Abstract
Background
There are very few long-term Canadian data on breast cancer outcomes by stage. We described the stage, treatment and outcomes of breast cancer at a population level for patients in British Columbia.
Methods
This population-based cohort study included almost all patients with incident breast cancer registered in 2002 (about 97.6% registry case completeness). For these patients, information on stage, primary local surgery, radiotherapy, chemotherapy, hormone therapy and survival outcome (based on registry date and cause-of-death data) were available. We calculated Kaplan–Meier curves for breast cancer–specific survival and overall survival by stage and analyzed prognostic and treatment factors with a multivariable Cox model.
Results
The 2927 incident cases of breast cancer identified in 2002 had the following distribution by stage: stage 0 (in situ), 424 (14%); stage I, 1118 (38%); stage II, 938 (32%); stage III, 233 (8%); stage IV, 123 (4%); unknown, 91 (3%). The distribution of patients’ ages was < 40 years, 127 (4%); 40–49, 538 (18%); 50–59, 719 (25%); 60–69, 660 (23%); 70–79, 583 (20%); ≥ 80, 300 (10%). Within the first year after diagnosis, radiotherapy was provided to 1649 patients (56%), chemotherapy to 928 (32%) and hormone therapy to 1664 (57%). Ten-year breast cancer–specific survival rates by stage were > 99% for stage 0, 95% for stage I, 81% for stage II, 55% for stage III and 4% for stage IV. Ten-year overall survival rates were 89% for stage 0, 81% for stage I, 68% for stage II, 43% for stage III and 2% for stage IV.
Interpretation
This analysis provides a Canadian benchmark for treatment rates and 10-year outcomes by stage for all incident cases of breast cancer in a single province. Outcomes in British Columbia compared well with published rates for the United States and Europe.
Although the 5-year overall survival rate for patients with breast cancer in the United States rose from 75% in the late 1970s to 90% by 2006, this disease remains the most common cancer and the second leading cause of cancer deaths among women.1 Many factors potentially contributing to these improvements in survival include the availability of breast screening2–4 and the increasingly multidisciplinary nature of cancer care; the availability of appropriate surgery, radiotherapy5–7 and systemic therapy;8,9 and various health system factors.10–12
British Columbia has a publicly funded cancer care system. The Screening Mammography Program of BC currently screens approximately 50% of its target population13,14 at no charge to the patient. The province also has a centralized organized cancer care program through the BC Cancer Agency, which provides all radiotherapy, chemotherapy and hormone therapy that is prescribed in the province, also at no charge to the patient. For the province as a whole, the rate of referral for patients with breast cancer to a BC Cancer Agency centre was 85% throughout the 2000s.15
For many decades, the BC Cancer Agency has developed treatment guidelines and disseminated them to all physicians in the province. BC data have been used in international studies assessing prognostic information and to validate prognostic models used in clinics internationally,16 but there has been no comprehensive study of outcomes and treatment rates in the province.
The objective of this cohort study was to describe patient characteristics, stage distribution, stage-specific treatment utilization and outcomes for all patients in whom breast cancer was diagnosed in British Columbia in 2002.
Methods
Data sources
The BC Cancer Agency has a mandate to deliver cancer care services to the population of British Columbia.17 Pathology departments in the province have a legal requirement to send all pathology reports and death notifications with a neoplastic diagnosis to the BC Cancer Registry, which thereby captures all incident cases of breast cancer. Death and cause-of-death information are collected by the BC Vital Statistics Agency. In most cases where cancer was the cause of death, a specific cancer is recorded on the death certificate. Although not all causes of death are adjudicated, for patients with more than one cancer whose death is caused by cancer, the chair of the Breast Cancer Outcomes Unit reviews the charts and the death certificate to confirm the particular type of cancer that was the cause of death. For the purposes of the breast cancer–specific survival analysis in this study, in cases where there was doubt about cause of death, the cause was assigned as breast cancer. The joint data quality report prepared by the Canadian Institute for Health Information and Statistics Canada18 (which assesses the quality of data in the Canadian Cancer Registry by contributing provinces and territories) gave the BC Cancer Registry a 97.6% case completeness score for 2005, the date of the report closest to the period of the study. All radiotherapy was provided at 1 of the 4 cancer centres of the BC Cancer Agency, which were the only providers of radiotherapy in the province in the study year, 2002. All funded anticancer drugs are reimbursed by the agency, and each drug, dose and dispensing date has been recorded in the agency’s pharmacy data repository since 1998. For cases referred to the BC Cancer Agency, pretreatment prognostic factors such as grade, stage, lymphatic and vascular invasion, estrogen receptor, tumour size and nodal status, as well as primary surgical therapies, are collected prospectively in the Breast Cancer Outcomes Unit.
This study was approved by the University of British Columbia — British Columbia Cancer Agency Research Ethics Board.
Cases
We identified all incident cases of breast cancer diagnosed between Jan. 1 and Dec. 31, 2002, from BC Cancer Registry records. We linked cases to radiotherapy records, records of the Breast Cancer Outcomes Unit and the BC Cancer Agency pharmacy data repository using the unique patient identifiers common to all datasets.
For cases not referred to the BC Cancer Agency (15% of incident cases), we reviewed registry pathology records to determine the grade, estrogen receptor status, tumour size, nodal status, and whether definitive local and regional surgery was performed. For these cases, we used pathology reports to determine the stage, and we extracted utilization of radiotherapy, chemotherapy and hormone therapy within 1 year after diagnosis by stage of disease. For cases not referred to the BC Cancer Agency, systemic therapy dispensed at other institutions is nonetheless captured by the agency’s pharmacy database.
Patients in whom breast cancer was diagnosed in British Columbia during 2002 were matched to screening program records, and screening information was extracted, including whether the cancer was detected by screening (i.e., diagnosed within 1 year after screening with abnormal results). Patients were considered attendees of the Screening Mammography Program of BC if they had a screening mammogram result listed in the program’s records. Those who had been screened within the 30 months before their diagnosis were considered active attendees.
The case mix, in terms of stage, prognostic factors and treatments used, was tabulated and summarized in terms of the numbers and percentages in relevant groups.
Statistical analysis
We calculated overall and disease-specific survival rates using the Kaplan–Meier method. We tested prognostic factors (i.e., age, stage, grade, estrogen receptor status, lymphatic and vascular invasion) and treatment factors (i.e., use of radiotherapy, chemotherapy and hormone therapy within 1 year of diagnosis) for significance using the log rank test. We incorporated factors that were significant (p < 0.05) or close to significant (p < 0.3) into a multivariable Cox model for overall survival and, for cases of invasive disease, breast cancer–specific survival. We did not incorporate screening attendance into the multivariable model, because of the known risk of lead time and length time bias in nonrandomized settings;19 nonetheless, we calculated outcomes by screening attendance. We calculated confidence intervals (CIs) from the standard error of the cumulative proportions surviving at specified intervals from survival table outputs. All statistical analyses were performed with the Statistical Package for Social Sciences (SPSS for Windows, version 14.0; SPSS Inc., Chicago, Ill.). We compared rates of radiotherapy, chemotherapy and hormone therapy within 1 and 5 years of diagnosis with benchmarked and modelled optimal utilization rates in the literature to facilitate an understanding of how survival results were achieved in British Columbia. We calculated expected survival for the relative survival analysis using life tables for British Columbia compiled by Statistics Canada. The analysis used the cohort relative survival approach and followed methodology identical with that used in other relative survival reports of Canadian Cancer Registry data.20,21
Results
Patient characteristics and stage distribution
In 2002, a total of 2927 incident cases of breast cancer (2909 in women and 18 in men) were diagnosed in British Columbia. The majority of cases (2412 [82%]) represented invasive disease (stages I to IV), whereas 424 (14%) were in situ (stage 0) and 91 (3%) had unknown stage (Table 1). About 70% of tumours were either stage I or stage II at diagnosis.
Table 1: Characteristics of study population.
| Stage of cancer; no. (%) of patients* |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All stages n = 2927 |
Stage 0 n = 424 |
Stage I n = 1118 |
Stage II n = 938 |
Stage III n = 233 |
Stage IV n = 123 |
Unknown n = 91 |
|||||||||||||
| % of all patients |
100 |
14 |
38 |
32 |
8 |
4 |
3 |
||||||||||||
| Sex, female |
2909 |
(99) |
422 |
(>99) |
1116 |
(>99) |
933 |
(99) |
229 |
(98) |
122 |
(99) |
87 |
(96) |
|||||
| Age at diagnosis |
|||||||||||||||||||
| Median (range) |
61 |
(27–102) |
58 |
(28–94) |
62 |
(29–98) |
58 |
(27–101) |
59 |
(30–95) |
64 |
(35–96) |
74 |
(39–102) |
|||||
| < 40 |
127 |
(4) |
6 |
(1) |
38 |
(3) |
62 |
(7) |
15 |
(6) |
5 |
(4) |
1 |
(1) |
|||||
| 40–49 |
538 |
(18) |
100 |
(24) |
176 |
(16) |
201 |
(21) |
46 |
(20) |
9 |
(7) |
6 |
(7) |
|||||
| 50–59 |
719 |
(25) |
124 |
(29) |
261 |
(23) |
226 |
(24) |
62 |
(27) |
33 |
(27) |
13 |
(14) |
|||||
| 60–69 |
660 |
(23) |
95 |
(22) |
290 |
(26) |
185 |
(20) |
40 |
(17) |
29 |
(24) |
21 |
(23) |
|||||
| 70–79 |
583 |
(20) |
74 |
(17) |
267 |
(24) |
164 |
(17) |
35 |
(15) |
23 |
(19) |
20 |
(22) |
|||||
| ≥ 80 |
300 |
(10) |
25 |
(6) |
86 |
(8) |
100 |
(11) |
35 |
(15) |
24 |
(20) |
30 |
(33) |
|||||
| Margin status |
|||||||||||||||||||
| Positive |
123 |
(4) |
14 |
(3) |
20 |
(2) |
39 |
(4) |
26 |
(11) |
15 |
(12) |
9 |
(10) |
|||||
| Negative |
2356 |
(80) |
371 |
(88) |
990 |
(89) |
795 |
(85) |
168 |
(72) |
32 |
(26) |
0 |
(0) |
|||||
| Close |
165 |
(6) |
29 |
(7) |
56 |
(5) |
61 |
(7) |
16 |
(7) |
3 |
(2) |
0 |
(0) |
|||||
| Unknown |
283 |
(10) |
10 |
(2) |
52 |
(5) |
43 |
(5) |
23 |
(10) |
73 |
(59) |
82 |
(90) |
|||||
| Size of tumour, cm |
|||||||||||||||||||
| Median (range) |
1.7 |
(0.1–9.9) |
1.5 |
(0.1–9.9) |
1.2 |
(0.1–2.0) |
2.5 |
(0.1–9.9) |
5.4 |
(0.1–9.9) |
4.3 |
(0.4–9.9) |
1.3 |
(1.1–1.5) |
|||||
| < 1.0 |
529 |
(18) |
122 |
(29) |
370 |
(33) |
27 |
(3) |
8 |
(3) |
2 |
(2) |
0 |
(0) |
|||||
| 1.0–2.0 |
1110 |
(38) |
139 |
(33) |
708 |
(63) |
227 |
(24) |
20 |
(9) |
14 |
(11) |
2 |
(2) |
|||||
| 2.1–5.0 |
822 |
(28) |
90 |
(21) |
0 |
(0) |
628 |
(67) |
70 |
(30) |
34 |
(28) |
0 |
(0) |
|||||
| > 5.0 |
204 |
(7) |
34 |
(8) |
0 |
(0) |
19 |
(2) |
115 |
(49) |
36 |
(29) |
0 |
(0) |
|||||
| Unknown |
262 |
(9) |
39 |
(9) |
40 |
(4) |
37 |
(4) |
20 |
(9) |
37 |
(30) |
89 |
(98) |
|||||
| ER status |
|||||||||||||||||||
| Positive |
1920 |
(66) |
30 |
(7) |
943 |
(84) |
707 |
(75) |
165 |
(71) |
63 |
(51) |
12 |
(13) |
|||||
| Negative |
457 |
(16) |
13 |
(3) |
139 |
(12) |
219 |
(23) |
57 |
(24) |
26 |
(21) |
3 |
(3) |
|||||
| Unknown |
550 |
(19) |
381 |
(90) |
36 |
(3) |
12 |
(1) |
11 |
(5) |
34 |
(28) |
76 |
(84) |
|||||
| Grade |
|||||||||||||||||||
| 1 |
784 |
(27) |
85 |
(20) |
487 |
(44) |
177 |
(19) |
22 |
(9) |
9 |
(7) |
4 |
(4) |
|||||
| 2 |
1050 |
(36) |
152 |
(36) |
413 |
(37) |
361 |
(38) |
82 |
(35) |
35 |
(28) |
7 |
(8) |
|||||
| 3 |
875 |
(30) |
147 |
(35) |
195 |
(17) |
383 |
(41) |
108 |
(46) |
38 |
(31) |
4 |
(4) |
|||||
| Unknown |
218 |
(7) |
40 |
(9) |
23 |
(2) |
17 |
(2) |
21 |
(9) |
41 |
(33) |
76 |
(84) |
|||||
| LVI |
|||||||||||||||||||
| Positive |
519 |
(18) |
0 |
(0) |
77 |
(7) |
297 |
(32) |
114 |
(49) |
28 |
(23) |
3 |
(3) |
|||||
| Negative |
1747 |
(60) |
18 |
(4) |
994 |
(89) |
600 |
(64) |
85 |
(36) |
40 |
(33) |
10 |
(11) |
|||||
| Unknown |
661 |
(23) |
406 |
(96) |
47 |
(4) |
41 |
(4) |
34 |
(15) |
55 |
(45) |
78 |
(86) |
|||||
| No. of positive nodes |
|||||||||||||||||||
| 0 |
1439 |
(49) |
71 |
(17) |
1005 |
(90) |
339 |
(36) |
17 |
(7) |
7 |
(6) |
0 |
(0) |
|||||
| 1–3 |
497 |
(17) |
0 |
(0) |
0 |
(0) |
422 |
(45) |
65 |
(28) |
10 |
(8) |
0 |
(0) |
|||||
| ≥ 4 |
252 |
(9) |
0 |
(0) |
0 |
(0) |
124 |
(13) |
107 |
(46) |
20 |
(16) |
1 |
(1) |
|||||
| Positive, no. unknown |
3 |
(<1) |
0 |
(0) |
0 |
(0) |
0 |
(0) |
3 |
(1) |
0 |
(0) |
0 |
(0) |
|||||
| Nodal status unknown |
736 |
(25) |
353 |
(83) |
113 |
(10) |
53 |
(6) |
41 |
(18) |
86 |
(70) |
90 |
(99) |
|||||
| SMPBC attender |
|||||||||||||||||||
| Yes |
1574 |
(54) |
302 |
(71) |
704 |
(63) |
431 |
(46) |
81 |
(35) |
33 |
(27) |
23 |
(25) |
|||||
| No |
1353 |
(46) |
122 |
(29) |
414 |
(37) |
507 |
(54) |
152 |
(65) |
90 |
(73) |
68 |
(75) |
|||||
| Screen detected† |
|||||||||||||||||||
| Yes |
971 |
(62) |
238 |
(79) |
499 |
(71) |
189 |
(44) |
25 |
(31) |
11 |
(33) |
9 |
(39) |
|||||
| No | 603 | (38) | 64 | (21) | 205 | (29) | 242 | (56) | 56 | (69) | 22 | (67) | 14 | (61) | |||||
Note: ER = estrogen receptor, LVI = lymphovascular invasion, SMPBC = Screening Mammography Program of British Columbia. *Unless otherwise indicated. †Defined as diagnosis of breast cancer within 1 year after abnormal results on screening. For patients with synchronous bilateral disease, the first diagnosis was used to define the screen-detection variable, which was then assigned to both diagnoses.
Most cases of breast cancer (2500 [85%]) were diagnosed in patients between the ages of 40 and 79 years (Table 1). Overall, the median age at diagnosis for all stages was 61, with only 127 (4%) patients younger than 40 and only 300 (10%) 80 or older. The percentage of those presenting with more advanced disease (i.e., stage III or IV) was greater among elderly patients than among younger patients (20% [59/300] of those 80 years or older v. 11% [297/2627] of those younger than 80 years).
More than half of all patients with breast cancer were attendees of the Screening Mammography Program of BC, and the majority of patients with a diagnosis of in situ (302/424 [71%]) or stage I (704/1118 [63%]) disease had been screened within the 30 months before diagnosis. In contrast, most patients with a diagnosis of stage II to IV breast cancer had not attended the screening program. Only 431 (46%) of 938 patients with stage II disease, 81 (35%) of 233 patients with stage III disease and 33 (27%) of 123 patients with stage IV disease had been screened within the 30 months before their diagnosis. Overall, 971 cases of breast cancer were considered to have been detected by screening; this represented 33% of all cancers diagnosed and 62% of cancers among the 1574 patients who attended screening.
Treatment characteristics
The majority of patients with disease ranging from in situ to stage III underwent surgery for their breast cancer (2546/2713 [94%]) (Table 2). Approximately two-thirds of patients with in situ disease underwent breast-conserving surgery, but only about one-third received radiotherapy. Less than 25% of patients with in situ disease received hormone therapy within 1 year of diagnosis.
Table 2: Characteristics of treatment.
| Stage of cancer; no. (%) of patients |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All stages n = 2927 |
Stage 0 n = 424 |
Stage I n = 1118 |
Stage II n = 938 |
Stage III n = 233 |
Stage IV n = 123 |
Unknown n = 91 |
||||||||
| Initial surgery |
||||||||||||||
| None |
121 |
(4) |
1 |
(<1) |
7 |
(1) |
13 |
(1) |
22 |
(9) |
65 |
(53) |
13 |
(14) |
| Breast-conserving |
1510 |
(52) |
281 |
(66) |
726 |
(65) |
445 |
(47) |
34 |
(15) |
22 |
(18) |
2 |
(2) |
| Mastectomy |
1086 |
(37) |
104 |
(25) |
344 |
(31) |
458 |
(49) |
154 |
(66) |
22 |
(18) |
4 |
(4) |
| Unknown |
210 |
(7) |
38 |
(9) |
41 |
(4) |
22 |
(2) |
23 |
(10) |
14 |
(11) |
72 |
(79) |
| Radiotherapy |
||||||||||||||
| Within 1 yr of diagnosis |
1649 |
(56) |
159 |
(38) |
655 |
(59) |
599 |
(64) |
179 |
(77) |
57 |
(46) |
0 |
(0) |
| Within 5 yr of diagnosis |
1715 |
(59) |
167 |
(39) |
679 |
(61) |
619 |
(66) |
184 |
(79) |
65 |
(53) |
1 |
(1) |
| Within 1 yr of breast-conserving surgery* |
1214 |
(80) |
155 |
(55) |
639 |
(88) |
394 |
(89) |
26 |
(76) |
NA |
NA |
||
| Chemotherapy |
||||||||||||||
| Within 1 yr of diagnosis |
928 |
(32) |
0 |
(0) |
159 |
(14) |
543 |
(58) |
166 |
(71) |
53 |
(43) |
7 |
(8) |
| Within 5 yr of diagnosis |
978 |
(33) |
0 |
(0) |
177 |
(16) |
558 |
(59) |
168 |
(72) |
61 |
(50) |
14 |
(15) |
| Hormone therapy |
||||||||||||||
| Within 1 yr of diagnosis (all) |
1664 |
(57) |
95 |
(22) |
709 |
(63) |
610 |
(65) |
156 |
(67) |
63 |
(51) |
31 |
(34) |
| Within 5 yr of diagnosis (all patients) |
1773 |
(61) |
109 |
(26) |
734 |
(66) |
657 |
(70) |
169 |
(73) |
67 |
(54) |
37 |
(41) |
| Within 1 yr of diagnosis (ER+) | 1493 | (78) | 7 | (23) | 695 | (74) | 590 | (83) | 143 | (87) | 52 | (83) | 6 | (50) |
Note: ER+ = estrogen receptor-positive, NA = not applicable (use of radiotherapy after breast-conserving surgery is not relevant for patients with stage IV or unknown stage cancer). *Percentages calculated on the basis of number who had breast-conserving surgery.
About two-thirds of patients with stage I breast cancer received breast-conserving surgery (Table 2). Of stage I and II patients treated with this type of surgery, 88% also received radiotherapy within 1 year of diagnosis. Patients with stage II disease at presentation were equally likely to undergo mastectomy or breast-conserving surgery, whereas two-thirds of stage III patients underwent mastectomy as their initial surgery. The percentage of patients who underwent various forms of therapy increased with increasing severity of disease at the time of diagnosis: for mastectomy as initial surgery, from 31% at stage I to 66% at stage III; for radiotherapy within 1 year after diagnosis, from 59% at stage I to 77% at stage III; and for chemotherapy, from 14% at stage I to 71% at stage III. Approximately two-thirds of patients with stage I to III cancer were treated with hormone therapy. When only estrogen receptor–positive patients were considered, the rate of treatment with hormone therapy ranged from 74% to 87% among those with stage I to IV cancer.
Patient outcomes
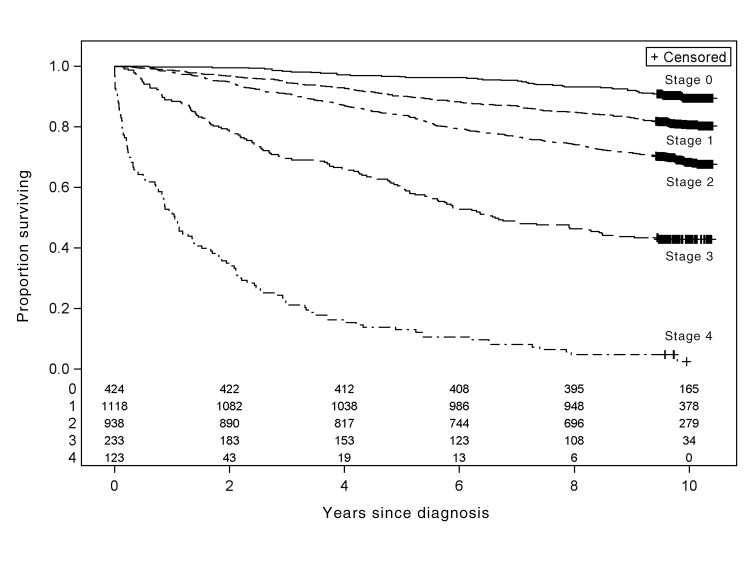
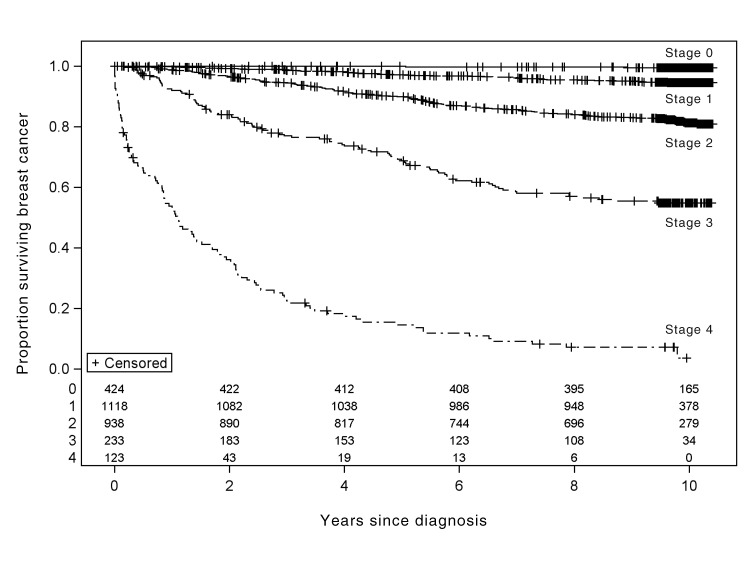
Overall survival and breast cancer–specific survival, for all stages and divided by stage, for patients in whom breast cancer was diagnosed in British Columbia in 2002 are shown in Figures 1 and 2, respectively. For all stages combined, overall 5-year survival was 83% (95% CI 81%– 84%) and 5-year breast cancer–specific survival was 89% (95% CI 88%– 90%). Overall 10-year survival was 71% (95% CI 69%–72%) and 10-year breast cancer–specific survival was 83.8% (95% CI 82%–85%). When the analysis was limited to women, survival estimates were the same (10-year overall survival 70.8%, 10-year breast cancer–specific survival 83.9%). For overall survival, all factors other than chemotherapy at 1 year after diagnosis were significant on univariable analysis and were included in the Cox models for overall survival and breast cancer–specific survival for invasive cases (see Table 3). All variables other than estrogen receptor status remained significant on multivariable analysis. Survival was better for people who attended screening than for those who did not (overall 10-year survival 82% v. 57%, p < 0.001; breast cancer–specific 10-year survival 91% v. 75%, p < 0.001). The 5-year relative survival rate for the entire cohort of patients with a diagnosis of breast cancer in British Columbia was 90% (95% CI 88%–91%).
Fig. 1:
Overall survival among 2927 patients in whom breast cancer was diagnosed in 2002 in British Columbia, by stage of cancer at diagnosis. The columns of numbers at the bottom of the graph show the number of patients at risk every 2 years, by stage of cancer (stage 0 to stage 4).
Fig. 2:
Breast cancer–specific survival among 2927 patients in whom breast cancer was diagnosed in 2002 in British Columbia, by stage of cancer at diagnosis. The columns of numbers at the bottom of the graph show the number of patients at risk every 2 years, by stage of cancer (stage 0 to stage 4).
Table 3: Multivariable Cox model for breast cancer–specific survival and overall survival.
| Variable | Breast cancer–specific survival |
Overall survival |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Age, per yr |
1.02 |
(1.00–1.03) |
1.06 |
(1.05–1.07) |
| Grade |
||||
| 2 v. 1 |
2.0 |
(1.3–2.9) |
1.3 |
(1.0–1.6) |
| 3 v. 1 |
3.5 |
(2.3–5.2) |
1.9 |
(1.5–2.4) |
| LVI, positive v. negative |
1.6 |
(1.2–2.0) |
1.5 |
(1.2–1.8) |
| Stage |
||||
| II v. I |
3.0 |
(2.2–4.3) |
1.5 |
(1.2–1.8) |
| III v. I |
9.8 |
(6.5–14.7) |
4.0 |
(3.1–5.2) |
| IV v. I |
47.0 |
(30.6–73.1) |
15.5 |
(11.0–22.0) |
| Radiotherapy* |
0.76 |
(0.60–0.96) |
0.68 |
(0.60–0.80) |
| Chemotherapy* |
0.58 |
(0.43–0.78) |
NA |
NA |
| Hormone therapy* | 0.74 | (0.59–0.95) | 0.70 | (0.59–0.83) |
Note: CI = confidence interval, LVI = lymphatic vascular space invasion, NA = not applicable. *Within 1 year of diagnosis, expressed as the hazard ratio of having had treatment v. not having had treatment.
Interpretation
This descriptive study of 2927 patients in whom breast cancer was diagnosed in British Columbia in 2002 shows a stage distribution heavily weighted toward early-stage disease, particularly stages I and II. Most early-stage cancers were diagnosed in patients aged 40 to 79 years. Most of these patients with early-stage disease underwent breast-conserving surgery and adjuvant radiotherapy, whereas most patients with stage III breast cancer were treated with mastectomy and adjuvant radiotherapy. Use of both chemotherapy and hormone therapy increased with increasing stage of disease, up to stage III.
Over 60% of in situ and stage I cases were diagnosed in patients who attended the Screening Mammography Program of BC, whereas the majority of stage III and IV cases were diagnosed in patients who had not been screened. Among patients who attended the Screening Mammography Program of BC, most cancers were detected by screening, including over 70% of in situ and stage I cancers. However, outcomes according to screening attendance should be interpreted with caution because of lead time and length time bias associated with screening.
The case mix presented here is similar to that reported by the US Surveillance Epidemiology and End Results registry22 (Table 4). The 5-year relative survival rate for the entire cohort of patients with a diagnosis of breast cancer in British Columbia (90%) is at the high end of the range seen in many European countries (from registry-based studies) and in the US Surveillance Epidemiology and End Results database (81%–89.3%).10,11,22,23 These findings suggest that the BC Cancer Agency is meeting its objective of providing timely, evidence-based cancer care services to provincial residents in the context of a widely accessible health care system. We have previously described how stage and treatment vary by region across the province.15
Table 4: Stage distribution of cases of breast cancer in British Columbia (this study) and US Surveillance Epidemiology and End Results (SEER)22.
| % of cases |
||
|---|---|---|
| Stage | British Columbia (this study) | SEER |
| In situ |
14 |
15 |
| I |
38 |
42 |
| II |
32 |
32 |
| II |
8 |
7 |
| IV |
4 |
4 |
| Unknown | 3 | - |
Models of optimal radiotherapy utilization have been developed,24,25 and we compared BC Cancer Agency data from the 2002 cohort with these ideal utilization rates (Table 5). The BC radiotherapy rate at 5 years (59% for all disease stages) was similar to the estimated ideal rate from a Canadian model (66%) but lower than the estimated ideal rate from Australia (83%).24,25 The difference between actual and ideal rates (the latter derived from evidence-based models of ideal treatment rates) likely relates to differences in patient preferences.24,25 Ideal utilization rates have also been published for chemotherapy.26 Chemotherapy use for all stages of disease was lower in the BC cohort than the ideal published rates, most notably for patients with stage I disease but also for those with stage III breast cancer (Table 5). Use of hormone therapy for invasive cancer in British Columbia was very similar to the published ideal rate.27
Table 5: Comparison of 5-year utilization rates for various therapies in British Columbia with optimal utilization*.
| Stage; % of cases |
|||||
|---|---|---|---|---|---|
| Type of therapy | All | I | II | III | IV |
| Radiotherapy |
|||||
| BCCA data (within 5 yr) |
59 |
61 |
66 |
79 |
52 |
| Ideal (Foroudi et al.24) |
66 |
69 |
82 |
95 |
64 |
| Ideal (Delaney et al.25) |
83 |
84 |
84 |
91 |
47 |
| Chemotherapy† |
|||||
| BCCA data (within 5 yr) |
34 (adjusted: 39) |
16 |
59 |
72 |
49 |
| Ideal (Ng et al.26) |
59 (adjusted: 69) |
56 |
56 |
90 |
29 |
| Hormone therapy† |
|||||
| BCCA data (within 5 yr) |
61 (adjusted: 68) |
66 |
70 |
73 |
54 |
| Ideal (Fong et al.27) | 57 (adjusted: 67) |
NR | NR | NR | NR |
Note: BCCA = BC Cancer Agency, NR = not reported. *Optimal (ideal) rates of utilization are based on evidence-based estimates of needs. Details of how these estimates were obtained are outlined in the cited references. †For chemotherapy and hormone therapy, the “all stages” rates were adjusted to account for exclusion of in situ cases; the parenthetical values are these adjusted rates, for invasive cancers only. In particular, Fong and associates27 assumed that hormone therapy is not indicated for in situ breast cancer.
Limitations
Despite the comprehensive nature of BC Cancer Agency records, our analysis had limitations. As mentioned above, the referral rate to the agency was 85%, so complete data were not available for all patients with breast cancer treated in British Columbia; however, for the year 2002, we reviewed pathology records in the BC Cancer Registry to gather information about non-referred patients. Although the registry captures all known cancers from clinical diagnoses made at cancer centres, pathology reports describing cancer diagnosis, and cancer diagnosis on death certificates, it is possible that the registry misses clinical diagnoses made in the community for patients who do not undergo subsequent biopsy or autopsy. Therefore, as with all cancer registries, there is some possibility that case ascertainment was incomplete. Although the ideal would be to analyze data from multiple and more recent years, to increase the sample size and hence generalizability, we were constrained by data availability to the year 2002. In that year, HER2/neu status, an important prognostic and predictive indicator, was not routinely measured in patients with early-stage breast cancer, because the evidence for efficacy of trastuzumab (anti-HER2 antibody) in adjuvant therapy had yet to emerge. Finally, the various forms of treatment were recorded as having been given if a patient received even 1 dose of chemotherapy or radiotherapy or a first prescription for hormone therapy within 1 year after diagnosis; as such, our data do not reflect completion of systemic therapy or the number of doses administered. The nonadherence rate for adjuvant hormone therapy has been reported as 40% for British Columbia,28 but compliance with chemotherapy and radiotherapy has not been reported. Differences in compliance among populations do have the potential to affect comparative patient outcomes, but the primary focus of this report was the description of patient characteristics, treatment and outcomes in British Columbia, so such differences were not a factor in our analysis. The management of breast cancer has evolved over the past decade, for example, through increasing use of taxane-based chemotherapy and less extensive node dissections (i.e., increased use of sentinel dissections). Such changes may affect long-term survival, and hence outcomes in the decade ahead may differ from those reported here.
Conclusions
According to the data reported here, breast cancer survival rates in British Columbia were at the high end of those reported from other regions of the world. Most patients with a diagnosis of early-stage disease were treated with breast-conserving surgery, as well as adjuvant radiotherapy and hormone therapy. This report also provides long-term outcomes for subgroups by stage, with detailed information on treatment at the population level; such data have only rarely been reported.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/1/4/E134/suppl/DC1
Supplementary Material
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36 [DOI] [PubMed] [Google Scholar]
- 2.Olivotto IA, Mates D, Kan L, et al. Prognosis, treatment, and recurrence of breast cancer for women attending or not attending the Screening Mammography Program of British Columbia. Breast Cancer Res Treat 1999;54:73-81 [DOI] [PubMed] [Google Scholar]
- 3.Schopper D, de Wolf C. How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer 2009;45:1916-23 [DOI] [PubMed] [Google Scholar]
- 4.Christensen LH, Engolm G, Cortes R, et al. Reduced mortality for women with mammography-detected breast cancer in east Denmark and south Sweden. Eur J Cancer 2006;42:2773-80 [DOI] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106 [DOI] [PubMed] [Google Scholar]
- 6.Dragun AE, Huang B, Tucker TC, et al. Disparities in the application of adjuvant radiotherapy after breast-conserving therapy for early stage breast cancer: impact on overall survival. Cancer 2011;117:2590-8 [DOI] [PubMed] [Google Scholar]
- 7.Martinez SR, Tseng WH, Canter RJ, et al. Do radiation use disparities influence survival in patients with advanced breast cancer? Cancer 2012;118:196-204 [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717 [DOI] [PubMed] [Google Scholar]
- 9.Chia SK, Speers CH, D’yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007;110:973-9 [DOI] [PubMed] [Google Scholar]
- 10.Coleman MP, Forman D, Bryant H, et al. ICBP Module 1 Working Group. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdecchia A, Francisci S, Brenner H, et al. EUROCARE-4 Working Group Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol 2007;8:784-96 [DOI] [PubMed] [Google Scholar]
- 12.Coleman MP, Quaresma M, Berrino F, et al. CONCORD Working Group Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730-56 [DOI] [PubMed] [Google Scholar]
- 13.Screening Mammography Program 2012 annual report Vancouver (BC): BC Cancer Agency; 2012. Available: www.screeningbc.ca/NR/rdonlyres/8CD1608D-BE23-41EC-A5E6-8ADE5119F6E4/61384/SMP_2012AR_WEB2.pdf (accessed 2013 Nov. 4).
- 14.Coldman AJ, Phillips N, Speers C. A retrospective study of the effect of participation in screening mammography on the use of chemotherapy and breast conserving surgery. Int J Cancer 2007;120:2185-90 [DOI] [PubMed] [Google Scholar]
- 15.Olson RA, Nichol A, Caron NR, et al. Effect of community population size on breast cancer screening, stage distribution, treatment use and outcomes. Can J Public Health 2012;103:46-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivotto IA, Bajdik CD. ADJUVANT! – a validated, web-based, systemic therapy decision aid for early-stage breast cancer. Am J Oncol Rev 2005;4(Suppl 13):29-31 [Google Scholar]
- 17.About BC Cancer Agency. Vancouver (BC): BC Cancer Agency. Available: www.bccancer.bc.ca/ABCCA/default.htm (accessed 2013 Oct 30).
- 18.Provincial, territorial data quality report, Canadian Cancer Registry 2005 Ottawa (ON): Statistics Canada; 2005. [Google Scholar]
- 19.Berry DA, Baines CJ, Baum M, et al. Flawed inferences about screening mammography’s benefit based on observational data. J Clin Oncol 2009;27:639-40 [DOI] [PubMed] [Google Scholar]
- 20.Ellison LF, Gibbons L. Leading cancers — changes in five-year relative survival. Health Rep 2004;15:19-32 [PubMed] [Google Scholar]
- 21.Ellison LF, Wilkins K. An update on cancer survival. Health Rep 2010;21:1-6 [PubMed] [Google Scholar]
- 22.Ries LAG, Eisner MP. Chapter 13: Cancer of the female breast. In: Ries LAG, Young JL Jr, Keel GE, et al., editors. SEER survival monograph: cancer survival among adults: U.S. SEER program, 1988–2001. Patient and tumour characteristics Bethesda (MD): National Cancer Institute. Publ. no. 07-6215. p. 101-10. Available: http://seer.cancer.gov/publications/survival/surv_toc.pdf (accessed 2012 Nov. 14).
- 23.Gondos A, Volker A, Holleczek B, et al. Cancer survival in Germany and the United States at the beginning of the 21st century: an up-to-date comparison by period analysis. Int J Cancer 2007;121:395-400 [DOI] [PubMed] [Google Scholar]
- 24.Foroudi F, Tyldesley S, Walker H, et al. An evidence-based estimate of appropriate radiotherapy utilization rate for breast cancer. Int J Radiat Oncol Biol Phys 2002;53:1240-53 [DOI] [PubMed] [Google Scholar]
- 25.Delaney G, Barbon M, Jacob S. Estimation of an optimal radiotherapy utilization rate for breast carcinoma. Cancer 2003;98:1977-86 [DOI] [PubMed] [Google Scholar]
- 26.Ng W, Delaney GP, Jacob S, et al. Estimation of optimal chemotherapy utilization rate for breast cancer: setting an evidence-based benchmark for the best-quality cancer care. Eur J Cancer 2010;46:703-12 [DOI] [PubMed] [Google Scholar]
- 27.Fong A, Ng W, Barton MB, et al. Estimation of an evidence-based benchmark for the optimal endocrine therapy utilization rate in breast cancer. Breast 2010;19:345-9 [DOI] [PubMed] [Google Scholar]
- 28.Chan A, Speers C, O’Reilly S, et al. Adherence of adjuvant hormonal therapies in post-menopausal hormone receptor positive (HR+) early stage breast cancer: a population based study from British Columbia [abstract 36]. Cancer Res 2009;69(Suppl 3):494s [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.