except for choline and total homocysteine (tHcy), which increased markedly. Within-person reproducibility in postmenopausal women was excellent (ICC > 0.75) for cotinine, all-trans retinol, cobalamin, riboflavin, α-tocopherol, Gly, pyridoxal, methylmalonic acid, creatinine, pyridoxal 5′-phosphate, and Ser; was good to fair (ICC of 0.74–0.40) for pyridoxic acid, kynurenine, tHcy, cholecalciferol, flavin mononucleotide, kynurenic acid, xanthurenic acid, 3-hydroxykynurenine, sarcosine, anthranilic acid, cystathionine, homoarginine, 3-hydroxyanthranilic acid, betaine, Arg, folate, total cysteine, dimethylglycine, asymmetric dimethylarginine, neopterin, symmetric dimethylarginine, and Trp; and poor (ICC of 0.39–0.15) for methionine sulfoxide, Met, choline, and trimethyllysine. Similar reproducibilities were observed in patients with coronary heart disease. Thus, most biomarkers investigated were essentially stable in cooled whole blood for up to 48 h and had a sufficient within-person reproducibility to allow one-exposure assessment of biomarker status in epidemiologic studies. The Western Norway B Vitamin Intervention Trial was registered at clinicaltrials.gov as NTC00354081.
Introduction
Modern analytical technologies, in particular methods based on MS, allow accurate and precise measurement of a large number of biomarkers in various matrices, including serum and plasma. In addition, multiplexing capabilities, high-throughput, and low-sample volume consumption of methods based on MS open new research possibilities for large epidemiologic studies of disease risk using precious specimens stored in biobanks (1–3).
Most prospective cohort studies collected 1 blood sample for each individual and thus rely on a single measurement to obtain biomarker status. Therefore, it is critical that the within-person variance in biomarker concentrations caused by sample handling and storage, as well as natural fluctuations, is small relative to the between-person variance. This reliability can be expressed as the intraclass correlation coefficient (ICC),11 which is the ratio of within-person variance/total variance (4); therefore, ICC also accounts for preanalytical and analytical variance components.
For large biobanks, centralized sample processing is recommended to reduce cost and secure uniform and optimal sample handling (5). This procedure implies transportation of whole blood to the central facility responsible for separation of serum/plasma and subsequent storage at low temperature. Many but not all clinical chemistry parameters are essentially stable during transportation at 4°C for up to 36 h (6), but such stability has to be investigated for each individual analyte to assess potential bias in biomarker studies caused by preanalytical variability. Conversely, storage is a minor source of error and bias, because a range of analytes, including labile molecules, are stable when stored under conditions currently recommended (i.e., at −80°C or below) (5).
We investigated the stability of 38 biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway in whole-blood samples from 12 men and women for up to 48 h under conditions mimicking those of transportation to the central biobank. We also assessed the reproducibility over a period of 1–2 y of the same biomarkers among 40 postmenopausal women enrolled in the Nurses’ Health Study (NHS). Because biomarker concentrations may vary according to lifestyle, nutritional, and clinical status, we also assessed the reproducibility of most biomarkers over 38 mo among 551 patients who had undergone coronary angiography for suspected coronary artery disease.
Participants and Methods
Study populations and sample collection.
Analyte stability was assessed from blood samples collected in EDTA and sodium heparin Vacutainers from 12 healthy volunteers (50% male) aged 24–56 y (median of 36 y). Samples from each individual were collected in 3 Vacutainers. The first sample was centrifuged immediately and then divided into aliquots and stored in a liquid nitrogen freezer (≤ −130°C). The second and third samples were stored with ice packs in Styrofoam containers for 24 and 48 h, respectively, before being processed and frozen.
We investigated the within-person reproducibility over time among postmenopausal women, aged 51–68 y (median of 62 y), enrolled in the NHS. The NHS is an ongoing cohort study that began in 1976 with 121,700 female registered nurses (7). Details about the blood collections in the NHS have been published previously (8). Briefly, in 1989–1990, 32,836 participants arranged to have their blood collected in 2 15-mL sodium heparin tubes. The tubes were placed in Styrofoam containers with an icepack (temperature of ∼4°C) and shipped via overnight mail to the central laboratory, where plasma was immediately separated and stored in liquid nitrogen freezers. A subset of 390 postmenopausal participants who returned a blood sample in 1989–1990 provided 2 additional blood samples over the following 1–2 y. For the current investigation, we randomly selected 40 women who provided at least 2 blood samples, each donated after fasting at least 8 h. The NHS was approved by the Committee on the Use of Human Subjects in Research at Harvard School of Public Health and Brigham and Women’s Hospital.
Within-person reproducibility was also investigated in a subset of patients enrolled in the Western Norway B Vitamin Intervention Trial (WENBIT) study. The patients had undergone coronary angiography for suspected coronary artery disease between 1999 and 2004. Details of the WENBIT study have been published previously (9). For the current investigation, we selected patients (n = 633) with stable angina recruited at the Haukeland University Hospitals in Bergen, Norway, who were allocated to receiving placebo and had data at baseline and after 1 month, 1 y, and 38 mo of follow-up. Their median (range) age was 62.8 y (31.8–84.7 y), and 75.8% were males. The number of participants with analyte data at all 4 time points varied somewhat by analyte, ranging from 402 to 551. Whole blood was collected into EDTA Vacutainer tubes and immediately centrifuged, and EDTA plasma was stored within 30 min at −80°C until analysis. The WENBIT study was approved by the Regional Committee for Medical and Health Research Ethics, the Data Inspectorate, and the Norwegian Directorate of Health and is registered at clinicaltrials.gov as NCT00354081.
Laboratory analyses.
Riboflavin, flavin mononucleotide, pyridoxal 5′-phosphate (PLP), pyridoxal, pyridoxic acid, kynurenic acid, anthranilic acid, 3-hydroxykynurenine, xanthurenic acid, 3-hydroxyanthranilic acid (HAA), neopterin, cotinine (10), free choline, betaine, dimethylglycine, creatinine, methionine sulfoxide, Arg, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), trimethyllysine (11), all-trans retinol (vitamin A), cholecalciferol (25-hydroxyvitamin D-3; vitamin D), and α-tocopherol (vitamin E) (12) were determined by liquid chromatography (LC)-MS/MS, and methylmalonic acid (MMA), total homocysteine (tHcy), total cysteine, Met, Ser, Gly, cystathionine, sarcosine, Trp, and kynurenine were determined by GC-MS/MS (2, 13). The kynurenine/Trp ratio (KTR) was calculated by dividing the plasma concentration of kynurenine (in nanomoles per liter) by the concentration of Trp (in micromoles per liter). Cobalamin (vitamin B-12) (14) and folate (15) were determined by microbiologic methods. All analyses were performed in the laboratory of Bevital, and the laboratory staff was unaware of the sample identities.
Statistical analyses.
Biomarker concentrations in plasma are presented as geometric means with 95% CIs. We examined the within-day precision of the methods for each analyte using blinded replicate samples from 3 large quality-control plasma pools created using discarded plasma from blood donation centers (8 replicates from 1 EDTA plasma pool and 4 replicates each from 2 different heparin plasma pools). Precision was expressed as analytical CV, which in this context is defined as the SD in percentage of the mean. Specifically, we calculated the CV among samples from the same quality-control pool and then averaged CVs across the 3 pools. We calculated changes in concentrations during storage with an icepack after normalizing the value to percentage of the baseline (time 0) concentration, which was defined as 100%. Because storage effects were investigated at only 3 time points (0, 24, and 48 h), degradation and accumulation kinetics were not modeled according to exponential functions (16) or by segmented regression but were summarized in terms of percentage changes (SD) per hour as obtained by simple linear regression. Storage effects were also evaluated by calculating the ICCs, defined as the between-person variance divided by the total variance (4), across all 3 time points for each analyte, using ln-transformed values. To assess whether participant ranking by analyte concentration changed according to storage, correlation of values obtained at 24 and 48 h with baseline values were calculated using the Spearman test (17). Within-person reproducibility for each analyte was assessed by determination of ICC (95% CI) from repeated participant samples using ln-transformed values and a random-effects mixed model with participant identification as the random variable. An ICC < 0.40 is considered as poor reproducibility, 0.40–0.75 as fair-to-good reproducibility, and ≥0.75 as excellent reproducibility (4).
A sample size of 40 people with 2 samples per person provides excellent power to estimate relatively high ICCs (4), which are of most scientific interest, e.g., the confidence interval width will be less than ±0.2 for an ICC ≥ 0.65. However, this sample size provides only modest power to estimate lower ICCs, e.g., the CI width for an estimated ICC of 0.35 will be ±0.28, indicating that a true ICC as high as 0.63 is consistent with the data. Thus, low ICC estimates in the NHS population should be interpreted cautiously because they could reflect a chance finding of low between-person variability among the selected 40 participants.
Approximate estimates of the within- and between-person CVs were determined by taking the square root of the within- and between-person variance components from the random-effects mixed model on the ln-transformed scale (4). The program R version 2.15.1 (18) was used for statistical analyses, and the package “ICC” was used to calculate ICC.
Results and Discussion
Principal findings.
We investigated stability of 38 biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway in chilled whole blood for up to 48 h and within-person reproducibility of biomarkers over 1–2 y in healthy postmenopausal women (NHS) and over 38 mo (for 32 biomarkers) in patients with stable coronary heart disease (WENBIT study). Most biomarkers were essentially stable, although pyridoxal, choline, tHcy, Arg, and HAA (in EDTA plasma) changed >1% per hour during storage. Within-person reproducibilities obtained from the NHS samples was fair to excellent for most (34 of 38) biomarkers with an ICC ≥ 0.75 for PLP, pyridoxal, riboflavin, cobalamin, Ser, Gly, all-trans retinol, α-tocopherol, MMA, creatinine, and cotinine and an ICC < 0.40 for choline, Met, methionine sulfoxide, and trimethyllysine. Similar within-person reproducibilities were obtained from the WENBIT samples.
Stability.
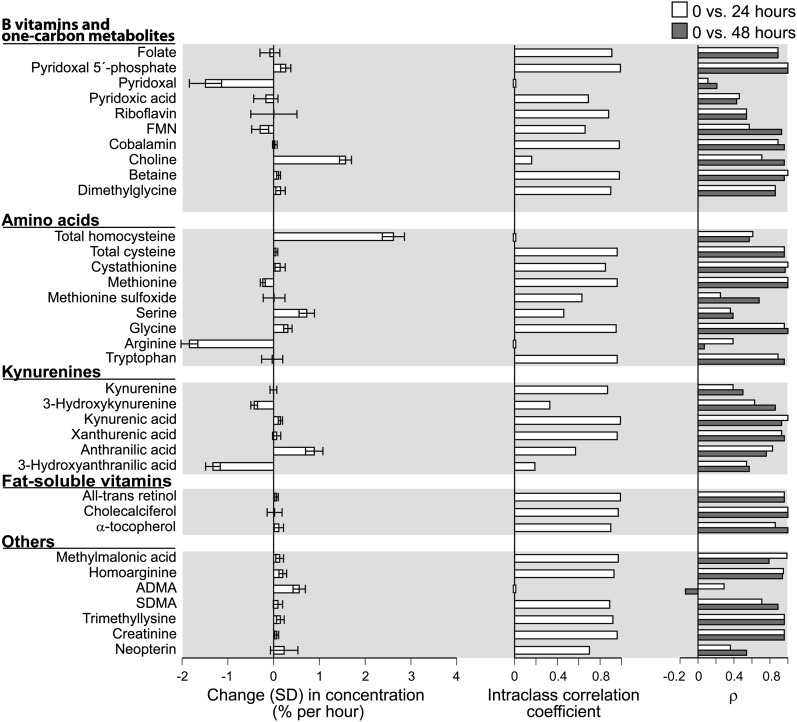
Among the biomarkers investigated, most were stable in cooled whole blood (i.e., change <1% per hour) (Fig. 1; Supplemental Fig. 1). The fat-soluble vitamins were stable in cooled blood (Fig. 1) as demonstrated previously for all-trans retinol and less so for α-tocopherol (19). tHcy and choline increased markedly, and for choline, the increase was more pronounced in heparin (Supplemental Fig. 1) than EDTA blood (Fig. 1). This is in agreement with published results and is explained by egress of homocysteine from intact blood cells (20) and enzymic conversion of phosphatidylcholine to free choline (21) catalyzed by phospholipase D, a calcium-dependent enzyme (22) inhibited by EDTA. Pyridoxal and Arg declined markedly, which for pyridoxal may reflect cellular uptake (23). HAA is known be unstable (24), and the concentration decreased markedly, in particular in the presence of EDTA. Otherwise, stability was similar in EDTA (Fig. 1) and heparin blood (Supplemental Fig. 1).
FIGURE 1.
Stability of biomarkers in EDTA blood stored in a Styrofoam container with an icepack for 24 or 48 h. Biomarkers (pyridoxal, choline, total homocysteine, Arg, and hydroxyanthranilic acid) that changed substantially over time (left panel) had a low intraclass correlation coefficient (<0.2) across the storage time (middle panel), but for some (choline and total homocysteine), concentrations at 24 or 48 h still showed a fair-to-good (ρ > 0.4) correlation with values at time 0 (right panel). This reflects a parallel increase in concentrations over time and implies that the ranking of the values at fixed time points was maintained. ADMA decreased moderately during storage, but both intraclass correlation coefficient and ranking were low, which is explained by the low between-person variability for ADMA. n = 12. ADMA, asymmetric dimethylarginine; FMN, flavin mononucleotide; SDMA, symmetric dimethylarginine.
Unstable biomarkers (choline, tHcy, Arg) had low ICC (<0.1) across storage time from 0 to 48 h. However, for choline and tHcy in particular, values at 24 and 48 h correlated well (ρ ≥ 0.5) with values at baseline (Fig. 1; Supplemental Fig. 1). This is explained by a parallel increase in blood concentrations as a function of time across samples (data not shown) and implies that participants were equally ranked by concentrations of these biomarkers in samples subjected to similar processing, although the absolute value of the biomarker changed markedly after a 24–48 h delay in processing. If there are variable transportation time and temperatures of whole blood in a study, this may bias results involving choline and tHcy.
We previously investigated stability in isolated sera and plasmas at room temperature over days or during storage at −20°C for decades for most biomarkers included in the present study (25). In general, most biomarkers that were stable under these conditions are also stable in chilled whole blood, but there are notable exceptions (summarized in Supplemental Fig. 2). The rapid degradation of folate in EDTA plasma (26) was not observed in cold EDTA blood (Fig. 1; Supplemental Fig. 1). Furthermore, PLP is dephosphorylated to pyridoxal in serum, citrate plasma, and heparin plasma but not EDTA plasma at room temperature and in frozen serum, leading to a concurrent increase in pyridoxal (25). However, in cold blood, PLP was stable and pyridoxal decreasing on storage (Fig. 1; Supplemental Fig. 1). tHcy is stable in serum and plasma, and Arg is stable in plasma and slightly increasing in serum (25) in contrast to the marked changes in these biomarkers observed in whole blood, which probably reflect cellular transport (20). The marked decrease in Met and its recovery as methionine sulfoxide that occur in serum samples during prolonged storage at −20°C was not observed in serum, plasma at room temperature (25), or whole blood (Fig. 1; Supplemental Fig. 1).
Within-person reproducibility in NHS participants.
The quality control CVs, medians, and Spearman correlations of values at 2 visits, within-person and between-person CVs (percentage), and ICCs for the 38 biomarkers measured in 40 NHS samples are listed in Table 1. The within-person reproducibility over 1–2 y was excellent (ICC range of 0.95–0.75) for the following biomarkers (in decreasing order of ICCs): cotinine, all-trans retinol, cobalamin, riboflavin, α-tocopherol, Gly, pyridoxal, MMA, creatinine, PLP, and Ser. Within-person reproducibility was good to fair (ICC range of 0.74–0.40) for pyridoxic acid, kynurenine, tHcy, vitamin D, flavin mononucleotide, kynurenic acid, xanthurenic acid, 3-hydroxykynurenine, sarcosine, KTR, anthranilic acid, cystathionine, homoarginine, HAA, betaine, Arg, folate, total cysteine, dimethylglycine, ADMA, neopterin, SDMA, and Trp and was poor (ICC range of 0.39–0.15) for methionine sulfoxide, Met, choline, and trimethyllysine (Table 1).
TABLE 1.
Concentrations and within-person reproducibility of biomarkers in plasma samples collected at 2 time points 1–2 y apart from 40 postmenopausal women in the Nurses’ Health Study1
| Geometric mean (95% CI) |
||||||||
| Biomarker | QC CV2 | First collection | Second collection | P3 | ρ | Within-person CV4 | Between-person CV4 | ICC (95% CI)5 |
| % | % | % | ||||||
| B vitamins and one-carbon metabolites | ||||||||
| Folate, nmol/L | 5.5 | 28.5 (22.5, 36.1) | 31.4 (24.5, 40.2) | 0.37 | 0.55 | 47.3 | 58.9 | 0.61 (0.37, 0.77) |
| Pyridoxal 5′-phosphate, nmol/L | 3.3 | 57.4 (45.0, 73.2) | 59.1 (46.4, 75.2) | 0.73 | 0.66 | 36.3 | 66.3 | 0.77 (0.61, 0.87) |
| Pyridoxal, nmol/L | 5.6 | 25.1 (19.2, 32.8) | 26.5 (20.7, 33.8) | 0.51 | 0.66 | 35.9 | 71.5 | 0.80 (0.65, 0.89) |
| Pyridoxic acid, nmol/L | 6.5 | 37.7 (29.2, 48.6) | 40.5 (32.3, 50.8) | 0.41 | 0.51 | 38.7 | 64.8 | 0.74 (0.56, 0.85) |
| Riboflavin, nmol/L | 7.4 | 26.2 (21.7, 31.5) | 28.5 (23.7, 34.2) | 0.08 | 0.82 | 21.3 | 54.0 | 0.87 (0.76, 0.93) |
| Flavin mononucleotide, nmol/L | 8.8 | 4.67 (4.11, 5.31) | 5.10 (4.56, 5.71) | 0.06 | 0.74 | 20.9 | 31.5 | 0.69 (0.49, 0.83) |
| Cobalamin, pmol/L | 2.7 | 469 (425, 518) | 461 (414, 514) | 0.53 | 0.87 | 11.6 | 30.2 | 0.87 (0.77, 0.93) |
| Choline, μmol/L | 3.7 | 16.4 (15.5, 17.4) | 15.3 (14.6, 16.2) | 0.05 | 0.33 | 14.8 | 9.4 | 0.29 (−0.02, 0.55)6 |
| Betaine, μmol/L | 4.1 | 36.1 (33.9, 38.5) | 36.4 (33.7, 39.3) | 0.82 | 0.64 | 13.5 | 17.5 | 0.63 (0.40, 0.79) |
| Dimethylglycine, μmol/L | 7.8 | 3.05 (2.78, 3.35) | 2.96 (2.73, 3.22) | 0.48 | 0.53 | 18.4 | 20.5 | 0.55 (0.30, 0.74) |
| Sarcosine, μmol/L | 4.6 | 1.09 (0.96, 1.25) | 1.13 (1.00, 1.26) | 0.57 | 0.73 | 21.9 | 32.0 | 0.68 (0.48, 0.82) |
| Amino acids | ||||||||
| Total homocysteine, μmol/L | 6.5 | 11.1 (10.2, 12.0) | 11.0 (10.1, 12.0) | 0.87 | 0.74 | 14.2 | 22.4 | 0.71 (0.52, 0.84) |
| Total cysteine, μmol/L | 5.7 | 325 (312, 338) | 330 (318, 344) | 0.34 | 0.49 | 8.1 | 9.2 | 0.56 (0.31, 0.74) |
| Cystathionine, μmol/L | 3.1 | 0.15 (0.14, 0.17) | 0.16 (0.14, 0.18) | 0.49 | 0.53 | 21.0 | 28.8 | 0.65 (0.43, 0.80) |
| Met, μmol/L | 1.9 | 25.4 (24.2, 26.6) | 26.1 (25.0, 27.2) | 0.30 | 0.31 | 11.1 | 8.4 | 0.36 (0.06, 0.60) |
| Methionine sulfoxide, μmol/L | 8.2 | 1.07 (0.99, 1.15) | 1.04 (0.96, 1.13) | 0.58 | 0.32 | 19.0 | 14.5 | 0.37 (0.07, 0.61) |
| Ser, μmol/L | 2.5 | 116 (110, 122) | 111 (105, 117) | 0.01 | 0.71 | 8.7 | 15.2 | 0.76 (0.59, 0.86) |
| Gly, μmol/L | 2.8 | 304 (280, 330) | 290 (267, 314) | 0.03 | 0.82 | 10.2 | 23.1 | 0.84 (0.71, 0.91) |
| Arg, μmol/L | 4.8 | 48.2 (41.5, 56.1) | 50.1 (44.1, 57.0) | 0.52 | 0.67 | 26.9 | 34.3 | 0.62 (0.39, 0.78) |
| Trp, μmol/L | 3.3 | 67.1 (64.6, 69.7) | 67.1 (64.0, 70.4) | 0.99 | 0.50 | 9.8 | 9.2 | 0.47 (0.19, 0.68) |
| Kynurenines | ||||||||
| Kynurenine, μmol/L | 2.2 | 1.55 (1.45, 1.67) | 1.59 (1.49, 1.69) | 0.43 | 0.70 | 11.5 | 18.0 | 0.71 (0.52, 0.84) |
| 3-Hydroxykynurenine, nmol/L | 3.7 | 34.2 (31.4, 37.2) | 36.0 (32.8, 39.6) | 0.14 | 0.65 | 15.8 | 23.2 | 0.68 (0.48, 0.82) |
| Kynurenic acid, nmol/L | 4.6 | 44.7 (38.8, 51.5) | 47.8 (42.0, 54.4) | 0.21 | 0.65 | 23.6 | 35.1 | 0.69 (0.49, 0.82) |
| Xanthurenic acid, nmol/L | 7.3 | 8.45 (6.97, 10.2) | 9.08 (7.63, 10.81) | 0.32 | 0.68 | 32.2 | 47.4 | 0.68 (0.48, 0.82) |
| Anthranilic acid, nmol/L | 4.8 | 15.7 (14.0, 17.7) | 17.0 (15.4, 18.8) | 0.07 | 0.63 | 19.8 | 28.2 | 0.67 (0.46, 0.81) |
| 3-Hydroxyanthralinic acid, nmol/L | 6.9 | 26.5 (23.8, 29.6) | 29.0 (26.0, 32.2) | 0.05 | 0.63 | 20.5 | 27.3 | 0.64 (0.42, 0.79) |
| Fat-soluble vitamins | ||||||||
| All-trans retinol, μmol/L | 3.8 | 2.30 (2.16, 2.45) | 2.32 (2.18, 2.45) | 0.62 | 0.82 | 6.8 | 17.7 | 0.87 (0.77, 0.93) |
| Cholecalciferol, nmol/L | 7.0 | 68.5 (62.9, 74.7) | 69.3 (63.6, 75.4) | 0.75 | 0.66 | 14.4 | 22.4 | 0.71 (0.51, 0.83) |
| α-Tocopherol, μmol/L | 2.4 | 35.6 (31.9, 39.6) | 37.7 (33.7, 42.1) | 0.04 | 0.75 | 12.7 | 32.1 | 0.86 (0.76, 0.93) |
| Others | ||||||||
| Methylmalonic acid, μmol/L | 4.9 | 0.16 (0.15, 0.18) | 0.17 (0.16, 0.18) | 0.11 | 0.78 | 12.0 | 22.9 | 0.79 (0.63, 0.88) |
| Homoarginine, μmol/L | 6.7 | 1.59 (1.41, 1.78) | 1.65 (1.47, 1.85) | 0.43 | 0.57 | 21.3 | 28.8 | 0.65 (0.43, 0.80) |
| ADMA, μmol/L | 7.2 | 0.61 (0.58, 0.63) | 0.61 (0.59, 0.64) | 0.69 | 0.47 | 8.6 | 9.5 | 0.55 (0.30, 0.74) |
| SDMA, μmol/L | 5.8 | 0.60 (0.58, 0.63) | 0.60 (0.58, 0.63) | 0.96 | 0.51 | 8.7 | 8.7 | 0.50 (0.23, 0.70) |
| Creatinine, μmol/L | 2.8 | 71.4 (67.8, 75.3) | 72.9 (69.5, 76.4) | 0.23 | 0.74 | 7.4 | 13.7 | 0.77 (0.62, 0.87) |
| Trimethyllysine, μmol/L | 6.3 | 0.63 (0.56, 0.72) | 0.65 (0.58, 0.73) | 0.81 | 0.38 | 34.7 | 14.6 | 0.15 (−0.16, 0.44)6 |
| KTR, nmol/μmol | 23.1 (21.6, 24.8) | 23.6 (21.9, 25.5) | 0.49 | 0.65 | 13.1 | 18.8 | 0.67 (0.47, 0.81) | |
| Neopterin, nmol/L | 6.8 | 15.3 (13.5, 17.3) | 16.7 (15.1, 18.5) | 0.11 | 0.53 | 24.6 | 25.7 | 0.52 (0.26, 0.71) |
| Cotinine,7 nmol/L | 2.2 | 2.21 (1.11, 4.40) | 2.48 (1.24, 4.98) | 0.27 | 0.44 | 46.9 | 211.4 | 0.95 (0.91, 0.97) |
ADMA, asymmetric dimethylarginine; ICC, intraclass correlation coefficient; KTR, kynurenine/Trp ratio; QC, quality control; SDMA, symmetric dimethylarginine.
From 16 blinded replicates from 3 quality-control plasma pools.
Paired t test comparing geometric mean at first vs. second blood collection.
Within- and between-person CVs were estimated by taking the square root of the within- and between-person variance components from random-effects mixed model on the ln-transformed scale (4).
Calculated using ln-transformed analyte values.
A lower value for 95% CI < 0 is often explained by the intraindividual variation being large compared with the interindividual variation and indicates that the computed ICC is not significantly different from 0.
Cotinine values below the detection limit (1 nmol/L) were set to 1.
The ICC values and Spearman correlation coefficients between the first and second collection showed similar variation across analytes (Supplemental Fig. 3). Furthermore, the ICC was 0.71 for tHcy and 0.29 for choline (Table 1), despite a substantial increase in both tHcy and choline during storage as also indicated by higher concentrations of tHcy and choline than expected (27) in healthy women. Together, these observations emphasize the advantage of uniform preanalytical sample handling, in particular for unstable analytes.
A few biomarkers, including total cysteine, Trp, ADMA, and SDMA, with low within-person CVs of <10% had only moderate ICCs (<0.6) because the between-person CV was low (Table 1). The low interindividual biologic variation of ADMA has been reported previously by others (28) who have emphasized the importance of accurate and precise analytical methods to obtain reproducible results that allow meta-analyses and implementation of ADMA in clinical diagnostics.
Within-person reproducibility in WENBIT participants.
The ICCs and the variance components for most analytes studied in NHS samples were also assessed among WENBIT participants (n = ∼550) donating samples at 4 visits for up to 38 mo (Table 2). The ICCs in the WENBIT were similar to those in the NHS, although somewhat lower for most B vitamins, amino acids, and kynurenines and equal or even higher than in NHS samples for choline, betaine, dimethylglycine, MMA, SDMA, creatinine, KTR, and neopterin (Supplemental Fig. 4). For choline, the relatively high ICC in the WENBIT probably reflects optimal sample handling (as indicated by a geometric mean for choline of ∼10 μmol/L) (Table 2). Higher prevalence of reduced renal function, cellular immune activation, metabolic syndrome, and coronary heart disease in WENBIT patients than in healthy NHS participants might have increased the between-person CVs for creatinine, SDMA (29), MMA (30), neopterin, KTR (31, 32), betaine (33), or dimethylglycine (34), thus increasing the ICC.
TABLE 2.
Concentrations and within-person reproducibility of biomarkers in plasma samples collected at 4 visits over 3.5 y from cardiovascular patients enrolled in the Western Norway B Vitamin Intervention Trial1
| Geometric mean (95% CI) |
|||||||
| Biomarker | Participants/time points | First visit | Last visit | P2 | Within-person CV3 | Between-person CV3 | ICC (95% CI)4 |
| n/n | % | % | |||||
| B vitamins and one-carbon metabolites | |||||||
| Folate, nmol/L | 551/4 | 10.6 (10.2, 11.0) | 11.8 (11.2, 12.4) | 0.0001 | 37.6 | 38.4 | 0.51 (0.47, 0.55) |
| Pyridoxal 5′-phosphate, nmol/L | 545/4 | 40.6 (39.1, 42.3) | 41.7 (40.0, 43.4) | 0.70 | 30.4 | 39.0 | 0.62 (0.59, 0.66) |
| Pyridoxal, nmol/L | 545/4 | 9.79 (9.43, 10.2) | 10.6 (10.2, 11.1) | 0.001 | 35.3 | 31.2 | 0.44 (0.40, 0.48) |
| Pyridoxic acid, nmol/L | 545/4 | 26.1 (25.2, 27.1) | 28.6 (27.3, 29.8) | 0.0001 | 36.7 | 31.3 | 0.42 (0.38, 0.47) |
| Riboflavin, nmol/L | 545/4 | 12.5 (11.8, 13.2) | 11.5 (10.8, 12.2) | 0.02 | 39.1 | 59.7 | 0.70 (0.67, 0.73) |
| Cobalamin, pmol/L | 551/4 | 334 (323, 345) | 328 (316, 340) | 0.06 | 19.9 | 36.7 | 0.77 (0.75, 0.80) |
| Choline, μmol/L | 551/4 | 9.52 (9.34, 9.70) | 10.5 (10.3, 10.7) | <0.0001 | 18.1 | 15.8 | 0.43 (0.39, 0.47) |
| Betaine, μmol/L | 551/4 | 38.2 (37.3, 39.2) | 41.3 (40.3, 42.2) | <0.0001 | 17.4 | 24.0 | 0.66 (0.62, 0.69) |
| Dimethylglycine, μmol/L | 551/4 | 4.09 (3.98, 4.20) | 4.27 (4.16, 4.38) | <0.0001 | 17.1 | 28.3 | 0.73 (0.70, 0.76) |
| Amino acids | |||||||
| Total homocysteine, μmol/L | 551/4 | 10.6 (10.4, 10.9) | 10.5 (10.3, 10.8) | 0.46 | 15.7 | 25.5 | 0.73 (0.70, 0.75) |
| Total cysteine, μmol/L | 551/4 | 289 (286, 292) | 291 (288, 294) | 0.02 | 7.2 | 10.5 | 0.68 (0.65, 0.71) |
| Cystathionine, μmol/L | 545/4 | 0.27 (0.26, 0.28) | 0.32 (0.30, 0.34) | <0.0001 | 39.3 | 48.2 | 0.60 (0.56, 0.64) |
| Met, μmol/L | 551/4 | 27.1 (26.5, 27.6) | 27.6 (27.1, 28.2) | 0.14 | 20.8 | 13.8 | 0.30 (0.26, 0.35) |
| Methionine sulfoxide, μmol/L | 551/4 | 0.94 (0.91, 0.97) | 0.99 (0.95, 1.02) | 0.21 | 36.0 | 26.0 | 0.34 (0.30, 0.39) |
| Ser, μmol/L | 477/4 | 93.2 (91.5, 95.0) | 92.4 (90.4, 94.3) | 0.13 | 14.1 | 19.5 | 0.66 (0.62, 0.69) |
| Gly, μmol/L | 551/4 | 202 (198, 206) | 204 (200, 208) | 0.32 | 11.8 | 21.4 | 0.77 (0.74, 0.79) |
| Arg, μmol/L | 551/4 | 44.8 (43.9, 45.7) | 45.9 (45.0, 46.8) | 0.03 | 19.7 | 17.1 | 0.43 (0.39, 0.47) |
| Trp, μmol/L | 402/4 | 58.5 (57.4, 59.6) | 58.0 (56.9, 59.2) | 0.16 | 14.3 | 15.6 | 0.54 (0.49, 0.59) |
| Kynurenines | |||||||
| Kynurenine, μmol/L | 545/4 | 1.62 (1.59, 1.65) | 1.72 (1.69, 1.76) | <0.0001 | 15.2 | 20.8 | 0.65 (0.62, 0.68) |
| 3-Hydroxykynurenine, nmol/L | 543/4 | 29.0 (28.1, 29.9) | 31.4 (30.4, 32.5) | <0.0001 | 28.9 | 31.9 | 0.55 (0.51, 0.59) |
| Kynurenic acid, nmol/L | 545/4 | 48.1 (46.7, 49.6) | 54.4 (52.6, 56.2) | <0.0001 | 22.7 | 32.2 | 0.67 (0.63, 0.70) |
| Xanthurenic acid, nmol/L | 545/4 | 13.9 (13.4, 14.4) | 16.2 (15.6, 16.8) | <0.0001 | 33.5 | 32.5 | 0.48 (0.44, 0.53) |
| Anthranilic acid, nmol/L | 543/4 | 13.8 (13.4, 14.2) | 14.8 (14.3, 15.3) | <0.0001 | 23.9 | 29.9 | 0.61 (0.57, 0.65) |
| 3-Hydroxyanthralinic acid, nmol/L | 543/4 | 33.9 (32.8, 35.0) | 37.8 (36.6, 39.2) | <0.0001 | 32.1 | 28.7 | 0.44 (0.40, 0.49) |
| Others | |||||||
| Methylmalonic acid, μmol/L | 551/4 | 0.17 (0.16, 0.17) | 0.18 (0.17, 0.18) | <0.0001 | 15.5 | 33.3 | 0.82 (0.80, 0.84) |
| ADMA, μmol/L | 551/4 | 0.71 (0.70, 0.72) | 0.71 (0.70, 0.72) | 0.67 | 12.9 | 13.1 | 0.51 (0.47, 0.55) |
| SDMA, μmol/L | 551/4 | 0.54 (0.53, 0.55) | 0.56 (0.55, 0.57) | <0.0001 | 12.3 | 20.6 | 0.74 (0.71, 0.76) |
| Creatinine, μmol/L | 551/4 | 73.6 (72.4, 74.9) | 74.7 (73.3, 76.0) | 0.007 | 9.6 | 19.4 | 0.80 (0.78, 0.82) |
| KTR, nmol/μmol | 396/4 | 26.8 (26.1, 27.5) | 29.4 (28.5, 30.3) | <0.0001 | 17.2 | 29.0 | 0.74 (0.71, 0.77) |
| Neopterin, nmol/L | 545/4 | 8.16 (7.97, 8.37) | 8.65 (8.42, 8.88) | <0.0001 | 19.1 | 27.4 | 0.67 (0.64, 0.70) |
| Cotinine,5 nmol/L | 545/4 | 9.09 (7.14, 11.6) | 7.24 (5.53, 9.47) | 0.25 | 103.9 | 298.5 | 0.89 (0.88, 0.90) |
ADMA, asymmetric dimethylarginine; ICC, intraclass correlation coefficient; KTR, kynurenine/Trp ratio; SDMA, symmetric dimethylarginine.
From paired t test comparing geometric mean at first vs. last visit.
Within- and between-person CVs were determined by taking the square root of the within- and between-person variance components from random-effects mixed model on the ln-transformed scale (4).
Calculated using ln-transformed analyte values.
Continine values below the detection limit (1 nmol/L) were set to 1.
We also investigated the change in ICC in the WENBIT samples for each analyte by narrowing the time period between sample collections from 38 mo to 1 y or 28 d. The ICC values for most biomarkers were remarkably stable across different periods, and only 4 biomarkers increased >10% when reducing the time span from 38 mo to 28 d, i.e., folate (from 0.51 to 0.71), ADMA (from 0.51 to 0.60), tHcy (from 0.72 to 0.85), and cystathionine (from 0.60 to 0.68) (Supplemental Fig. 5).
Comparison with published reliability data.
Reproducibility over time has been assessed previously in healthy individuals for some biomarkers that were investigated in the present study. Compared with reproducibility data obtained among the NHS participants, similar ICC values for Arg, Met, and Trp and lower values for Gly and Ser have been reported from a study based on European Prospective Investigation into Cancer and Nutrition–Potsdam samples collected 4 mo apart (35). One study based on samples collected over 30 mo demonstrated similar ICC for tHcy (36), whereas a higher ICC of 0.90 for tHcy over 2–5 y was reported from a European Prospective Investigation into Cancer and Nutrition–Dutch study, which also reported lower reproducibility for folate, vitamin B-6, vitamin B-12, and α-tocopherol (37). A recent study on intra-individual variations of 19 biomarkers related to one-carbon metabolism (38) demonstrated similar reproducibility for most biomarkers as we observed for NHS participants, except for better reproducibility for choline and DMG and lower for folate, cobalamin, tHcy, and cystathionine. Reproducibility over 2–4 y has been investigated previously for fat-soluble vitamins in postmenopausal women participating in the NHS (8), whereas similar ICCs were reported for α-tocopherol and 25-hydroxyvitamin D and lower ICC for all-trans retinol compared with the values obtained in the present study. Thus, our results are essentially in agreement with published data on within-person reproducibility for 21 biomarkers, which demonstrate fair-to-excellent reproducibility.
In conclusion, most biomarkers investigated were essentially stable in whole blood stored with ice for up to 48 h, and biomarkers that were stable under these conditions were generally found to be stable in plasma and serum, with notable exceptions for folate (in EDTA plasma stored for days at 23°C) and PLP (in heparin plasma for days at 23°C) (Supplemental Fig. 2). Biomarkers that are unstable in whole blood, such as choline and tHcy, maintain ranking only if samples are undergoing uniform preanalytical handling. Thus, biomarker stability should be assessed under conditions close to those occurring during sample transportation and handling.
Most biomarkers also have a fair-to-excellent within-person reproducibility over 1–2 y in healthy postmenopausal women and a similar reproducibility over 38 mo in patients with stable angina pectoris. However, some differences in reproducibility were noted between healthy women and patients with coronary artery disease. Such differences may partly be explained by increased between-person variance for biomarkers reflecting clinical conditions that are common among cardiovascular patients, such as renal dysfunction, inflammation, and metabolic syndrome. Consequently, a precise or valid correction of regression dilution bias in epidemiologic research based on a single biomarker measurement (39) should be assessed using ICC values determined from a population with demographic and clinical characteristics similar to those of the study population.
Biomarker stability during sample collection, transportation, and storage reflects the chemical structure of the actual compound. Low stability is a major source of preanalytical variability, which should be minimized by adequate sample handling based on stability data for each biomarker. For stable biomarkers determined by a precise method, within-person reproducibility mainly reflects the biologic variability over time due to changing pathophysiologic processes and altered lifestyle, including nutrition. Knowledge on within-person reproducibility is paramount for adequate study design, data interpretation, and statistical analysis, including adjustment for important confounders.
Supplementary Material
Acknowledgments
M.K.T., S.S.T., O.N., P.B., and M.J. designed the research; Ø.M., M.K.T., and P.M.U. conducted the research; Ø.M., M.K.T., and P.M.U. analyzed the data; Ø.M. and P.M.U. wrote the paper; and Ø.M. and P.M.U. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ADMA, asymmetric dimethylarginine; HAA, 3-hydroxyanthranilic acid; ICC, intraclass correlation coefficient; KTR, kynurenine/Trp ratio; MMA, methylmalonic acid; NHS, Nurses’ Health Study; PLP, pyridoxal 5′-phosphate; SDMA, symmetric dimethylarginine; tHcy, total homocysteine; WENBIT, Western Norway B Vitamin Intervention Trial.
Literature Cited
- 1.Kussmann M, Affolter M, Nagy K, Holst B, Fay LB. Mass spectrometry in nutrition: understanding dietary health effects at the molecular level. Mass Spectrom Rev. 2007;26:727–50. [DOI] [PubMed] [Google Scholar]
- 2.Ueland PM, Midttun O, Windelberg A, Svardal A, Skalevik R, Hustad S. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med. 2007;45:1737–45. [DOI] [PubMed] [Google Scholar]
- 3.Kushnir MM, Rockwood AL, Bergquist J. Liquid chromatography-tandem mass spectrometry applications in endocrinology. Mass Spectrom Rev. 2010;29:480–502. [DOI] [PubMed] [Google Scholar]
- 4.Rosner B. One-way ANOVA—the random-effects model. Fundamentals of biostatistics. Belmont, CA: Duxbury; 2006;613–8. [Google Scholar]
- 5.Elliott P, Peakman TC, Biobank UK. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–44. [DOI] [PubMed] [Google Scholar]
- 6.Jackson C, Best N, Elliott P. UK Biobank Pilot Study: stability of haematological and clinical chemistry analytes. Int J Epidemiol. 2008;37(Suppl 1):i16–22. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 8.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygard O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. [DOI] [PubMed] [Google Scholar]
- 10.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1371–9. [DOI] [PubMed] [Google Scholar]
- 11.Midttun Ø, Kvalheim G, Ueland PM. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem. 2013;405:2009–17. [DOI] [PubMed] [Google Scholar]
- 12.Midttun Ø, Ueland PM. Determination of vitamins A, D and E in a small volume of human plasma by a high-throughput method based on liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2011;25:1942–8. [DOI] [PubMed] [Google Scholar]
- 13.Windelberg A, Arseth O, Kvalheim G, Ueland PM. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin Chem. 2005;51:2103–9. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher BP, Broin SD. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol. 1991;44:592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 16.FOCUS. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU registration. Report of the FOCUS work group on degradation kinetics. 2006. Available from: http://focus.jrc.ec. europa.eu/dk.
- 17.Bartko JJ. Measurement and reliability: statistical thinking considerations. Schizophr Bull. 1991;17:483–9. [DOI] [PubMed] [Google Scholar]
- 18.Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008..
- 19.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, Sacks FM, Stampfer MJ. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35:2313–6. [PubMed] [Google Scholar]
- 20.Fiskerstrand T, Refsum H, Kvalheim G, Ueland PM. Homocysteine and other thiols in plasma and urine: automated determination and sample stability. Clin Chem. 1993;39:263–71. [PubMed] [Google Scholar]
- 21.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49:286–94. [DOI] [PubMed] [Google Scholar]
- 22.Anthes JC, Eckel S, Siegel MI, Egan RW, Billah MM. Phospholipase D in homogenates from HL-60 granulocytes: implications of calcium and G protein control. Biochem Biophys Res Commun. 1989;163:657–64. [DOI] [PubMed] [Google Scholar]
- 23.Solomon LR. Considerations in the use of B6 vitamers in hematologic disorders: I. Red cell transport and metabolism of pyridoxal. Blood. 1982;59:495–501. [PubMed] [Google Scholar]
- 24.Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N, Stone TW. On the biological importance of the 3-hydroxyanthranilic acid:anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hustad S, Eussen S, Midttun O, Ulvik A, van de Kant PM, Morkrid L, Gislefoss R, Ueland PM. Kinetic modeling of storage effects on biomarkers related to B vitamin status and one-carbon metabolism. Clin Chem. 2012;58:402–10. [DOI] [PubMed] [Google Scholar]
- 26.Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr. 2009;139:1415–8. [DOI] [PubMed] [Google Scholar]
- 27.Velzing-Aarts FV, Holm PI, Fokkema MR, vanderDijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr. 2005;81:1383–9. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann Clin Biochem. 2010;47:17–28. [DOI] [PubMed] [Google Scholar]
- 29.Vanholder R, Eloot S, Schepers E, Neirynck N, Glorieux G, Massy Z. An obituary for GFR as the main marker for kidney function? Semin Dial. 2012;25:9–14. [DOI] [PubMed] [Google Scholar]
- 30.Lewerin C, Ljungman S, Nilsson-Ehle H. Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J Intern Med. 2007;261:65–73. [DOI] [PubMed] [Google Scholar]
- 31.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–7. [DOI] [PubMed] [Google Scholar]
- 32.Sulo G, Vollset SE, Nygard O, Midttun O, Ueland PM, Eussen SJ, Pedersen ER, Tell GS. Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol. 2013;168:1435–40. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–20. [DOI] [PubMed] [Google Scholar]
- 34.Svingen GFT, Ueland PM, Pedersen EKR, Schartum-Hansen H, Seifert R, Ebbing M, Løland KH, Tell GS, Nygård O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33:2041–8. [DOI] [PubMed] [Google Scholar]
- 35.Floegel A, Drogan D, Wang-Sattler R, Prehn C, Illig T, Adamski J, Joost H-G, Boeing H, Pischon T. Reliability of serum metabolite concentrations over a 4-month period using a targeted metabolomic approach. PLoS One. 2011;6:e21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg UC, Zheng ZJ, Folsom AR, Moyer YS, Tsai MY, Mcgovern P, Eckfeldt JH. Short-term and long-term variability of plasma homocysteine measurement. Clin Chem. 1997;43:141–5. [PubMed] [Google Scholar]
- 37.Leenders M, Ros MM, Sluijs I, Boshuizen HC, van Gils CH, Jansen EHJM, Bueno-de-Mesquita HB. Reliability of selected antioxidants and compounds involved in one-carbon metabolism in two dutch cohorts. Nutr Cancer. 2013;65:17–24. [DOI] [PubMed] [Google Scholar]
- 38.Cope EL, Shrubsole MJ, Cohen SS, Cai Q, Wu J, Ueland PM, Midttun O, Sonderman JS, Blot WJ, Signorello LB. Intra-individual variation in one-carbon metabolism plasma biomarkers. Cancer Epidemiol Biomarkers Prev. 2013;22:1894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berglund L. Regression dilution bias: tools for correction methods and sample size calculation. Ups J Med Sci. 2012;117:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.