Abstract
Mild traumatic brain injury (MTBI) is difficult to accurately assess with conventional imaging because such approaches usually fail to detect any evidence of brain damage. Recent studies of MTBI patients using diffusion-weighted imaging and diffusion tensor imaging suggest that these techniques have the potential to help grade tissue damage severity, track its development, and provide prognostic markers for clinical outcome. Although these results are promising and indicate that the forensic diagnosis of MTBI might eventually benefit from the use of diffusion-weighted imaging and diffusion tensor imaging, healthy skepticism and caution should be exercised with regard to interpreting their meaning because there is no consensus about which methods of data analysis to use and very few investigations have been conducted, of which most have been small in sample size and examined patients at only one time point after injury.
Keywords: axonal injury, diffusion tensor imaging, diffusion-weighted imaging, magnetic resonance imaging, mild traumatic brain injury
Mild traumatic brain injury (MTBI) is one of the most significant public health problems confronting the modern world.1 In the United States alone, current estimates indicate that it accounts for about 75% of the 1.5 million brain trauma cases reported annually by hospital emergency departments and costs more than $17 billion per year in health care utilization and lost productivity.2 Mild traumatic brain injury is variously defined3–5 as a sudden and violent acceleration, deceleration, rotatory, or blunt trauma to the head with no skull fractures,3,4 which results in a possible4 or definite3 loss of consciousness, transient memory dysfunction,3,4 confusion,3,4 disorientation,3,4 focal neurologic deficits that may or may not be transient,3 a Glasgow Coma Scale (GCS) score of 13 to 15,3,4 and a hospital stay lasting less than 48 hours.3 The leading causes are falls, transportation crashes, collisions with stationary or moving objects, and assaults, respectively.6 Particularly at high risk are children aged 0 to 4 years, adolescents aged 15 to 19 years, and adults aged 35 to 44 years and 75 years or older.1,7 Patients may experience a disabling array of somatic, cognitive, and affective sequelae.1,2,8,9 Although approximately 70% eventually recover to normal levels, it is estimated that 7% to 33% continue to have long-term or permanent deficits with serious social and economic consequences.1,2,8,10–12 Ability to forecast outcome is of central importance to the management of MTBI because early medical and rehabilitative intervention may reduce the risk of long-term deficits.13,14 Clinical and cognitive predictors, however, are suboptimal for this purpose in accuracy because of the wide intrapatient and interpatient variability in clinical progression and the confounding effects of psychological and motivational factors. The problem is further complicated by the fact that, in most cases, more objective measures such as computed tomography (CT) and conventional magnetic resonance imaging (MRI) usually fail to detect any evidence of brain injury.
The discrepancy in MTBI between clinical and radiographic findings has been attributed to the inability of conventional imaging modalities to reveal microscopic injury in brain tissue. On the basis of results from postmortem studies of MTBI, the primary form of injury is thought to be the widespread presence of multifocal lesions called diffuse axonal injury (DAI) that occur over a continuum of white matter regions including the corpus callosum, the internal capsule, the gray-white junction, and the cerebral peduncles.15 Yet, it has been reported that 70% of patients with a GCS score of 13 display no evidence of abnormalities on CT. This number increases to 95% for patients with a GCS score of 15.16 It is estimated that the total prevalence of abnormalities on CT is only 16% to 21% for a GCS score of 13 to 15 based on a study of 912 patients.17 Similar results have been reported with the use of conventional MRI to visualize MTBI. In the rare cases where positive findings do occur, they fail to identify the more widespread microstructural component of DAI and reveal only cortical contusions and multifocal hyperintensites on fluid-attenuated inversion recovery sequences or microhemorrhages on T2*-weighted sequences.18
A great deal of research in recent years has led to the development of diffusion-weighted imaging (DWI)19–21 and diffusion tensor imaging (DTI),22–24 2 advanced MRI techniques that have the potential to help identify and quantify microstructural changes that cannot be detected by CT and conventional MRI. There is growing evidence to suggest that both of these approaches could help grade tissue damage severity, track its development, and provide prognostic markers for clinical outcome in MTBI.
DWI AND DTI METHODS OF DATA ANALYSIS
There are currently 3 methods that can be used to analyze DWI and DTI data, namely, histogram analysis,25 region-of-interest (ROI) analysis,26 and voxel-based analysis (VBA),27,28 and 1 additional method that can be used to analyze DTI data, which is quantitative tractography.29 No consensus currently exists, however, on which approach to use.
Histogram analysis provides a summary of tissue changes occurring throughout the whole brain.25 It is fast and easy to implement, does not require spatial normalization, has minimal subjective involvement, and is easily reproducible. A limitation of this method is that it lacks sensitivity to tissue changes when only a few voxels differ between comparison groups.
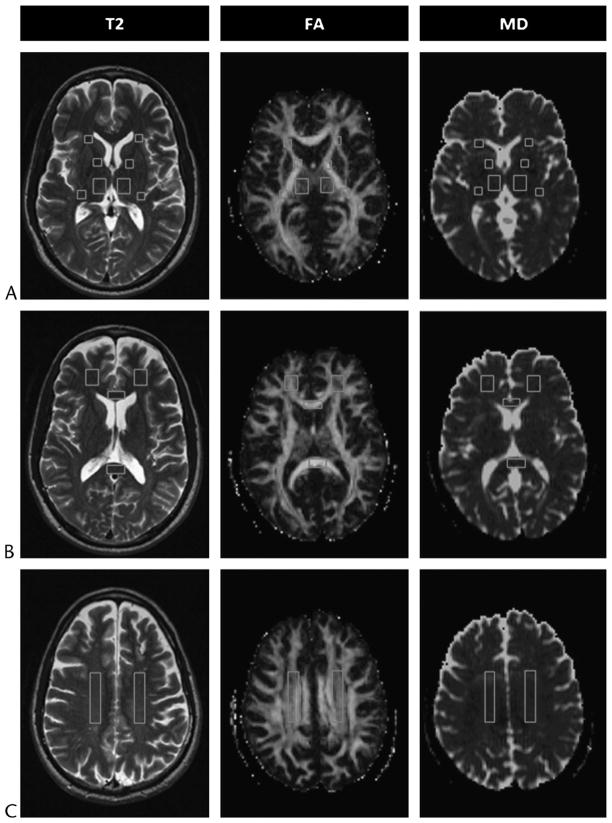
Region-of-interest analysis (Fig. 1) provides measurements from manually specified regions of brain.26 It is relatively easy to implement, does not require spatial normalization, and is designed to test tract specific a priori hypotheses. A limitation of this method is that it introduces error as a result of interobserver and intraobserver variability. Furthermore, results can be significantly weakened by statistical correction for multiple comparisons depending on the number of regions examined, and large subject groups may be required to adequately power a study. Also, only small sections of much larger tracts are measured, which reduces the generalizability of results. Moreover, information about tissue integrity is supplied from only a few specified sites and possible locations for damage in the rest of the brain are completely overlooked.
FIGURE 1.
Regions of interest shown on selected axial T2-weighted (T2) images and corresponding FA and MD maps (A, B, and C) from a 49-year-old man with MTBI who showed no visible evidence of brain damage on conventional MRI and was scanned 54 days after injury. Locations of ROIs indicated are as follows: the thalamus and the anterior limb, genu, and posterior limb of the internal capsule (A); the genu and splenium of the corpus callosum and frontal white matter (B); and the centrum semiovale (C). Figure 1 can be viewed online in color at www.topicsinmri.com.
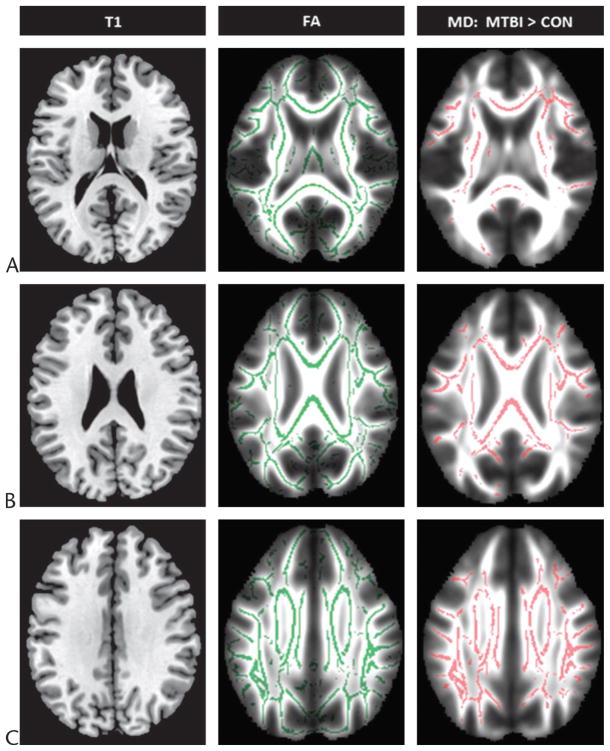
Voxel-based analysis (Fig. 2) provides a fully automated evaluation of the whole brain that can identify specific focal regions where tissue changes are occurring.27,28 It has minimal subjective involvement, is easily reproducible, and is designed for exploratory investigations. A limitation of this method is that it requires spatial normalization that may introduce errors if coregistration is performed inaccurately.30,31 Also, spatial smoothing may reduce the sensitivity for detection to only large tracts or lesions. A recent approach that has been developed for the purpose of improving the application of VBA to the evaluation of DTI data is tract-based spatial statistics (Fig. 3), which uses a nonlinear registration projected onto the mean white matter tract skeleton.32 Results from the application of tract-based spatial statistics in several studies show that it decreases intersubject variability and leads to more precise analyses.26,32
FIGURE 2.
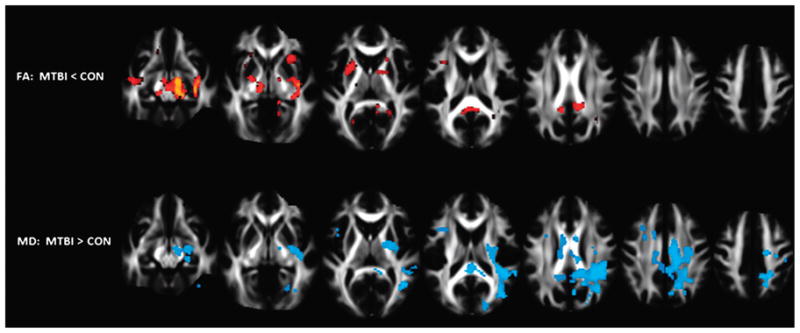
Voxel-based analysis results overlaid on selected axial slices from the mean FA template derived using 40 healthy controls (CON) and 60 patients with MTBI scanned within a few days of injury. The top and bottom rows depict voxel clusters where the mean FA and MD of patients were significantly greater and less than controls, respectively, at P < 0.05 corrected for age, sex, education, and multiple comparisons. Figure courtesy of Arnaud Messé, PhD, Laboratoire d’Imagerie Fonctionnelle, Paris, France. Figure 2 can be viewed online in color at www.topicsinmri.com.
FIGURE 3.
Tract-based spatial statistics results shown in reference to selected axial T1-weighted (T1) images from the Montreal Neurological Institute 152 standard brain and overlaid on corresponding maps from the mean FA template normalized to the same space (A, B, and C) derived using 16 healthy controls (CON) and 20 patients with MTBI scanned within 1 month of injury. The skeletons on the FA maps in the middle and the right columns depict alignment-invariant tract projections representing the mean FA of all subjects and voxel clusters where the MD of patients were significantly greater than controls at P < 0.05 corrected for multiple comparisons, respectively. Figure 3 can be viewed online in color at www.topicsinmri.com.
Finally, quantitative tractography (Fig. 4) provides a 3-dimensional reconstruction of white matter fiber trajectories throughout the brain.29 It has fairly complementary advantages and disadvantages to VBA. An additional limitation of this method is that it cannot distinguish between kissing and crossing fibers.33 Several recent advances in diffusion imaging techniques might, however, have the potential to more accurately measure complex fiber structure. These include q-ball imaging34 and other high angular resolution diffusion imaging techniques35 as well as diffusion spectrum imaging,36 although no MTBI studies have thus far been conducted using these methods.
FIGURE 4.
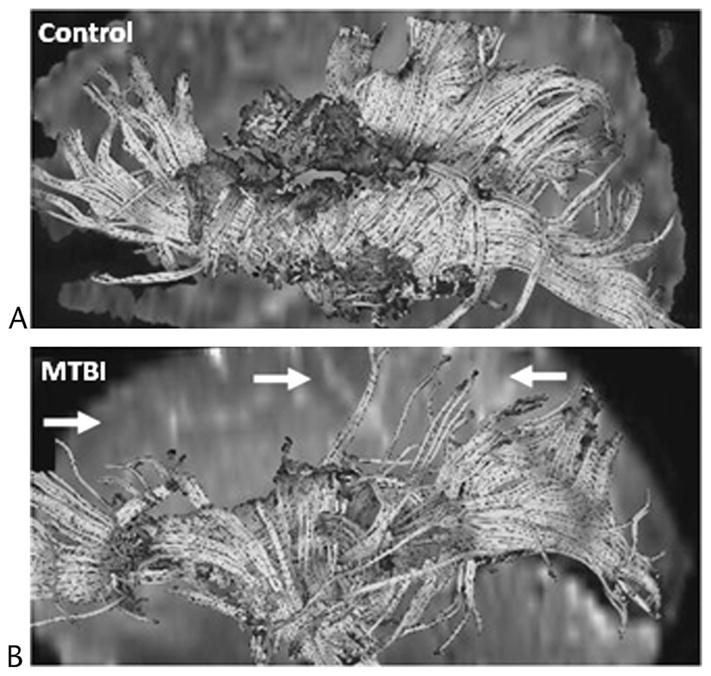
Diffusion tensor imaging tractography results generated from seed voxels placed around the corpus callosum and viewed from the midsagittal region of the left hemisphere for a 56-year-old healthy male control (A) and a 52-year-old male patient with MTBI (B) who showed no visible evidence of brain damage on conventional MRI and was scanned 22 days after injury. In comparison to the control, the patient exhibited fewer frontal, parietal, and occipital white matter fibers tracts (arrows). Figure courtesy of Kelly A. Mcgorty, RT, New York University School of Medicine, New York, NY. Figure 4 can be viewed online in color at www.topicsinmri.com.
DWI STUDIES OF ADULTS WITH MTBI
Several groups that have applied DWI in both experimental disease models of MTBI37–40 and humans with MTBI41–48 reported that it shows promise for the evaluation of DAI and might be possible to use as an adjunct tool in determining early intervention treatment strategies. In each of these studies, DWI was found to detect more white matter lesions than could be identified using either T2-weighted fast spin echo or T2*-weighted gradient echo MR sequences, although it was less sensitive at depicting microhemorrhages than T2*-weighted gradient echo sequences. These investigations and others involving rodent models of ischemic damage49–52 also indicate that DWI can reflect an increase or decrease of diffusion in lesions, which suggests that the technique may enable differentiation between cytotoxic and vasogenic edema. This has important clinical implications. Cytotoxic edema, which is caused by hypoxic injury resulting in decreased sodium pump functioning and abnormal accumulation of intracellular water, is considered irreversible. Vasogenic edema, however, which is caused by increased capillary and endothelial cell permeability resulting in abnormal accumulation of extracellular water, is considered reversible.53 In some cases, DWI detected changes of diffusion in patients at very early stages of MTBI that correlated with indicators for outcome and cognitive functioning even before neurological symptoms become manifest or unalterable.45,47 Schaefer et al47 found that, for MTBI patients examined less than 48 hours after injury, both the total volume and the number of lesions detected by DWI provided a stronger correlation with their modified Rankin scale score than did conventional MR sequences. Kurča et al45 observed that, for MTBI patients examined less than 4 days after injury, the total number of lesions detected by DWI was correlated with performance on neuropsychological measures for memory.
DTI STUDIES OF ADULTS WITH MTBI
There is a growing body of literature that suggests DTI is more sensitive to detecting white matter microstructural damage in MTBI than conventional imaging and could potentially serve as a biomarker for tracking the effects of injury and predicting cognitive outcome.54–85 Diffusion tensor imaging studies of MTBI, however, have been small in sample size and usually examined patients at either short-term or long-term time points after injury. The few studies that have included patients from both time points mostly consisted of mixed cohorts, separate cohorts not tracked longitudinally,59,64 or cohorts with neuropsychological testing but no imaging at follow-up.55,60,62,67,74,75 Despite these difficulties, DTI metrics have been correlated with patient behavioral and cognitive measures.55,58–63,65–67,70–78,81,82,84 Results suggest that there is a natural disease progression, but its timing remains unclear.
DTI Measures of MTBI
Almost as many DTI studies of MTBI have used 1.5-T scanners54,56,60–64,67–69,74–76,79,80,82 as have used 3-T scanners.55,58,59,65,66,70–73,77,78,81,85 Most of these studies54–56,58,60–63,65,67–72,74,76–82,85 were conducted using a conventional diffusion spin echo sequence with an echo planar imaging readout at either 6 diffusion gradient-encoding directions56,61,68,76 or more.54,55,58,60,62,63,65,67,69–72,74,77–82,85 Some studies,59,64,66,73,75 however, have reported using a twice-refocused spin echo86 sequence to diminish eddy current–induced geometric distortions at either 6 diffusion gradient-encoding directions64,75 or more.59,66,73
Two groups using histogram analysis to analyze DTI data in the study of MTBI reported conflicting results, finding that, when they both compared patients with controls, there were no differences in 1 case64 and globally decreased fractional anisotropy (FA), a measure of directional selectivity in water molecule movement, in the other.69 It may be that histogram analysis lacks the sensitivity needed to provide regionally specific information in MTBI and cannot be used to establish correlations with impairment in particular behavioral and cognitive domains.
The most common methods that have been used to analyze DTI data in the study of MTBI are ROI analysis54,55, 58–60,62,64,66,67,71,73,75–78 and VBA.65,69,70,74,81 Both of these approaches have yielded significant results. Several groups have reported that patients with short-term MTBI show decreased FA54,62,64,67,70,75 and patients with long-term MTBI with post-concussion symptoms show decreased FA and increased mean diffusivity (MD), a measure of the average displacement of water molecules.58,59,64–66,69,71,74,76,77,81 Interestingly, Bazarian et al55 reported finding increased FA and decreased MD in the splenium of the corpus callosum of patients at a mean of 72 hours after injury. Mayer et al73 also found increased FA in the corpus callosum and several left hemisphere tracts of patients at a mean of 12.5 days after injury. One possible explanation for these occurrences, which remains to be validated, is that axonal swelling during early stages of injury could restrict both the interstitial and overall movement of water leading to increased FA and decreased MD, respectively.
A number of MTBI studies have recently begun to apply axial diffusivity (AD), a measure of the magnitude of diffusion along the fiber orientation within a tract, and radial diffusivity (RD), a measure of mean rate of diffusion orthogonal to the fiber orientation within a tract.66,67,73 On the basis of results from investigations of rodent models, these 2 metrics could provide more specific information about axonal and myelin pathologic diseases, respectively.87,88 Kraus et al66 examined patients at a mean of 92.55 months after injury and found increased AD and normal RD in the sagittal striatum and the superior longitudinal fasciculus, suggesting this demonstrated less irreversible myelin damage than could be observed in moderate-to-severe head trauma patients who exhibited increased AD and RD in both these regions as well as the corticospinal tract and the whole brain. Kumar et al67 examined patients at a mean of 8.9 days after injury and found normal AD and increased RD in the genu and splenium of the corpus callosum. Mayer et al73 examined patients at a mean of 12.5 days after injury and found decreased RD in the corpus callosum and several left hemisphere tracts. These differences could arise from the fact that Kraus et al examined patients with long-term MTBI, whereas Kumar et al and Mayer et al examined patients with short-term MTBI. Changes in RD might have been observed only in patients with short-term MTBI because they are at a stage when demyelination is occurring while the discrepancy in RD between the patients from the 2 immediate studies might reflect varying inflammatory responses that can cause fluctuations in the water content of myelin sheath.89–91
Results from the ROI analysis show that the most commonly damaged tracts in MTBI include the frontal association pathways such as the corona radiata, uncinate fasciculus, the superior longitudinal fasciculus, and the commissural fibers of the corpus callosum.54,55,58–60,62,64,66,67,71,73,75–78 Most studies, however, have focused on patients with long-term MTBI and examined only a small number of regions. The few studies of patients with short-term MTBI that have been performed identified damage in the corpus callosum, the internal capsule, the external capsule, and the centrum semiovale.54,55,64,67,75 Results from VBA have also focused mostly on patients with long-term MTBI and identified damage in the commissural fibers of the corpus callosum as well as various supratentorial association fibers.29,33,34,38,45
Only a few groups have used quantitative tractography to analyze DTI data in the study of MTBI, and these were limited to case studies of 1 to 2 tracts.61,63,68,72,76,79,80,82 In an attempt to demonstrate that tractography can visualize traumatic axonal shearing, Le et al68 performed serial scans at 3 days and 12 weeks after injury on a patient with blunt head injury that exhibited posterior callosal disconnection syndrome. Tractography revealed interruption of the white matter fibers in the posteroinferior aspect of the splenium that correlated with the patient’s left hemialexia, suggesting that this approach may have prognostic value for evaluating cognitive and neurological sequelae associated with MTBI. Unfortunately, no lesion was found at follow-up. Rutgers et al79,80 conducted 2 tractography investigations of patients in which it was found that only a minority of sites with decreased FA were associated with fiber bundles showing evidence of discontinuity. This suggests that decreases in FA could be related to edema, hemosiderin deposition, axonal degeneration, or fiber misalignment rather than fiber disruption.
DTI Correlations With MTBI Clinical Measures
A number of studies suggest that the severity of white matter damage detected by DTI correlates with behavioral and cognitive measures of impairment in MTBI.55,58–63,65–67,70–78,81,82,84 Some studies have identified associations between global extent of damage and cognitive impairment.55,56,66,75,77 Kraus et al66 found that the total number of structures with decreased FA in patients was correlated with measures for attention, memory, and executive functioning. Miles et al75 reported that, for patients examined at a mean of 4.5 days after injury, decreased FA levels averaged as a single measure from the centrum semiovale, the genu and splenium of the corpus callosum, and the posterior limb of the internal capsule were correlated with measures for executive functioning acquired 6 months later. Most studies, however, have investigated more specific relationships between damage to individual structures and cognitive impairment.55,58–63,65–67,70–72,74,76,78,81,82 In general, investigations indicate that the integrity of frontal and temporal white matter pathways are associated with attention, memory, learning, and executive functioning deficits. Salmond et al81 found that increased MD in the left posterior cingulate, the left hippocampal formation, and the left temporal, frontal, and occipital cortex were correlated with memory and learning impairment. Lipton et al70 observed that decreased FA in frontal white matter, particularly the dorsolateral prefrontal cortex, was correlated with executive functioning impairment. Niogi et al78 used correlational double-dissociation analysis to show patients lacked the relationship between average FA in the left anterior corona radiata and attention and average FA in the bilateral uncinate fasciculus and verbal memory that existed in controls. Huang et al63 integrated the use of magnetoencephalography and DTI to test the hypothesis that delta waves, pathological low-frequency (1–4 Hz) neuronal magnetic signals, arise from gray matter neurons that experience deafferentation caused by injury to white matter fiber tracts. The results of this experiment confirmed the hypothesis and were also consistent with diverse postconcussion symptoms reported by patients.
DTI Evidence for Thalamic Injury in MTBI
Results from several recent DTI studies of MTBI suggest that, in addition to white matter, the thalamus may be an important further site of damage in MTBI.59,71,74 Little et al71 found that decreased FA from seed voxels placed within the thalamus of patients was correlated with variance in performance on neuropsychological tests for attention, memory, and executive functioning. Messe et al74 reported that, for patients examined at a mean of 17.2 days after injury, decreased MD in the anterior thalamic radiations was associated with persistent postconcussion symptoms based on a behavioral and cognitive assessment given 3 and 4 months after injury. The thalamus projects to the entire cerebral cortex and if damaged could produce various clinical nonfocalized sequelae.92 Its possible function in MTBI, however, has remained largely unexplored.
DTI Longitudinal Studies of MTBI
Diffusion tensor imaging longitudinal studies of MTBI are lacking. There have only been two such investigations,54,73 both of which were small in sample size and only examined patients at 2 imaging points spanning a very limited time frame. In the first investigation, Arfanakis et al54 examined 5 patients within 24 hours after injury and 2 patients who returned 1 month later for a longitudinal follow-up visit and compared them with 10 healthy controls. Although patients displayed decreased FA in a number of white matter regions at the immediate phase of injury, these changes were partially or completely corrected several weeks later. In the second investigation, Mayer et al73 examined 22 patients at a mean of 12 days after injury and 10 patients who returned 3 to 5 months later for a longitudinal follow-up visit and compared them with 21 matched healthy controls. Although patients demonstrated evidence of cytotoxic edema at the semi-immediate phase of injury, there was partial normalization in several white matter tracts several months later. More longitudinal studies will be needed to determine if DTI can successfully elucidate how microstructural pathologic disease and symptoms evolve over time and whether this information can be used to help predict which patients are at risk for long-term or permanent disability.
DWI AND DTI STUDIES OF CHILDREN AND ADOLESCENTS WITH MTBI
Most DWI and DTI studies of humans with MTBI have focused on an adult population. There have, however, been a few investigations involving adolescents.57,83,84 Wilde et al83 performed quantitative tractography in the corpus callosum of 10 adolescents at a mean of 2.7 days after injury and compared them with 10 matched healthy controls. The injured group displayed increased FA and decreased MD and RD consistent with the presence of cytotoxic edema. Increased FA and decreased RD were correlated with the severity of postconcussion symptoms. Chu et al57 used VBA to examine 10 adolescents at a mean of 3.4 days after injury and compared them with 10 matched healthy controls. The injured group displayed decreased MD in several white matter regions including, interestingly, the left thalamus. Wu et al84 performed ROI analysis together with quantitative tractography in the cingulum bundles of 12 adolescents at a mean of 2.92 days after injury and compared them with 11 matched healthy controls. A correlation was identified in the injured group between increased FA in the left cingulum bundle and poor performance on episodic verbal learning and memory tasks. The authors concluded that these preliminary findings suggest it is likely damage to the cingulum bundles contributes to cognitive sequelae during the early phases of injury.
CONCLUSIONS
The lack of evidence for injury in MTBI on conventional imaging has led to the examination of DWI and DTI as possible approaches to revealing microstructural changes that have the potential to help grade tissue damage severity, track its development, and provide prognostic markers for clinical outcome. Although several studies of MTBI patients have yielded promising results, healthy skepticism and caution should be exercised with regard to interpreting their meaning because there is no consensus about which methods of data analysis to use and very few investigations have been conducted of which most have been small in sample size and examined patients at only 1 time point after injury. Although forensic diagnosis of MTBI might eventually benefit from the use of DWI and DTI, it must be accepted that these techniques are not yet ready for prime time.
Acknowledgments
The authors thank Kelly A. Mcgorty, RT, of the Department of Radiology at the New York University School of Medicine, New York, NY, and Arnaud Messé, PhD, of the Laboratoire d’Imagerie Fonctionnelle, Paris, France, for kindly providing figures used in this article.
Footnotes
Disclosure: The authors have no conflict of interest to declare.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.US National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 3.Esselman PC, Uomoto JM. Classification of the spectrum of mild traumatic brain injury. Brain Inj. 1995;9:417–424. doi: 10.3109/02699059509005782. [DOI] [PubMed] [Google Scholar]
- 4.Leclerc S, Lassonde M, Delaney JS, et al. Recommendations for grading of concussion in athletes. Sports Med. 2001;31:629–636. doi: 10.2165/00007256-200131080-00007. [DOI] [PubMed] [Google Scholar]
- 5.Servadei F, Teasdale G, Merry G. Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma. 2001;18:657–664. doi: 10.1089/089771501750357609. [DOI] [PubMed] [Google Scholar]
- 6.Langlois JA, Rutland-Brown W, Thomas KE US National Center for Injury Prevention and Control, Division of Injury and Disability Outcomes and Programs. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Acute Care, Rehabilitation Research and Disability Prevention, National Center for Injury Prevention and Control; 2004. [Google Scholar]
- 7.Bazarian JJ, McClung J, Shah MN, et al. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 2005;19:85–91. doi: 10.1080/02699050410001720158. [DOI] [PubMed] [Google Scholar]
- 8.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 9.Kushner D. Mild traumatic brain injury: toward understanding manifestations and treatment. Arch Intern Med. 1998;158:1617–1624. doi: 10.1001/archinte.158.15.1617. [DOI] [PubMed] [Google Scholar]
- 10.Binder LM, Rohling ML, Larrabee GJ. A review of mild head trauma. Part I: Meta-analytic review of neuropsychological studies. J Clin Exp Neuropsychol. 1997;19:421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- 11.Rimel RW, Giordani B, Barth JT, et al. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- 12.Ruff RM, Camenzuli L, Mueller J. Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10:551–565. doi: 10.1080/026990596124124. [DOI] [PubMed] [Google Scholar]
- 13.Cicerone KD, Smith LC, Ellmo W, et al. Neuropsychological rehabilitation of mild traumatic brain injury. Brain Inj. 1996;10:277–286. doi: 10.1080/026990596124458. [DOI] [PubMed] [Google Scholar]
- 14.Mittenberg W, Tremont G, Zielinski RE, et al. Cognitive-behavioral prevention of postconcussion syndrome. Arch Clin Neuropsychol. 1996;11:139–145. [PubMed] [Google Scholar]
- 15.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126(Pt 3):515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 16.Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):61–75. doi: 10.1080/16501960410023822. [DOI] [PubMed] [Google Scholar]
- 17.Iverson GL, Lovell MR, Smith S, et al. Prevalence of abnormal CT-scans following mild head injury. Brain Inj. 2000;14:1057–1061. doi: 10.1080/02699050050203559. [DOI] [PubMed] [Google Scholar]
- 18.Hammoud DA, Wasserman BA. Diffuse axonal injuries: pathophysiology and imaging. Neuroimaging Clin N Am. 2002;12:205–216. doi: 10.1016/s1052-5149(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 20.Merboldt KD, Hanicke W, Frahm J. Self-diffusion NMR imaging using stimulated echoes. J Magn Reson. 1985;64:479–486. [Google Scholar]
- 21.Taylor DG, Bushell MC. The spatial mapping of translational diffusion coefficients by the NMR imaging technique. Phys Med Biol. 1985;30:345–349. doi: 10.1088/0031-9155/30/4/009. [DOI] [PubMed] [Google Scholar]
- 22.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 24.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Buchem MA, McGowan JC, Kolson DL, et al. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med. 1996;36:632–636. doi: 10.1002/mrm.1910360420. [DOI] [PubMed] [Google Scholar]
- 26.Ellis CM, Simmons A, Jones DK, et al. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology. 1999;53:1051–1058. doi: 10.1212/wnl.53.5.1051. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 28.Good CD, Johnsrude IS, Ashburner J, et al. Avoxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 29.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friston KJ, Ashburner J, Frith CD, et al. Spatial registration and normalization of images. Hum Brain Map. 1995;3:165–189. [Google Scholar]
- 31.Shen S, Szameitat AJ, Sterr A. VBM lesion detection depends on the normalization template: a study using simulated atrophy. Magn Reson Imaging. 2007;25:1385–1396. doi: 10.1016/j.mri.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Watts R, Liston C, Niogi S, et al. Fiber tracking using magnetic resonance diffusion tensor imaging and its applications to human brain development. Ment Retard Dev Disabil Res Rev. 2003;9:168–177. doi: 10.1002/mrdd.10077. [DOI] [PubMed] [Google Scholar]
- 34.Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52:1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- 35.Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med. 2002;47:1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- 36.Wedeen VJ, Hagmann P, Tseng WY, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 37.Assaf Y, Holokovsky A, Berman E, et al. Diffusion and perfusion magnetic resonance imaging following closed head injury in rats. J Neurotrauma. 1999;16:1165–1176. doi: 10.1089/neu.1999.16.1165. [DOI] [PubMed] [Google Scholar]
- 38.Barzo P, Marmarou A, Fatouros P, et al. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 39.Henninger N, Sicard KM, Li Z, et al. Differential recovery of behavioral status and brain function assessed with functional magnetic resonance imaging after mild traumatic brain injury in the rat. Crit Care Medi. 2007;35:2607–2614. doi: 10.1097/01.CCM.0000286395.79654.8D. [DOI] [PubMed] [Google Scholar]
- 40.Ito J, Marmarou A, Barzo P, et al. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg. 1996;84:97–103. doi: 10.3171/jns.1996.84.1.0097. [DOI] [PubMed] [Google Scholar]
- 41.Chan JH, Tsui EY, Peh WC, et al. Diffuse axonal injury: detection of changes in anisotropy of water diffusion by diffusion-weighted imaging. Neuroradiology. 2003;45:34–38. doi: 10.1007/s00234-002-0891-y. [DOI] [PubMed] [Google Scholar]
- 42.Goetz P, Blamire A, Rajagopalan B, et al. Increase in apparent diffusion coefficient in normal appearing white matter following human traumatic brain injury correlates with injury severity. J Neurotrauma. 2004;21:645–654. doi: 10.1089/0897715041269731. [DOI] [PubMed] [Google Scholar]
- 43.Hergan K, Schaefer PW, Sorensen AG, et al. Diffusion-weighted MRI in diffuse axonal injury of the brain. Eur Radiol. 2002;12:2536–2541. doi: 10.1007/s00330-002-1333-2. [DOI] [PubMed] [Google Scholar]
- 44.Huisman TA, Sorensen AG, Hergan K, et al. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr. 2003;27:5–11. doi: 10.1097/00004728-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Kurca E, Sivak S, Kucera P. Impaired cognitive functions in mild traumatic brain injury patients with normal and pathologic magnetic resonance imaging. Neuroradiology. 2006;48:661–669. doi: 10.1007/s00234-006-0109-9. [DOI] [PubMed] [Google Scholar]
- 46.Liu AY, Maldjian JA, Bagley LJ, et al. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol. 1999;20:1636–1641. [PMC free article] [PubMed] [Google Scholar]
- 47.Schaefer PW, Huisman TA, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial Glasgow Coma Scale score and score on modified Rankin scale at discharge. Radiology. 2004;233:58–66. doi: 10.1148/radiol.2323031173. [DOI] [PubMed] [Google Scholar]
- 48.Topal NB, Hakyemez B, Erdogan C, et al. MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurol Res. 2008;30:974–978. doi: 10.1179/016164108X323799. [DOI] [PubMed] [Google Scholar]
- 49.Gass A, Niendorf T, Hirsch JG. Acute and chronic changes of the apparent diffusion coefficient in neurological disorders—biophysical mechanisms and possible underlying histopathology. J Neurol Sci. 2001;186(Suppl 1):S15–S23. doi: 10.1016/s0022-510x(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 50.Hanstock CC, Faden AI, Bendall MR, et al. Diffusion-weighted imaging differentiates ischemic tissue from traumatized tissue. Stroke. 1994;25:843–848. doi: 10.1161/01.str.25.4.843. [DOI] [PubMed] [Google Scholar]
- 51.Unterberg AW, Stroop R, Thomale UW, et al. Characterisation of brain edema following “controlled cortical impact injury” in rats. Acta Neurochir Suppl. 1997;70:106–108. doi: 10.1007/978-3-7091-6837-0_33. [DOI] [PubMed] [Google Scholar]
- 52.Yuan XQ, Prough DS, Smith TL, et al. The effects of traumatic brain injury on regional cerebral blood flow in rats. J Neurotrauma. 1988;5:289–301. doi: 10.1089/neu.1988.5.289. [DOI] [PubMed] [Google Scholar]
- 53.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217:331–345. doi: 10.1148/radiology.217.2.r00nv24331. [DOI] [PubMed] [Google Scholar]
- 54.Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 55.Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 56.Benson RR, Meda SA, Vasudevan S, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24:446–459. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- 57.Chu Z, Wilde EA, Hunter JV, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geary EK, Kraus MF, Pliskin NH, et al. Verbal learning differences in chronic mild traumatic brain injury. J Int Neuropsychol Soc. 2010;16:506–516. doi: 10.1017/S135561771000010X. [DOI] [PubMed] [Google Scholar]
- 59.Grossman EJ, Ge Y, Jensen JH, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study [published online ahead of print] J Neurotrauma. 2011 doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartikainen KM, Waljas M, Isoviita T, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J Clin Exp Neuropsychol. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto K, Okumura A, Shinoda J, et al. Tensor magnetic resonance imaging in a case of mild traumatic brain injury with lowered verbal intelligence quotient. J Rehabil Med. 2007;39:418–420. doi: 10.2340/16501977-0065. [DOI] [PubMed] [Google Scholar]
- 62.Holli KK, Waljas M, Harrison L, et al. Mild traumatic brain injury: tissue texture analysis correlated to neuropsychological and DTI findings. Acad Radiol. 2010;17:1096–1102. doi: 10.1016/j.acra.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Huang MX, Theilmann RJ, Robb A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma. 2009;26:1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- 64.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 65.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kraus MF, Susmaras T, Caughlin BP, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 67.Kumar R, Gupta RK, Husain M, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23:675–685. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- 68.Le TH, Mukherjee P, Henry RG, et al. Diffusion tensor imaging with three-dimensional fiber tractography of traumatic axonal shearing injury: an imaging correlate for the posterior callosal “disconnection” syndrome: case report. Neurosurgery. 2005;56:189. [PubMed] [Google Scholar]
- 69.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 70.Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- 71.Little DM, Kraus MF, Joseph J, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mao H, Polensek SH, Goldstein FC, et al. Diffusion tensor and functional magnetic resonance imaging of diffuse axonal injury and resulting language impairment. J Neuroimaging. 2007;17:292–294. doi: 10.1111/j.1552-6569.2007.00146.x. [DOI] [PubMed] [Google Scholar]
- 73.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Messe A, Caplain S, Paradot G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Map. 2011;32:999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miles L, Grossman RI, Johnson G, et al. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- 76.Nakayama N, Okumura A, Shinoda J, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Niogi SN, Mukherjee P, Ghajar J, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131(Pt 12):3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 79.Rutgers DR, Fillard P, Paradot G, et al. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rutgers DR, Toulgoat F, Cazejust J, et al. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salmond CH, Menon DK, Chatfield DA, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 82.Sugiyama K, Kondo T, Higano S, et al. Diffusion tensor imaging fiber tractography for evaluating diffuse axonal injury. Brain Inj. 2007;21:413–419. doi: 10.1080/02699050701311042. [DOI] [PubMed] [Google Scholar]
- 83.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 84.Wu TC, Wilde EA, Bigler ED, et al. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang K, Johnson B, Pennell D, et al. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reese TG, Heid O, Weisskoff RM, et al. Reduction of eddy-current–induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 87.Mac Donald CL, Dikranian K, Bayly P, et al. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Wu EX, Qiu D, et al. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res. 2009;69:1190–1198. doi: 10.1158/0008-5472.CAN-08-2661. [DOI] [PubMed] [Google Scholar]
- 89.Cederberg D, Siesjo P. What has inflammation to do with traumatic brain injury? Child’s Nerv Syst. 2010;26:221–226. doi: 10.1007/s00381-009-1029-x. [DOI] [PubMed] [Google Scholar]
- 90.Morganti-Kossmann MC, Satgunaseelan L, Bye N, et al. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 91.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. 2. Cambridge, MA: MIT Press; 2006. [Google Scholar]