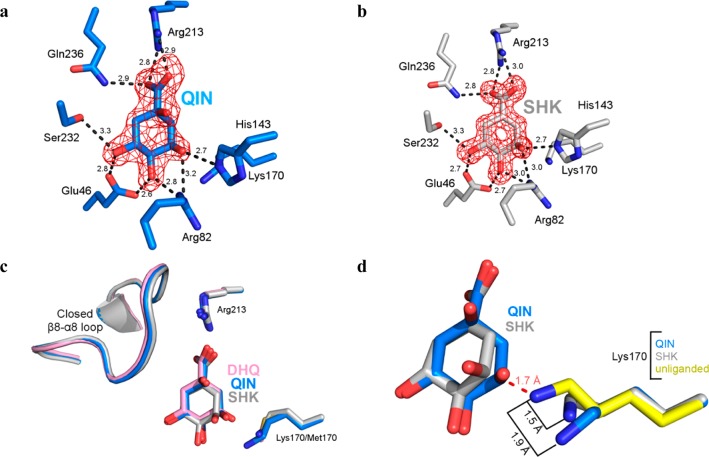
Figure 3.
Forms of protomer A of the quinate and shikimate complexes. (a) Stick model of the quinate complex active site (protomer A). (b) Stick model of the shikimate complex active site (protomer A). The Fo – Fc map was calculated with ligand omitted and is contoured at 3σ. (c) Superposition of forms of protomer A of quinate (blue) and shikimate (gray) to the K170M–DHQ complex (pink, PDB entry 3NNT) illustrates the similar mode of ligand binding and closed loop conformational states. (d) Superposition of forms of protomer A of quinate (rmsd = 0.31 Å over 196 Cα atoms) and shikimate (rmsd = 0.20 Å over 196 Cα atoms) to the unliganded structure (yellow, PDB entry 3L2I). The retraction of Lys170 from its unliganded state conformation prevents a clash (dashed red line) with quinate or shikimate.