Abstract
Long (31.5 mm) electrode arrays are inserted deeper into the cochlea than the typical 1.25 turn insertion. With these electrode arrays, the apical electrodes are closer to (and possibly extend past) the end of the spiral ganglion. Using multi-dimensional scaling with patients implanted with a 31.5 mm electrode array, the perceptual space between electrodes was measured. The results suggest that deeper insertion increases the range of place pitches, but the perceptual differences between adjacent electrodes become smaller in the apex.
Introduction
Pitch can be provided by a cochlear implant by the selection of an electrode used to provide stimulation. Because of the tonotopic arrangement of the cochlea, each electrode in a cochlear implant is usually perceived as having a different pitch arranged in order from lowest at the apical end to highest at the basal end. Cochlear implant processing strategies therefore code pitch information by the selection of electrode or electrodes to encode an external sound. Each electrode that provides a unique place pitch is used to provide a channel of information. Most cochlear implant electrode arrays, such as the Nucleus Contour Advance 24, the Advanced Bionics HiFocus 1J, and the MED-EL FLEX24 are inserted up to approximately 1.25 turns into the cochlea (Boyd, 2011). In the normal human ear, 1.25 turns into the cochlea typically represents a characteristic frequency of approximately 500 Hz (Greenwood, 1990). Therefore, the full range of frequencies presented by a cochlear implant are presented to cochlear locations representing approximately 500 Hz or higher. If a longer electrode array inserted deeper into the cochlea were able to extend the range of place pitches then it would allow a greater cochlear extent for which to provide channels of information. However, it is currently unknown if perceptual changes in the cochlear apex correspond to changes in place pitch.
Two long (31.5 mm) electrode arrays (the MED-EL STANDARD and MED-EL FLEXSOFT arrays) are currently on the market. These arrays are typically inserted 1.75 to 2 full turns into the cochlea (Boyd, 2011), allowing for stimulation of regions typically representing lower frequencies than shorter electrode arrays. With these electrode arrays, electrodes 1, 2, and 3 are typically inserted deeper than 1.25 cochlear turns. The longer electrode array has a few potential benefits. First, the place of stimulation encoded by a speech processor would provide a closer match to the place of stimulation in a normal hearing acoustic hearing ear. This could potentially make the sound quality more natural and reduce the required adaptation to the implant. Second, it could reduce the mismatch (and therefore improve performance and sound quality) with any residual acoustic hearing in the ipsilateral or contralateral ear. Third, a longer electrode array could provide better spectral resolution. If a fixed number of channels is used (as is done in the MED-EL Maestro system), a longer array provides greater spacing between the channels, potentially reducing channel interaction. If a fixed cochlear distance between channels is used, a longer array would provide an increased number of channels without potentially increasing channel interaction.
However, there are likely to be limits to the optimal insertion depth into the cochlea. It is generally accepted that cochlear implants primarily stimulate the cell bodies of neurons in the spiral ganglion (SG). The SG only extends approximately between 1.5 to 2 turns into the cochlea (Spoendlin and Schrott, 1988; Kawano et al., 1996). In the normal hearing ear, peripheral neurites extend deeper into the cochlea. After hair-cell loss, the loss of peripheral neurites soon follows (Nadol, 1997; Otte et al., 1978), suggesting that there might not be a sufficient local neural population to stimulate with deeply inserted electrodes. Therefore, one possible effect of stimulation in the apical end of a long array is that the most apical electrodes are perceptually similar as they mostly stimulate the same neural population in the SG. Baumann and Nobbe (2004) showed that subjects could reliably discriminate electrode 3 from either electrode 1 or electrode 2 using MED-EL COMBI 40+ implants with 31.5 mm electrode arrays. These results suggest that electrodes positioned more apically than electrode 3 stimulate different neural populations than electrode 3. However, this data does not provide evidence that the perceptual differences in the apex are changes in place pitch or if there is additional perceptual benefit stimulating more apically than the location corresponding to electrode 2. Pitch matching, scaling, and ranking tasks have provided mixed results. Baumann and Nobbe (2006) and Dorman et al. (2007) found no pitch differences between the most apical 2 electrodes. Hamzavi and Arnoldner (2006) presented data suggesting that the most apical 2 electrodes had different pitches. Gani et al. (2007) and Boyd (2011) present data with mixed results on the pitch difference between the most apical electrodes.
It is possible that the mixed psychophysical results are caused by the most apical electrodes being perceptually different from each other in some perceptual quality other than place pitch. One potential explanation is that the most apical electrode would differ in sound quality because it stimulates a different ratio of peripheral neurites to SG cell bodies. Another potential explanation is that the peak of stimulation from the most apical two or three electrodes are at the same location (from the same SG cell bodies) but the spread of excitation from the most apical electrode would reach less basally than the second-most apical electrode. Using multi-dimensional scaling (MDS), Tong et al. (1983) showed that a change in rate of stimulation and a change in place of stimulation represent different perceptual dimensions, even though subjects describe changes in rate, place, or a combination of the two as a change in pitch (e.g., Luo et al., 2012). Therefore, if the perceptual changes in the apex are not changes in place pitch, a pitch related task (such as pitch scaling, ranking, or matching) may not adequately describe the perceptual attributes of the apex.
In the present study, the perceptual distances between electrodes in cochlear implant users with fully inserted 31.5 mm MED-EL electrode arrays were measured. Using MDS, the magnitude of perceptual differences between adjacent apical electrodes can be compared across the cochlea. Furthermore, an MDS analysis can indicate if any perceptual changes observed in the apex are changes along the same perceptual dimension as the rest of the cochlea, which would indicate that the perceptual changes in the apex are indeed changes in place pitch.
Methods
Subjects
Fourteen subjects with a MED-EL cochlear implant and either a STANDARD or FLEXSOFT 31.5 mm electrode array participated in the experiment. Specific subject demographics are presented in Table Table 1.. Data from 1 subject (P14) was collected at the House Research Institute in Los Angeles, CA, while the data from the other 13 subjects were collected at the Universitair Ziekenhuis Antwerpen (Antwerp University Hospital or UZA) in Antwerp, Belgium. It is worth noting that three of the patients (P11, P12, and P13) received their implants for tinnitus treatment and have normal or near-normal hearing in the contralateral ear. All subjects provided informed consent in accordance with either the IRB regulations for St. Vincent's Hospital in Los Angeles (P14) or the University Hospital of Antwerp (all other subjects) in Antwerp.
Table 1.
Subject demographics. For simplicity, subjects are referred to in this manuscript by subject code (i.e., P1–P14). The internal lab code for each subject is also provided to allow for comparison of the same subjects in different publications.
| Subject | Lab code | Gender | Age | Implant | Electrode | Strategy | Duration of deafness | Etiology |
|---|---|---|---|---|---|---|---|---|
| P1 | UZA-M1 | M | 68 | COMBI 40+ | STANDARD | FSP | Unknown, progressive | Unknown |
| P2 | UZA-M2 | F | 70 | COMBI 40+ | STANDARD | FS4 | Unknown, progressive | Unknown |
| P3 | UZA-M3 | M | 35 | SONATA | STANDARD | FS4 | 2 yrs | Bacterial meningitis |
| P4 | UZA-M4 | F | 57 | COMBI 40+ | STANDARD | FSP | Start at 20 yrs old | Meniere's disease |
| P5 | UZA-M5 | F | 46 | PULSAR | STANDARD | FSP | Unknown, progressive | Unknown |
| P6 | UZA-M6 | M | 80 | COMBI 40+ | STANDARD | FSP | Unknown, progressive | Unknown |
| P7 | UZA-M7 | M | 70 | PULSAR | FLEXSOFT | FS4 | Unknown, progressive | Unknown |
| P8 | UZA-M8 | F | 59 | SONATA | FLEXSOFT | FSP | Start in 1983 | Unknown |
| P9 | UZA-M9R | M | 61 | PULSAR | STANDARD | FS4 | Start at 13 yrs old | Unknown |
| P10 | UZA-M10 | F | 50 | PULSAR | FLEXSOFT | FS4 | Start in 1996 | Unknown |
| P11 | UZA-SSD-6 | M | 50 | PULSAR | FLEXSOFT | FS4 | 22 months | Temporal bone fracture |
| P12 | UZA-SSD-7 | F | 64 | SONATA | FLEXSOFT | FS4 | 18 months | Meniere's disease, gentamyacine |
| P13 | UZA-SSD-10 | M | 70 | PULSAR | FLEXSOFT | FSP | 16 months | Sudden deafness |
| P14 | HEI-M2 | F | 58 | SONATA | STANDARD | FSP | Unknown, progressive | Genetic/sensorineural |
Stimuli
Stimuli consisted of 600 ms single electrode, cathodic-first, bi-phasic pulse-trains. Phase durations were 50 μs and pulses were presented without an inter-phase gap in monopolar mode at 5000 pulses per second (pps). The 5000 pps stimulation rate was selected to minimize any potential temporal cues, and was used to test all 14 subjects. Stimuli were presented directly to patients using custom written software on a windows computer through a research interface (Research Interface Box 2, RIB2, University of Innsbruck). All stimuli were presented at an equally loud level described as “most comfortable.”
Procedure
The loudness growth for each stimulus was coarsely estimated. On a single electrode, a stimulus was played initially with 5 μa amplitude, and gradually increased in 5 μa steps until subjects indicated that the sound was at the maximum comfort level. Subjects reported loudness by pointing to values on an 11-point loudness scale provided by Advanced Bionics. The amplitude of the pulses were recorded when the subjects indicated that the loudness corresponded to the most comfortable level as well as the maximum acceptable loudness. The process was repeated for single electrode pulse-trains on electrodes numbered 1 through 9, corresponding to the 9 most apical electrodes (out of 12) and covering a cochlear range of 19.2 mm.
Adjustments were made to the amplitudes of the pulse trains on each electrode to ensure that estimates of the most comfortable level were actually the same loudness across electrodes. Four stimuli (on four adjacent electrodes) were played in order for 600 ms with a 300 ms inter-stimulus interval (ISI). Subjects were asked if all four sounds were the same loudness. If they were not, the amplitudes of the stimuli were adjusted, and the four sounds were played again until the subject reported that the sounds were all of the same loudness. The procedure was then repeated with a new set of electrodes until the amplitudes to require equal loudness on all nine electrodes were recorded.
To measure dissimilarity scores, a typical MDS protocol was used (e.g., Lawless, 1986; Tong et al., 1983). In a given trial, two of the nine single-electrode stimuli were randomly presented with a 300 ms ISI. Subjects were asked to scale how different the two sounds were by using the computer mouse to click on a bar on the screen, which represented a continuum from “Most Similar” to “Most Different.” Clicks on the bar were converted to a numerical value ranging from 0 (at the left side, corresponding to Most Similar) to 100 (on the right side, corresponding to Most Different.) After each trial, the location of the entire response scale randomly moved to a new location on the screen to force the subject to move the mouse to a new location to select the next response, preventing the subject from repeatedly clicking at the same location. In a block of trials, each possible pair of stimuli from the 9 stimuli set was compared once, totaling 81 trials. For each subject, five blocks of data were collected.
Results
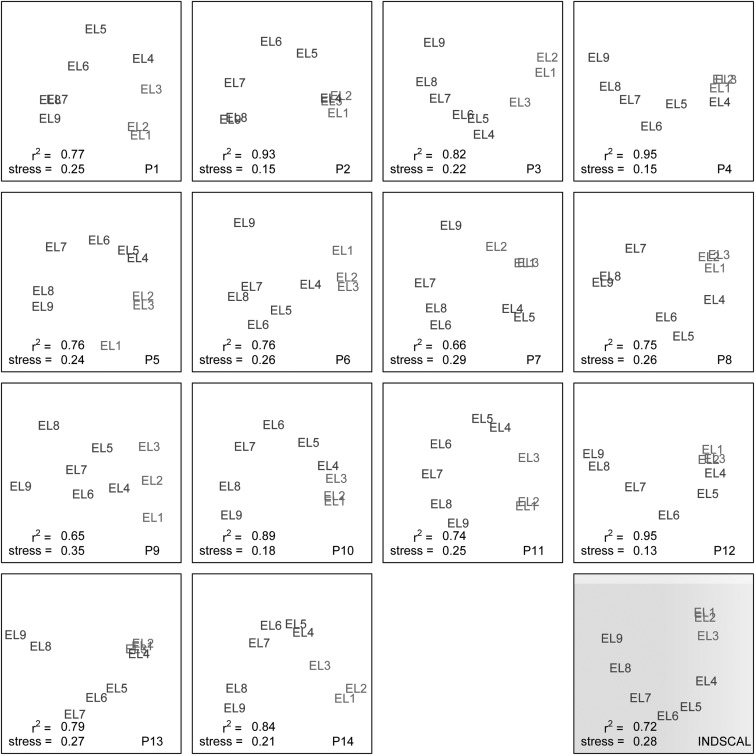
A two-dimensional alternating least squares scaling (ALSCAL) algorithm (Young and Lewyckyj, 1979) was used to analyze the response from each subject. The results are plotted in Fig. 1. The r2 values, which range from 0.65 to 0.95, suggest that the analyses are good fits to the data. Although there is a great deal of variability across subjects, most subjects perceptually organize each of the nine electrodes in order in a horse-shoe shape. A horse-shoe shape is commonly seen as the output of an MDS analysis of a one-dimensional space because patients are likely to overestimate the magnitude of small perceptual differences causing the single-dimensional perceptual space to bend (Kendall, 1971). Subject P9 was the noticeable exception to the horse-shoe configuration. In P9's data, the x axis clearly corresponds to the electrode number but the y axis does not correspond to any obvious dimension. Perhaps the y axis for subject P9 corresponds to a dimension of loudness for poorly balanced stimuli. One common observation across subjects is that although a change in place corresponds to a change on a continuous perceptual dimension, the perceptual distances between electrodes in the apex are often smaller than the perceptual changes in the rest of the array.
Figure 1.
(Color online) MDS plots (in two dimensions). Each of the 14 squares with a white background represents individual subject data. The perceptual location of each electrode is indicated. The bottom-left corner of each plot indicates both the stress and the r2 fit. The bottom-right corner of each plot indicates the subject code. The bottom-right plot (with the gray background) contains an INDSCAL plot that indicates the best perceptual space fit for all 14 subjects.
Although the physical distance between electrodes is a fixed 2.4 mm, the perceptual distance between adjacent electrodes varies both across subjects and electrode locations. For example, subject P2 perceives electrodes 8 and 9 as being quite similar, subject P1 perceives electrodes 6 and 7 as being quite similar, and subject P11 has a clear perceptual separation across these electrodes. It is difficult to determine to what extent the variability across subjects is due to the variability inherent in scaling responses instead of variability in perceptual qualities across subjects. Nevertheless, a consistent pattern can be seen across subjects. In the lower-right corner of Fig. 1, an Individual Differences Scaling (INDSCAL) plot is presented with a gray background. The INDSCAL plot presents the best fit for the multi-dimensional space across all subjects. Because the INDSCAL analysis uses the data from all subjects, the resulting output is more reliable than any of the individual ALSCAL plots. Similar to the individual subjects, a horse-shoe shape is revealed, suggesting that a change in place corresponds to a change in place pitch throughout the electrode array. However, it is worth noting that the apical electrodes (most notably electrodes 1 and 2) are perceptually clustered together.
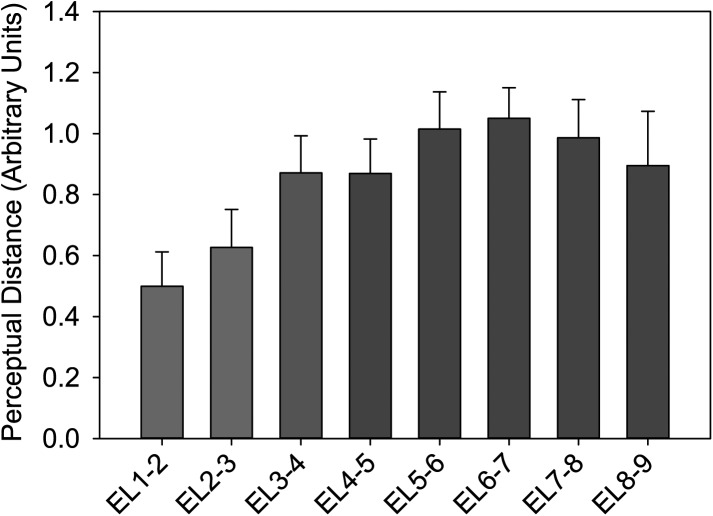
The average perceptual distance between each pair of adjacent electrodes is calculated from the Cartesian distances in the ALSCAL plot across all subjects (Fig. 2). Figure 2 suggests that the perceptual distances between adjacent electrodes in the apex (i.e., electrodes 1 and 2 and electrodes 2 and 3) are smaller than the perceptual distances along the rest of the cochlea. Because the motivation for the experiment was to examine the perceptual changes with place of stimulation in the apex relative to changes in the remainder of the cochlea, the mean distances for each adjacent electrode pair between electrodes 4 and 9 were compared to the perceptual distances between electrodes in the apex. Specifically, paired t-tests were used to compare the mean adjacent distances between electrodes 4 and 9 with the perceptual distances between electrodes 1 and 2 (t13 = −4.111, p = 0.001), electrodes 2 and 3 (t13 = −2.310, p = 0.038), and the mean perceptual adjacent distances between electrodes 1 to 3 (t13 = −4.114, p = 0.001). Using Rom's method (Rom, 1990) to correct for family-wise type-I error, all three comparisons were found to be statistically significant. However, no statistically significant differences were found between the perceptual distances between electrodes 1 and 2 and the distances between electrodes 2 and 3 (t13 = −0.735, p = 0.476).
Figure 2.
(Color online) The average distances between adjacent electrodes across all 14 subjects. Error bars indicate ±1 standard error of the mean.
Discussion
The horse-shoe shape observed in the ALSCAL plots for most subjects as well as in the summary INDSCAL plot are consistent with a one-dimensional perceptual space (Kendall, 1971). Therefore, if the perceptual differences between adjacent electrodes in the middle/basal region of the cochlea (i.e., represented by electrodes 4–9) are place pitch, then the perceptual changes between adjacent electrodes in the cochlear apex are also likely to be place pitch. However, it is worth noting that the horse-shoe shape plot might mask a much more subtle second dimension in the apex. If so, then the data would suggest that a change in the apex is a change of place pitch as well as a change in some other perceptual quality. However, the change in the “other perceptual quality” would be very small relative to the change in place pitch. It is therefore expected that an electrode insertion deeper than the typical 1.25 turn depth would allow for a greater range of place pitches, as well as improved spectral resolution. Furthermore, a deeper insertion can be used to reduce the mismatch between the place of stimulation provided by a cochlear implant and the place of stimulation provided by acoustic hearing and expected by the auditory system in a newly implanted post-lingually deafened patient.
Although a deeper insertion improves the range of place pitch coding, it is important to note that the perceptual distances between adjacent electrodes tend to decrease in the apex. To compensate, an electrode array could be designed such that the spacing between electrodes in the apex is greater than the spacing between electrodes along the rest of the array. A related observation is that the perceptual differences between the most apical two electrodes are typically very small. If a benefit is dependent on place pitch independence, little additional benefit would be provided by the addition of the most apical electrode with a 31.5 mm array. Performance improvement might be predicted from disabling either electrode 1 or electrode 2. It is worth noting that Arnoldner et al. (2007) tested speech performance with users of MED-EL 31.5 mm arrays and found that performance was best when a subset of apical electrodes were deactivated, although they did not specifically test the condition where only electrode 1 or electrode 2 was disabled. Furthermore, Arnoldner et al. (2007) similarly proposed a new electrode array designed in which the spacing between electrodes in the cochlear apex is increased. An alternate electrode array design would be to slightly shorten the electrode array by 2.4 mm to 29.1 mm such that the most apical contact in the new 29.1 mm array reaches the location represented by electrode 2 on a FLEXSOFT array. MED-EL presently produces a 28 mm array (the FLEX28), which is only slightly shorter than the proposed 29.1 mm array. Perhaps the FLEX28 would provide the optimal balance between extended place pitch coding from a long insertion and place pitch confusion from overly apical stimulation.
Acknowledgments
We thank our participants for their time and effort. We also thank John J. Galvin III, Justin Aronoff, and Laurel Fisher for their helpful comments on the manuscript. This work was supported by a MED-EL Hearing Solutions grant, NIH grant from the National Organization for Hearing Research, and NIDCD Grant Nos. R03-DC010064, R01-DC012152, and P30-DC0062710, and a TOPBOF grant from the University of Antwerp.
References and links
- Arnoldner, C., Riss, D., Baumgartner, W. D., Kaider, A., and Hamzavi, J. S. (2007). “Cochlear implant channel separation and its influence on speech perception–implications for a new electrode design,” Audiol. Neuro-Otol. 12, 313–324. 10.1159/000103212 [DOI] [PubMed] [Google Scholar]
- Baumann, U., and Nobbe, A. (2004). “Pitch ranking with deeply inserted electrode arrays,” Ear Hear. 25, 275–283. 10.1097/00003446-200406000-00008 [DOI] [PubMed] [Google Scholar]
- Baumann, U., and Nobbe, A. (2006). “The cochlear implant electrode-pitch function,” Hear. Res. 213, 34–42. 10.1016/j.heares.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Boyd, P. J. (2011). “Potential benefits from deeply inserted cochlear implant electrodes,” Ear Hear. 32, 411–427. 10.1097/AUD.0b013e3182064bda [DOI] [PubMed] [Google Scholar]
- Dorman, M. F., Spahr, T., Gifford, R., Loiselle, L., McKarns, S., Holden, T., Skinner, M., and Finley, C. (2007). “An electric frequency-to-place map for a cochlear implant patient with hearing in the nonimplanted ear,” J. Assoc. Res. Otolaryngol. 8, 234–240. 10.1007/s10162-007-0071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani, M., Valentini, G., Sigrist, A., Kos, M. I., and Boex, C. (2007). “Implications of deep electrode insertion on cochlear implant fitting,” J. Assoc. Res. Otolaryngol. 8, 69–83. 10.1007/s10162-006-0065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, D. D. (1990). “A cochlear frequency-position function for several species—29 years later,” J. Acoust. Soc. Am. 87, 2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- Hamzavi, J., and Arnoldner, C. (2006). “Effect of deep insertion of the cochlear implant electrode array on pitch estimation and speech perception,” Acta Oto-Laryngol. 126, 1182–1187. 10.1080/00016480600672683 [DOI] [PubMed] [Google Scholar]
- Kawano, A., Seldon, H. L., and Clark, G. M. (1996). “Computer-aided three-dimensional reconstruction in human cochlear maps: Measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal's canal,” Ann. Otol., Rhinol., Laryngol. 105, 701–709. [DOI] [PubMed] [Google Scholar]
- Kendall, D. (1971). “Seriation from abundance matrices,” in Mathematics in the Archaeological and Historical Sciences, edited by Hodson F., Kendall D., and Tautu P. (Edinburgh University Press, Edinburgh: ), pp. 215–252. [Google Scholar]
- Lawless, H. T. (1986). “Multidimensional scaling,” in Clinical Measurement of Taste and Smell, edited by Meiselman H. L. and Rivlin R. S. (MacMillan Publishing Company, New York), pp. 87–103. [Google Scholar]
- Luo, X., Padilla, M., and Landsberger, D. M. (2012). “Pitch contour identification with combined place and temporal cues using cochlear implants,” J. Acoust. Soc. Am. 131, 1325–1336. 10.1121/1.3672708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol, J. B., Jr. (1997). “Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation,” Otolaryngol.—Head Neck Surg. 117, 220–228. 10.1016/S0194-5998(97)70178-5 [DOI] [PubMed] [Google Scholar]
- Otte, J., Schunknecht, H. F., and Kerr, A. G. (1978). “Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation,” Laryngoscope 88, 1231–1246. [DOI] [PubMed] [Google Scholar]
- Rom, D. M. (1990). “A sequentially rejective test procedure based on a modified Bonferroni inequality,” Biometrika 77, 663–665. 10.1093/biomet/77.3.663 [DOI] [Google Scholar]
- Spoendlin, H., and Schrott, A. (1988). “The spiral ganglion and the innervation of the human organ of Corti,” Acta Oto-Laryngol. 105, 403–410. 10.3109/00016488809119493 [DOI] [PubMed] [Google Scholar]
- Tong, Y. C., Blamey, P. J., Dowell, R. C., and Clark, G. M. (1983). “Psychophysical studies evaluating the feasibility of a speech processing strategy for a multiple-channel cochlear implant,” J. Acoust. Soc. Am. 74, 73–80. 10.1121/1.389620 [DOI] [PubMed] [Google Scholar]
- Young, F., and Lewyckyj, R. (1979). ALSCAL-4 User's Guide, University of North Carolina, Chapel Hill, NC. [PubMed]