Abstract
Optical stimulation of the inner ear has recently attracted attention, suggesting a higher frequency resolution compared to electrical cochlear implants due to its high spatial stimulation selectivity. Although the feasibility of the effect is shown in multiple in vivo experiments, the stimulation mechanism remains open to discussion. Here we investigate in single-cell measurements the reaction of spiral ganglion neurons and model cells to irradiation with a nanosecond-pulsed laser beam over a broad wavelength range from 420 nm up to 1950 nm using the patch clamp technique. Cell reactions were wavelength- and pulse-energy-dependent but too small to elicit action potentials in the investigated spiral ganglion neurons. As the applied radiant exposure was much higher than the reported threshold for in vivo experiments in the same laser regime, we conclude that in a stimulation paradigm with nanosecond-pulses, direct neuronal stimulation is not the main cause of optical cochlea stimulation.
OCIS codes: (110.5125) Photoacoustics; (170.1065) Acousto-optics; (170.4940) Otolaryngology; (170.1530) Cell analysis; (140.3600) Lasers, tunable; (350.5340) Photothermal effects
1. Introduction
Cochlear implants can restore auditory function in large parts by electrical stimulation of the auditory nerve, helping people suffering from sensorineural hearing loss to achieve proper speech perception. However, hearing performance is dramatically reduced in noisy environments, which has been partially attributed to the low spatial precision of the electric stimulation. Due to the tonotopy of the cochlea, low spatial specificity corresponds to low frequency specificity. Laser light, on the other hand, can be focused to stimulate tissue very site-specific and has therefore recently attracted attention for the stimulation of the cochlea [1]. Fridberger and Ren [2] demonstrated local mechanical stimulation of the hearing organ by laser irradiation at a wavelength of 813 nm with a pulse duration of 50 µs and measured resulting basilar membrane motions as well as cochlear microphonic potentials. Richter et al. furthermore showed that the spread of cochlear excitation during stimulation with pulsed infrared radiation at a wavelength of 1860 nm and a pulse duration of 100 µs is comparable to that produced by acoustic tone pips by recording spike activity in the central nucleus of the inferior colliculus (ICC) [3]. They suggested a direct stimulation of spiral ganglion neurons by laser irradiation due to a thermal effect.
Laser tissue interaction can be categorized as follows: no confinement, thermal confinement and stress confinement. This depends mainly on the duration of the laser pulse and if it is short compared to the thermal (thermal confinement) or stress (stress confinement) relaxation time in the tissue [4]. Successful optical stimulation of the cochlea has been shown in both thermal [5] and stress [6] confinement with the benefit of low thresholds of only a few µJ pulse energy for short pulse durations [7,8]. Low pulse energy means also low energy deposition in the tissue and therefore reduced heating effects which could be beneficial for the long-term application of the technique in a future device.
Although optical stimulation of the cochlea was shown in multiple in vivo experiments, the stimulation mechanism remains unclear. Recently, it was reported that irradiation of cells with infrared laser light excites them by changing their membrane capacitance by temperature increase of the surrounding medium due to the absorption of the laser light [9]. This would be a possible photo-thermal mechanism to stimulate spiral ganglion neurons in the cochlea. Other studies suggest an optoacoustic effect, where irradiation with laser light results in pressure pulses, finally stimulating hair cells in the cochlea [8,10,11]. Furthermore, it was shown that pulsed infrared irradiation can trigger the intracellular release of Ca2+ ions, probably modulating the mitochondrial Ca2+ transport [12]. In general, laser irradiation and subsequent temperature changes can modulate intracellular signaling [13,14].
In this study we investigated the basic effects and mechanisms of optical cell stimulation in stress confinement with a nanosecond-pulsed laser beam over a broad wavelength range from 420 nm up to 1950 nm. Single-cell measurements on irradiated HEK 293 cells, on HEK 293 cells stably transfected with the human ClC-4 channel [15] as model cells for voltage-gated cells as well as on spiral ganglion neurons from Sprague-Dawley rats were performed using the patch clamp technique. Due to the short pulse duration and therefore very low pulse energy needed to reach thresholds of optical in vivo cochlea stimulation [6,8], heating effects were minimal. Cell reactions due to the laser irradiation were wavelength- and pulse-energy-dependent but too small to elicit action potentials in the investigated spiral ganglion neurons.
2. Materials and methods
2.1 Spiral ganglion cell preparation and cultivation
Spiral ganglion cells were dissected from the cochleae of decapitated P3 – P6 neonatal Sprague-Dawley rats of both sexes and prepared as previously described [16]. The procedure was approved by the Laboratory Animal Science Center of the Hannover Medical School and conducted in accordance with the German ‘Law on Protecting Animals’ (§4/03 TierSchG) and with the European Communities Council Directive 2010/63/EU. Briefly, the isolated spiral ganglia were incubated for 18 – 20 min at 37°C in 2 ml Ca2+/Mg2+-free HBSS (Invitrogen) solution which contained 0.1% trypsin (Biochrom) and 0.01% DNase I (Roche). After adding 200 µl FCS (Invitrogen) to stop the enzymatic reaction, the solution was centrifuged, the supernatant discarded and the cells rinsed three times in Panserin 401 (PAN-Biotech). The cells were then dissociated mechanically by gently pipetting the solution.
The cell suspension was plated onto poly-DL-ornithine (0.1 mg/ml, Sigma-Aldrich) and laminin (0.01 mg/ml, Invitrogen) coated coverslips (10 mm diameter) as described previously [17,18]. The coverslips were placed in 35 mm culture dishes (Thermo Scientific) with 2 ml of serum-free culture medium. The Panserin 401 culture medium was supplemented as described for the DMEM-based medium used in previous experiments for spiral ganglion cell cultivation [19]. Dissociated cells were maintained at 37°C, 5% CO2 in a humidified atmosphere for 1 to 2 days.
2.2 HEK 293 cell culture
The HEK 293 cell line, stably transfected with the human ClC-4 channel [15], was kindly provided by Prof. Dr. Christoph Fahlke (Forschungszentrum Jülich, Cellular Biophysics). Furthermore the untransfected HEK 293 cell line was used. Both were grown at 37°C, 5% CO2 in a humidified atmosphere in Dulbeccos’s modified Eagle’s medium supplemented with 10% fetal calf serum. For the untransfected cell line 100 U/ml penicillin and 0.1 mg/ml streptomycin were added and for the transfected cell line 0.9 mg/ml Geneticin G418 (all components from Biochrom AG).
2.3 Laser stimulation
For optical stimulation a tunable laser system, based on an optical parametric oscillator (OPO), was used (NT342A, Ekspla). The system delivered laser pulses with a pulse length of 3-5 ns and a repetition rate of 10 Hz. The laser beam was coupled into a multimode optical fiber of 105 µm core diameter (AFS105/125Y, Thorlabs). Pulse energies were measured directly behind the tip of the optical fiber in air with a pyroelectric energy detector (DPJ8, Spectrum Detector Inc / Gentec-EO.). For the wavelength study we investigated the wavelength range between 420 - 1950 nm at a constant pulse energy of 15 µJ at all wavelengths. For the other measurements, pulse energies of up to 25 µJ were applied, which corresponds to a maximum radiant exposure of 289 mJ/cm2, calculated with the nominal fiber core diameter.
The optical fiber was mounted to a micromanipulator (MHW-3, Narishige) and additionally stabilized by leading it through a glass pipette (GB150EFT, Science Products) to overcome the surface tension of the water. It was cleaved prior to each experiment and brought close to the cell to a distance of approximately 100 µm. The reactions of the irradiated cells were measured via the whole-cell patch clamp technique [20].
2.4 Electrophysiology
Thin-walled, borosilicate capillary glass patch pipettes with filament (GB150EFT, Science Products) were produced using a micropipette puller (P97, Sutter Instrument) and fire-polished on a microforge (MF-830, Narishige). Pipette resistances were 2 - 4 MΩ.
Measurements were carried out with the patch clamp amplifier EPC10 (HEKA Elektronik Dr. Schulze GmbH). Usually, currents were recorded with a 20 µs time resolution and low-pass filtered with a 4-pole Bessel filter at 2.9 kHz. Additionally, due to the series resistance and cell capacitance in the whole-cell configuration, the cell itself acted as a low-pass filter with a typical cutoff frequency in our experiments of 1.5-3.0 kHz. Therefore, one has to bear in mind that the measured current responses are not the actual currents evoked by the laser stimulation, but are filtered and delayed. It is not possible to detect currents with a nanosecond or microsecond resolution in whole-cell experiments but the recorded currents still describe the behavior of the cell over a longer timescale. The patch pipette was mounted to a micromanipulator (MHW-3, Narishige) and the patch clamp procedure optically controlled by an upright microscope (BX51WI, Olympus), additionally equipped with a digital camera (UI-1450-M, IDS Imaging Development Systems GmbH).
The external solution for spiral ganglion neurons consisted of (in mM) NaCl 155, KCl 5.8, CaCl2 1.3, MgCl2 0.9, HEPES 10, D-glucose 5.6, pH adjusted to 7.4 with NaOH, the internal solution of (in mM) KCl 135, MgCl2 3.5, HEPES 5, EGTA 5, CaCl2 0.1, Na2-ATP 2.5, Na2-GTP 1, pH adjusted to 7.2 with KOH (composition adopted from [21]).
The external solution for HEK293 cells (transfected and untransfected) consisted of (in mM) NaCl 145, KCl 4, CaCl2 2, MgCl2 1, HEPES 5, pH adjusted to 7.4 with NaOH, the internal solution of (in mM) NaCl 120, MgCl2 2, HEPES 10, EGTA 5, pH adjusted to 7.4 with NaOH. Chemicals were purchased from Th. Geyer Hamburg, Carl Roth and Merck KGaA. All experiments were conducted at room temperature.
2.5 Temperature measurements
The temperature change due to laser irradiation was measured using the method of Yao et al. [22], which employs the temperature dependent conductance change of an open pipette. To calibrate the system, the electrode current I of an open pipette was measured in bath solution of known temperature T and fitted to
| (1.1) |
where T 0 is the room temperature, I 0 is the pipette current at T 0 and a = Ea / R the fit parameter with Ea the activation energy and R the gas constant. In order to minimize errors due to long-time voltage fluctuations, the electrode current was always determined for a 5 mV voltage jump.
For temperature measurements, the pipette was positioned at approximately the same distance to the optical fiber as in the actual cell measurements and was held at a potential of 5 mV. The tip of the pipette was irradiated with laser pulses and the current time courses were collected. The corresponding temperature time courses were calculated by
| (1.2) |
3. Results
A representative whole-cell measurement in the voltage-clamp configuration of a HEK 293 cell, stably transfected with the human ClC-4 channel, is shown in Fig. 1 . The cell was held at 0 mV and test potentials were applied from –80 mV to + 70 mV in 10 mV intervals. For clarification not all traces are shown. The irradiation of the cell with a laser pulse of 1450 nm wavelength and pulse energy of 15 µJ elicited inward currents, their magnitudes depending linearly on the holding potential (Fig. 2(a) ). For better comparability between different cells, the currents were normalized to cell capacitance. The amplification (Fig. 1(b)) demonstrates that the cell reaction starts directly after the irradiation of the cell reaching the maximum current (0.17 ± 0.01) ms after the laser pulse. Voltage-dependent activation of the ClC-4 channel could be detected for voltages more positive than + 50 mV in accordance with [15]. As optical stimulation for subsequent analyses was performed at resting potential, we could be sure that the majority of ClC-4 channels were still closed.
Fig. 1.

(a) Current response of a HEK-hClC4 cell in voltage-clamp configuration to irradiation with a laser pulse (1450 nm wavelength, 15 µJ pulse energy). The red arrow represents the time point of the laser irradiation. (b) Amplification of the time at which the laser light irradiates the cell.
Fig. 2.

(a) Dependence of the current change on the holding potential (1450 nm wavelength, 15 µJ pulse energy, normalized to cell capacitance) for HEK cells. (b) Dependence of the current change on the energy of the laser pulse (1450 nm wavelength, normalized to cell capacitance). Error bars depict standard deviation.
The amplitudes of the current spikes show a linear dependence on laser pulse energy (Fig. 2(b)). This is true for all investigated cell types, i.e. transfected and untransfected HEK 293 cells and spiral ganglion neurons (SGN). The current response was always investigated near resting potential, which means due to the composition of the solution 0 mV for the transfected and untransfected HEK 293 cells and around −60 mV for the SGN. Every measurement was repeated 20 times, error bars depict standard deviation. The slopes of the single examined cells differ, but we could not discover a significant difference between the cell types.
The cell reaction due to the laser irradiation depends strongly on the wavelength of the laser light (Fig. 3(a) ). We investigated wavelengths from 420 nm up to 1950 nm, whereas every wavelength measurement was repeated 20 times. Only for wavelengths of at least 1375 nm we could detect a significant change in current flow through the cell membrane. For a comparison of 9 different cells, their reactions were normalized to each cells current change at a wavelength of 1450 nm (Fig. 3(b)). The cell reaction has a first maximum at 1450 nm, diminishes for larger wavelengths, increases again after 1700 nm and finally starts to decrease for wavelengths larger than 1890 nm. Over the wavelength range up to 1890 nm, the reaction is in good agreement with the wavelength dependence of the absorption coefficient of water μ (H20) [23]. For larger wavelengths, when the absorption coefficient rises steeply, they clearly differ from each other. For wavelengths above 1890 nm, the cell reaction decreases although the absorption coefficient still rises to 126 cm−1 at 1950 nm. This can be seen more clearly in Fig. 3(c), which shows the pooled arithmetic means over all cells. For the measurements at the highest wavelengths of 1900 nm and 1950 nm the variance between the cell reactions was considerably larger than for all other wavelengths. All cells, independent of their cell type (transfected and untransfected HEK 293 cells and spiral ganglion neurons), showed in the discussed wavelength range a good agreement between the wavelength dependence of the laser-induced current change and the absorption coefficient of water.
Fig. 3.
(a) Dependence of the current change on the wavelength of the laser pulse (15 µJ pulse energy) for different cells. Each cell was measured 20 times, error bars depict standard deviation. (b) Each cell was normalized to its current change at 1450 nm and compared to the absorption coefficient of water. (c) Pooled normalized arithmetic means of all cells (n = 9) compared to the absorption coefficient of water. The grey shaded area represents the variation of the current change without laser irradiation. It is calculated from all cells and shows the arithmetic mean ± standard deviation.
3.1 Spiral ganglion neurons
Before spiral ganglion neurons were stimulated optically, their electrophysiological behavior was investigated. Only cells that showed a normal resting potential and were capable of generating action potentials were included in the study. Figure 4 represents a typical spiral ganglion neuron with the expected ionic currents [24] and the ability to generate action potentials after electrical depolarization.
Fig. 4.

(a) Spiral ganglion neuron in voltage clamp configuration. The cell was held at −60 mV near its resting potential and was depolarized for 100 ms (starting at 50 ms) to 0mV. The cell showed the expected Na+ inward current, followed by a K+ outward current. (b) The same spiral ganglion neuron in current clamp configuration. Current injection of + 300 pA for 100 ms (starting at 50 ms) generated an action potential.
Irradiated spiral ganglion neurons in voltage clamp configuration (Fig. 5 ) showed marked differences in their reactions to the laser irradiation for positive test potentials in contrast to the investigated HEK 293 cells (Fig. 1). Spiral ganglion neurons were held at −60 mV and test potentials were applied from –100 mV to + 80 mV in 20 mV intervals. For negative potentials up to −40 mV all cells showed the typical inward current as a reaction to the laser irradiation. In combination with more positive potentials, however, the laser irradiation resulted in outward currents of up to 186 pA with a much longer decay constant of (6.7 ± 4.9) ms (n = 4) at + 80 mV. This corresponds with the thermal relaxation time which is measured and calculated in section 3.2 for the given setup. For cells at resting potential this was not the case as the whole response of the cell took place in less than 0.5 ms.
Fig. 5.
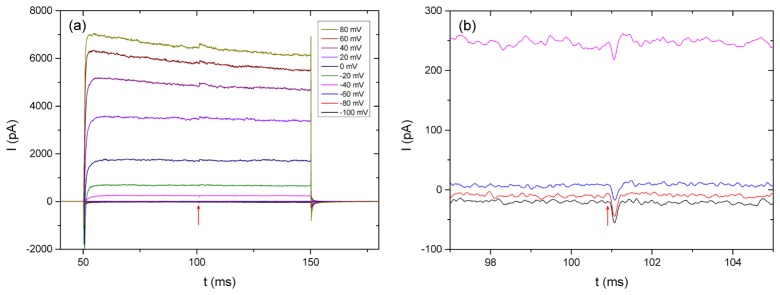
(a) Current response of a spiral ganglion neuron in voltage-clamp configuration to irradiation with a laser pulse (1450 nm wavelength, 15 µJ pulse energy). (b) Amplification of the −100 mV to −40 mV trace at the time point of the irradiation (red arrow).
While all cells exhibited strongly voltage dependent inward sodium and outward potassium currents during depolarizing voltage steps, the quasi steady-state current-voltage relations show a considerable spread for the different spiral ganglion neurons (Fig. 6(a) ). Whereas the laser induced current change of the HEK 293 cells depended linearly on the holding potential (Fig. 2(a)), this is only partially true for the spiral ganglion neurons (Fig. 6(b)). For negative voltages up to −40 to −20 mV, depending on the cell, the laser induced current change also shows the linear dependence on the holding potential. But for more positive potentials, the gradient of the slope changes to a significant higher value. This is consistent with the current-voltage relation in Fig. 6(a) where the current increase due to voltage-gated channel openings takes place at the same voltage. For these more positive potentials changes the laser induced inward current to an outward current.
Fig. 6.

(a) Quasi steady-state current-voltage relations for different spiral ganglion neurons (currents measured at the end of the 100 ms test potentials). Each class of symbols represents one spiral ganglion neuron. (b) Dependence of the laser induced current change on the holding potential (1450 nm wavelength, 15 µJ pulse energy).
Current clamp measurements of irradiated spiral ganglion neurons (Fig. 7 ) show a depolarizing effect of the laser light to the cells around resting potential. This effect is pretty small and results for the maximal laser pulse energy of 25 µJ in a depolarization of (1.2 ± 0.9) mV (n = 5) with a typical natural voltage fluctuation of 0.2 mV (root mean square error for 1 ms). The depolarization is therefore by far not big enough to elicit an action potential. Even when superimposing optical stimulation with electrical depolarization (just below the threshold to generate an action potential) we could not observe the generation of action potentials due to laser pulses. In very few cells, when large positive currents were injected that led to positive membrane potentials, laser irradiation resulted in hyperpolarization. For most spiral ganglion neurons, however, no reaction to the laser irradiation, discriminable from natural voltage fluctuations, could be identified in this condition.
Fig. 7.

Voltage response of a spiral ganglion neuron in current-clamp configuration at resting potential to irradiation with a laser pulse (1450 nm wavelength, 25 µJ pulse energy). The red arrow represents the time point of the laser irradiation.
3.2 Temperature change
The comparison of the increased pipette current due to heated bath solution of known temperature with the pipette current at room temperature (295.8 K) resulted in the calibration curve in Fig. 8(a) (see Eq. 1.1). With the obtained fit parameter a = (1677 ± 108) K, the current time course of an irradiated pipette could be converted to a temperature time course (Fig. 8(b)). The applied laser pulses of 25 µJ pulse energy produced an almost linear temperature rise (rise time of 0.15 ms) of (1.4 ± 0.2) K at 1450 nm wavelength and (0.8 ± 0.1) K at 1850 nm (n = 10). After reaching the maximum, the temperature decreased with a time constant of τ 1/2 ~8 ms. This is in good agreement with the estimation of the thermal relaxation time t r of water. For a cylinder of heated medium with the diameter d, the thermal relaxation time can be approximated by tr = d 2 /16κ [25] with the thermal diffusivity of water κ = 1.43 × 10−7 m2s−1. Due to beam expansion, the irradiated area 100 µm behind the optical fiber has a diameter of approximately 140 µm, which leads to a calculated thermal relaxation time t r of 8.6 ms.
Fig. 8.
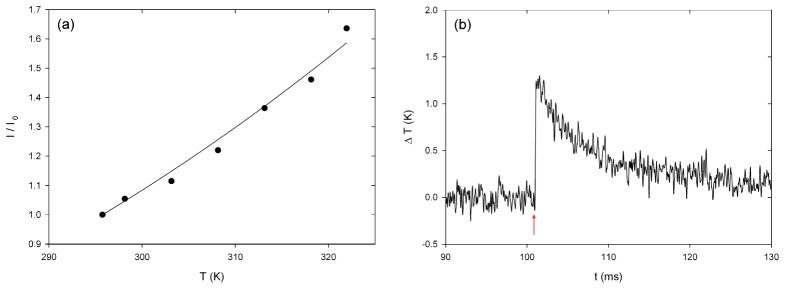
(a) Calibration curve of pipette current at different temperatures normalized to current at room temperature. (b) Temperature change due to irradiation with a laser beam (λ = 1450 nm, 25 µJ pulse energy), 100 µm off the optical fiber. The red arrow represents the time point of the laser irradiation.
4. Discussion
Successful optical stimulation of the cochlea has been shown in several in vivo studies. Stimulations with short laser pulses in the stress [6,8,10] and thermal [1,7] confinement regime (here for pulse durations < 300 µs) seem to be especially promising. Compared to stimulations with longer pulse durations one can use lower laser pulse energies of only a few µJ to elicit hearing sensations which lead to a lower temperature rise in the irradiated area. Multiple explanations for the stimulation mechanism have been proposed [26]. The most noted are (a) a general cell excitation mechanism due to a laser induced change of the membrane capacitance by local heating [9], which could lead to the generation of action potentials in spiral ganglion neurons and (b) the generation of a laser induced pressure pulse which finally stimulates the inner hair cells in the cochlea [8]. Zhang et al. demonstrated that pulsed laser irradiation induces optoacoustic waves within the cochlea and localized vibrations of the basilar membrane in close proximity to the optoacoustic wave’s source [10]. This may replace outer hair cell motility effects [27] and lead to the stimulation of inner hair cells.
Single-cell measurements on spiral ganglion neurons of the inner ear, irradiated over a broad wavelength range in order to determine the optical stimulation mechanism are still missing. In this in vitro study, we could demonstrate that irradiation with laser light does trigger similar electrophysiological responses in very different cell types at resting potential: in spiral ganglion neurons and in transfected (ClC-4) and untransfected HEK 293 cells, suggesting that the cellular response does not depend on a special cell type or ion channel. Laser stimulation resulted in inward currents for cells hold at their resting potential. These currents depended linearly on laser pulse energy and led to a depolarization of the cells. This depolarization effect is very small, reaching maximally (1.2 ± 0.9) mV for spiral ganglion neurons irradiated with laser pulses of 25 µJ pulse energy at a wavelength of 1450 nm and is therefore not high enough to cause action potentials. In our experiments, depolarizations to resting potential between 20 mV and 40 mV were needed to activate sodium channels and with it generate action potentials in SGN which is in good agreement with Moore et al. [24].
In vivo studies on guinea pigs with nanosecond (ns) laser pulse stimulation showed the generation of compound action potentials for laser pulse energies as low as 3 µJ [8], which corresponds to a radiant exposure of 35 mJ/cm2. In this study, we applied laser pulses with energies of up to 25 µJ corresponding to a maximum radiant exposure of 289 mJ/cm2, calculated with the nominal fiber core diameter. Due to the widening of the beam after the optical fiber, the radiant exposure in 100 µm distance to the fiber (distance between fiber and investigated cell) is reduced to 167 mJ/cm2. As the fiber-cell-distance in the cochlea in in vivo experiments is much larger than 100 µm [28], which corresponds to an even larger beam widening, the obtained radiant exposure in our experiments was considerably higher than 8 times the reported threshold for in vivo experiments. Despite these much higher radiant exposure levels, action potential generation in spiral ganglion neurons was by far not possible. Therefore, our results suggest that in a stimulation paradigm with nanosecond-pulses, direct neuronal stimulation is not the main cause of the optical stimulation of the cochlea.
Even if direct neuronal stimulation is too small to account for in vivo cochlea stimulation with low energy nanosecond laser pulses, it cannot be ruled out that for longer pulse durations, corresponding to considerably more energy deposition in the tissue, it may play a role. It has been shown that action potentials in retinal and vestibular primary neurons can be generated with near infrared laser pulses of 2 ms to 30 ms duration [29]. However, the radiant exposure to reach stimulation threshold was higher than 15 J/cm2, which is around two orders of magnitude higher than the maximum radiant exposure we used in our experiments. The disadvantage of this stimulation configuration is the temperature rise from room temperature to 55 – 60°C in the vicinity of the cell. Short-pulsed laser stimulation can prevent this problem. Our experiments demonstrated for laser pulses with 25 µJ pulse energy at 1850 nm wavelength a temperature rise of (0.8 ± 0.1) K, which could be suitable even for long term application. Albert et al. [30] showed that in retinal and vestibular ganglion neurons the generation of action potentials is possible for high radiant exposures of 20-60 J/cm2. This stimulation depended on the function of the temperature sensitive TRPV4 channel, which is expressed in these cells. Pulsed infrared irradiation with a radiant exposure of more than 10 J/cm2 can also trigger the intracellular release of Ca2+ ions (shown in cardiomyocytes [12]). These effects seem to rely on the temperature change, induced by the absorbed laser irradiation. A similar mechanism could be possible for spiral ganglion neurons, too.
In this study, the demonstrated, laser light induced cell reaction, is strongly wavelength dependent (Fig. 3) and shows only small distinctions between different cell types at resting potential. The magnitude of the cell reaction follows clearly the optical absorption coefficient of water and with it the laser induced temperature change. Only for wavelengths bigger than 1890 nm (corresponding to the water absorption coefficient μ (H20) > 50 cm−1), this behavior changes and the cell reaction decreases with increasing water absorption coefficient. Most likely, this is due to the high absorption of the laser light in the water even before it reaches the cell, since the penetration depth in water at 1950 nm is only about 80 µm. Concordantly, Thompson et al. calculated, that because of the absorption loss in the medium before the cell, the maximal energy absorption at cell level (for a fiber-cell-distance of 250 µm) occurs at a wavelength of 1885 nm [31]. In the wavelength range between 420 nm and 1350 nm, where the absorption coefficient is small (μ (H20) < 4 cm−1), we could not detect a significant cell reaction. This findings support the theory of cell excitation based on a laser induced temperature change. Furthermore, we demonstrated that this effect is not big enough to explain positive in vivo results, especially in vivo results where visible green laser light was used [6] resulting in a water absorption coefficient of μ (H20) < 0.001 cm−1 [32].
Therefore we propose a split mechanism: Local heating with a possible subsequent capacitance change of the cell membrane seems to affect all irradiated cells but appears to be, for low energy pulses with a duration in the nanosecond regime, not the main reason for optical cochlea stimulation. Typical radiant exposure levels used in in vivo experiments with positive results are not high enough to cause action potentials in spiral ganglion neurons, irradiated with ns-pulsed laser pulses, which would be a requirement for direct neuronal stimulation in the cochlea with laser pulses in the same regime. Our results rather suggest that the stimulation mechanism in the cochlea does not require large temperature changes. One possible explanation could therefore be an optoacoustic effect, relying on hair cell stimulation due to laser-generated pressure pulses, supporting the results from Wenzel et al. [6] and Schultz et al. [8].
Acknowledgments
We would like to thank the German Research Foundation (SFB Transregio 37, A5) and the state of Lower Saxony (Georg-Christoph-Lichtenberg Scholarship) for funding and Athanasia Warnecke, Jana Schwieger, Katharina Kranz and Melanie Steffens for their support.
References and links
- 1.Izzo A. D., Richter C. P., Jansen E. D., Walsh J. T., Jr, “Laser Stimulation of the Auditory Nerve,” Lasers Surg. Med. 38(8), 745–753 (2006). 10.1002/lsm.20358 [DOI] [PubMed] [Google Scholar]
- 2.Fridberger A., Ren T., “Local mechanical stimulation of the hearing organ by laser irradiation,” Neuroreport 17(1), 33–37 (2006). 10.1097/01.wnr.0000195665.22714.ee [DOI] [PubMed] [Google Scholar]
- 3.Richter C. P., Rajguru S. M., Matic A. I., Moreno E. L., Fishman A. J., Robinson A. M., Suh E., Walsh J. T., Jr, “Spread of cochlear excitation during stimulation with pulsed infrared radiation: inferior colliculus measurements,” J. Neural Eng. 8(5), 056006 (2011). 10.1088/1741-2560/8/5/056006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L. V. Wang and H. Wu, Biomedical optics: principles and imaging (Wiley-Interscience, Hoboken, NJ, 2007) [Google Scholar]
- 5.Richter C. P., Bayon R., Izzo A. D., Otting M., Suh E., Goyal S., Hotaling J., Walsh J. T., Jr, “Optical stimulation of auditory neurons: Effects of acute and chronic deafening,” Hear. Res. 242(1-2), 42–51 (2008). 10.1016/j.heares.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel G. I., Balster S., Zhang K., Lim H. H., Reich U., Massow O., Lubatschowski H., Ertmer W., Lenarz T., Reuter G., “Green laser light activates the inner ear,” J. Biomed. Opt. 14(4), 044007 (2009). 10.1117/1.3174389 [DOI] [PubMed] [Google Scholar]
- 7.Izzo A. D., Walsh J. T., Jr, Ralph H., Webb J., Bendett M., Wells J., Richter C. P., “Laser Stimulation of Auditory Neurons: Effect of Shorter Pulse Duration and Penetration Depth,” Biophys. J. 94(8), 3159–3166 (2008). 10.1529/biophysj.107.117150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz M., Baumhoff P., Maier H., Teudt I. U., Krüger A., Lenarz T., Kral A., “Nanosecond laser pulse stimulation of the inner ear-a wavelength study,” Biomed. Opt. Express 3(12), 3332–3345 (2012). 10.1364/BOE.3.003332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro M. G., Homma K., Villarreal S., Richter C. P., Bezanilla F., “Infrared light excites cells by changing their electrical capacitance,” Nat. Commun. 3, 736 (2012). 10.1038/ncomms1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K. Y., Wenzel G. I., Balster S., Lim H. H., Lubatschowski H., Lenarz T., Ertmer W., Reuter G., “Optoacoustic induced vibrations within the inner ear,” Opt. Express 17(25), 23037–23043 (2009). 10.1364/OE.17.023037 [DOI] [PubMed] [Google Scholar]
- 11.Teudt I. U., Maier H., Richter C. P., Kral A., “Acoustic events and “optophonic” cochlear responses induced by pulsed near-infrared laser,” IEEE Trans. Biomed. Eng. 58(6), 1648–1655 (2011). 10.1109/TBME.2011.2108297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittami G. M., Rajguru S. M., Lasher R. A., Hitchcock R. W., Rabbitt R. D., “Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes,” J. Physiol. 589(6), 1295–1306 (2011). 10.1113/jphysiol.2010.198804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajith Karunarathnea W. K., Giri L., Kalyanaramana V., Gautama N., “Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension,” Proc. Natl. Acad. Sci. 110(17), 1565–1574 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderwood S. K., Stevenson M. A., Hahn G. M., “Heat Stress Stimulates Inositol Trisphosphate Release and Phosphorylation of Phosphoinositides in CHO and Balb C 3T3 Cells,” J. Cell. Physiol. 130(3), 369–376 (1987). 10.1002/jcp.1041300309 [DOI] [PubMed] [Google Scholar]
- 15.Hebeisen S., Heidtmann H., Cosmelli D., Gonzalez C., Poser B., Latorre R., Alvarez O., Fahlke C., “Anion Permeation in Human ClC-4 Channels,” Biophys. J. 84(4), 2306–2318 (2003). 10.1016/S0006-3495(03)75036-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warnecke A., Sasse S., Wenzel G. I., Hoffmann A., Gross G., Paasche G., Scheper V., Reich U., Esser K.-H., Lenarz T., Stöver T., Wissel K., “Stable release of BDNF from the fibroblast cell line NIH3T3 grown on silicone elastomers enhances survival of spiral ganglion cells in vitro and in vivo,” Hear. Res. 289(1-2), 86–97 (2012). 10.1016/j.heares.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Hegarty J. L., Kay A. R., Green S. H., “Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range,” J. Neurosci. 17(6), 1959–1970 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wefstaedt P., Scheper V., Lenarz T., Stöver T., “Brain-derived neurotrophic nfactor/glial cell line-derived neurotrophic factor survival effects are not limited by dexamethasone,” Neuroreport 16, 2011–2014 (2005). 10.1097/00001756-200512190-00008 [DOI] [PubMed] [Google Scholar]
- 19.Berkingali N., Warnecke A., Gomes P., Paasche G., Tack J., Lenarz T., Stöver T., “Neurite outgrowth on cultured spiral ganglion neurons induced by erythropoietin,” Hear. Res. 243(1-2), 121–126 (2008). 10.1016/j.heares.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 20.Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J., “Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches,” Pflugers Arch. 391(2), 85–100 (1981). 10.1007/BF00656997 [DOI] [PubMed] [Google Scholar]
- 21.Rutherford M. A., Chapochnikov N. M., Moser T., “Spike Encoding of Neurotransmitter Release Timing by Spiral Ganglion Neurons of the Cochlea,” J. Neurosci. 32(14), 4773–4789 (2012). 10.1523/JNEUROSCI.4511-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J., Liu B., Qin F., “Rapid Temperature Jump by Infrared Diode Laser Irradiation for Patch-Clamp Studies,” Biophys. J. 96(9), 3611–3619 (2009). 10.1016/j.bpj.2009.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kou L., Labrie D., Chylek P., “Refractive indices of water and ice in the 0.65- to 2.5-µm spectral range,” Appl. Opt. 32(19), 3531–3540 (1993). 10.1364/AO.32.003531 [DOI] [PubMed] [Google Scholar]
- 24.Moore E. J., Hall D. B., Narahashi T., “Sodium and Potassium Currents of Type I Spiral Ganglion Cells from Rat,” Acta Otolaryngol. 116(4), 552–560 (1996). 10.3109/00016489609137888 [DOI] [PubMed] [Google Scholar]
- 25.Anderson R. R., Parrish J. A., “Selective Photothermolysis: Precise Microsurgery by Selective Absorption of Pulsed Radiation,” Science 220(4596), 524–527 (1983). 10.1126/science.6836297 [DOI] [PubMed] [Google Scholar]
- 26.Wells J., Kao C., Konrad P., Milner T., Kim J., Mahadevan-Jansen A., Jansen E. D., “Biophysical Mechanisms of Transient Optical Stimulation of Peripheral Nerve,” Biophys. J. 93(7), 2567–2580 (2007). 10.1529/biophysj.107.104786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuter G., Zenner H. P., “Active radial and transverse motile responses of outer hair cells in the organ of Corti,” Hear. Res. 43(2-3), 219–230 (1990). 10.1016/0378-5955(90)90230-M [DOI] [PubMed] [Google Scholar]
- 28.Goyal V., Rajguru S., Matic A. I., Stock S. R., Richter C. P., “Acute Damage Threshold for Infrared Neural Stimulation of the Cochlea: Functional and Histological Evaluation,” Anat. Rec. (Hoboken) 295(11), 1987–1999 (2012). 10.1002/ar.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bec J. M., Albert E. S., Marc I., Desmadryl G., Travo C., Muller A., Chabbert C., Bardin F., Dumas M., “Characteristics of Laser Stimulation by Near Infrared Pulses of Retinal and Vestibular Primary Neurons,” Lasers Surg. Med. 44(9), 736–745 (2012). 10.1002/lsm.22078 [DOI] [PubMed] [Google Scholar]
- 30.Albert E. S., Bec J. M., Desmadryl G., Chekroud K., Travo C., Gaboyard S., Bardin F., Marc I., Dumas M., Lenaers G., Hamel C., Muller A., Chabbert C., “TRPV4 channels mediate the infrared laser-evoked response in sensory neurons,” J. Neurophysiol. 107(12), 3227–3234 (2012). 10.1152/jn.00424.2011 [DOI] [PubMed] [Google Scholar]
- 31.Thompson A. C., Wade S. A., Brown W. G. A., Stoddart P. R., “Modeling of light absorption in tissue during infrared neural stimulation,” J. Biomed. Opt. 17(7), 0750021 (2012). 10.1117/1.JBO.17.7.075002 [DOI] [PubMed] [Google Scholar]
- 32.Pegau W. S., Gray D., Zaneveld J. R. V., “Absorption and attenuation of visible and near-infrared light in water: dependence on temperature and salinity,” Appl. Opt. 36(24), 6035–6046 (1997). 10.1364/AO.36.006035 [DOI] [PubMed] [Google Scholar]



