Abstract
Geometric distortions and poor image resolution are well known shortcomings of single-shot echo-planar imaging (ss-EPI). Yet, due to the motion immunity of ss-EPI, it remains the most common sequence for diffusion-weighted imaging (DWI). Moreover, both navigated DW interleaved EPI (iEPI) and parallel imaging (PI) methods, such as sensitivity encoding (SENSE) and generalized autocalibrating parallel acquisitions (GRAPPA), can improve the image quality in EPI. In this work, DW-EPI accelerated by PI is proposed as a self-calibrated and unnavigated form of interleaved acquisition. The PI calibration is performed on the b = 0 s/mm2 data and applied to each shot in the rest of the DW data set, followed by magnitude averaging. Central in this study is the comparison of GRAPPA and SENSE in the presence of off-resonances and motion. The results show that GRAPPA is more robust than SENSE against both off-resonance and motion-related artifacts. The SNR efficiency was also investigated, and it is shown that the SNR/scan time ratio is equally high for one- to three-shot high-resolution diffusion scans due to the shortened EPI readout train length. The image quality improvements without SNR efficiency loss, together with motion tolerance, make the GRAPPA-driven DW-EPI sequence clinically attractive.
Keywords: diffusion, susceptibility, motion, GRAPPA, SENSE
During the last decade diffusion-weighted imaging (DWI) has demonstrated great utility for the diagnostic workup of acute stroke (1). In addition, it has been used successfully for the differential diagnosis of several other abnormalities, such as tumors and brain abscesses (2–4). DWI in combination with single-shot EPI (ssDW-EPI) is still the method that is most commonly used in clinical practice, primarily due to its immunity to bulk motion and its short acquisition time. However, the image quality of ssDW-EPI is generally impaired by significant geometric distortions, intravoxel dephasing, blurring, and poor spatial resolution, which can reduce DWI’s sensitivity to acute stroke and decrease the diagnostic confidence of a DWI study.
The source and presentation of EPI-related artifacts has been well characterized (5). In general, these artifacts occur because of an intrinsically low bandwidth along the phase-encode dimension and a considerable decay during the course of the EPI readout. To overcome these artifacts, one must sample k-space more quickly, so that phase accruals caused by off-resonant spins and -induced signal loss will diminish. One popular method is to use interleaved EPI (iEPI) (6,7). This technique reduces distortions proportionately to the number of interleaves (Ninter). However, DWI using iEPI suffers from severe shot-to-shot ghosting artifacts that are induced by bulk physiologic motion during the diffusion-encoding period. Therefore, phase navigation techniques of various complexity have been suggested to overcome the ghosting artifacts (8–11). However, a solution is not guaranteed, as navigation cannot overcome the gaps that are introduced in k-space in the ky direction. Recently, parallel imaging (PI) has demonstrated great utility to reduce distortions in ssDWI-EPI (12–16). This allows formation of full-FOV images using a fraction of the phase-encoding steps compared to conventional gradient encoding alone, and thus benefits from the faster k-space traversal in the same fashion as iEPI. While several studies (12–16) have already demonstrated the great utility of sensitivity encoding (SENSE) (17) for DWI, only little is known thus far about the implementation details of generalized autocalibrating parallel acquisitions (GRAPPA) (18) for DWI, as well as its utility, strengths, and weaknesses. Recently, it was shown that GRAPPA and SENSE differ fundamentally in the way they achieve unaliased images (19), and evidence exists that with regard to residual aliasing and failure tolerance, GRAPPA may be even more robust in clinical routine than SENSE (20). For most MRI applications, many of these benefits have to do with the fact that GRAPPA scans are normally carried out in a self-calibrated fashion, and GRAPPA reconstruction artifacts are more subtle and less discrete than corresponding SENSE artifacts. Note that the original SENSE concept suggested retrieving coil sensitivity information from an additional calibration scan, whereas initial GRAPPA reconstructions suggested a variable-density k-space sampling approach in order to retrieve GRAPPA weights from the scan data itself. However, it can be shown that GRAPPA weights can be also derived from separate calibration scans and likewise SENSE coil sensitivity maps can be retrieved from variable-density k-space data (e.g., modified SENSE (mSENSE) (21)). In addition, there are other PI techniques that make use of variable-density k-space trajectories (22–25). One may now question whether or not there is actually a quality difference in DWI scans reconstructed with SENSE or GRAPPA methods, or whether or not there are any advantages in using a self-calibrated approach for DWI. In this context, it also remains unclear as to what extent a variable k-space sampling density might cause artifacts in PI reconstructions of EPI.
It was the focus of this study to address the aforementioned questions and comparatively evaluate the strengths and weaknesses of both reconstruction methods. Specifically, we focused our investigations on problems that are associated with acute-stroke patients, such as involuntary motion and the need for rapid scanning, and developing a method to simultaneously reduce geometric distortions and achieve a spatial resolution that is closer to that of conventional MRI without introducing a new class of artifacts from PI. A multishot iEPI sequence (introduced in the next section) was developed. This method uses all interleaves for PI calibration, and each interleaf is then reconstructed separately using a PI reconstruction. For DW scanning, this approach offers the improved image quality associated with a multishot acquisition, without the need for phase navigation and correction.
MATERIALS AND METHODS
Using iEPI As a Variant for Self-Calibration
Common to all PI methods is the collection of a calibration data set, either by performing a separate scan or adding extra lines to the center of k-space. For the latter, a “band” of autocalibration signal (ACS lines) is sampled around the origin of k-space at the normal Nyquist rate, while the rest of k-space is undersampled by the outer reduction factor (ORF). Due to the resulting variable sampling scheme, the net scan acceleration is lower than what would be achieved with a separate calibration scan played out up front. Of particular interest for EPI scans is the variable k-space velocity that is associated with this variable k-space sampling scheme. EPI scans carried out in this fashion may experience a greater sensitivity to off-resonances at different portions of k-space: thus, unwanted artifacts may occur in the final reconstruction. Therefore, an alternative method to extract PI calibration data for EPI that somewhat resembles the temporal GRAPPA (TGRAPPA) approach (26) is proposed. Instead of using an external calibration scan or a variable sampling density scheme, this approach uses a conventional DW iEPI acquisition as the basis sequence, with the number of interleaves being equal to the desired reduction factor. Typically in a DWI experiment, images with the diffusion-gradients turned off (b = 0 s/mm2, “b0”) are also acquired to allow for apparent diffusion coefficient (ADC) computation. In our current approach, this b0 scan is used to extract the required PI calibration information. By combining all interleaves of the b0 scan, a full k-space is formed from which either coil sensitivity maps (for SENSE) or GRAPPA weights can be extracted. Both of these can subsequently be applied to each interleave in the entire data set. Although there is no net gain in total acquisition speed over conventional iEPI acquisitions, the individual PI reconstruction of each interleave allows one to abandon phase navigation, which is otherwise needed for DW multishot sequences in order to correct for motion-induced phase perturbations. By magnitude averaging the unfolded “shot-images” originating from each interleave in the image domain, the absence of motion-related ghosting is guaranteed provided that the coil sensitivity maps and GRAPPA weights are accurate. This DW image, which is magnitude-averaged from different interleaves, produces the same image quality as if only the first interleave had been acquired (like conventional PI) and magnitude-averaged R = Ninter times.
Alternatively, if SNR permits, an optional speed improvement is to precede each DWI interleave with a different diffusion-encoding direction. With, e.g., a tetrahedral diffusion encoding scheme (27) (1 × b0 + 4 × diffusion directions; [1,1,1]T, [−1,1,1]T, [1,−1,1]T [1,1,−1]T) and, e.g., R = Ninter = 4 can be performed with a minimum of eight interleaves (four for the b0 image, and one for each of the four diffusion-encoded scans). For the current focus, which includes high spatial resolution, this shortcut was not used. Instead, some of the time penalty due to the interleaved acquisition was reduced by skipping the time-consuming (~30 s) obligate EPI reference scan for FOV/(2Ninter) ghost correction by relying on iterative postprocessing methods based on image entropy (28).
The subsequent sections compare results obtained with SENSE and GRAPPA reconstructions and investigate various types of motion and susceptibility effects to find the most favorable acquisition and reconstruction method for PI-driven DW iEPI. If not stated otherwise, the following common imaging parameters were used for the experiments: FOV = 24 cm, 10 slices with thickness/spacing = 4/1 mm, half Fourier imaging using 24 extra lines (a.k.a. overscans), acquisition matrix = 192 × 192, b = 1000 s/mm2, and TE/TR = 65–69/3–5000 ms. This iEPI approach was combined with a twice-refocused spin-echo (SE) preparation for diffusion weighting to eliminate the eddy currents generated by the diffusion-encoding gradients (29). The tetrahedral encoding scheme was used as it allows significantly reduced TEs for a given b-value. The 1.5T and 3T GE HDx research systems used for the nonclinical scans were equipped with 50- and 40-mT/m gradients, respectively. The clinical 1.5T GE system had a 40-mT/m gradient system. An eight-channel brain head coil (Invivo Corp., Peewaukee, WI, USA) was used for all nonclinical scans and some clinical scans. Alternatively, an eight-channel neurovascular array coil (Invivo Corp.), which had a somewhat lower SNR and a less optimal sensitivity profile for PI, was used for clinical scans. All procedures were approved by the review board at our institution, and written informed consent was obtained from both volunteers and stroke patients before the MR scans were conducted.
GRAPPA calibration was performed with a 2D kernel spanning two ky lines and five kx locations, in a manner similar to that described in Refs. 30 and 31. For SENSE, coil sensitivity maps were generated from the same b0 images after a rectangular smoothing kernel of 20 × 20 voxels was applied to the coil images to suppress the noise in the sensitivity maps. Other smoothing kernel sizes were also tested, and none improved the results (data not shown). No regularization was performed for the SENSE and GRAPPA reconstructions.
On Perturbations Induced Through Off-Resonances
The major motivation for adding PI to DWI is distortion reduction. In this section the reduction of these distortions is weighed against new artifacts introduced by PI. First, it is hypothesized that the variable sampling density acquisitions normally employed in GRAPPA and mSENSE are not optimal for EPI. Acquiring the center of k-space with full sampling density while sampling the outer parts sparsely with a certain ORF would lead to a greater off-resonant spin phase accrual in the low-spatial-frequency range than in the high-spatial-frequency range. In the image domain, the low spatial frequencies would be a factor ORF times more distorted than the high spatial frequencies (such as edges and tissue boundaries), thus rendering the reconstructed image self-inconsistent. To investigate this effect systematically, an experiment was performed at the 1.5T system using a cylindrical phantom containing doped water. Three scans were acquired: conventional SE, ss-EPI, and PI-accelerated ss-EPI. Both EPI scans used half Fourier, and all scans used a matrix of 256 × 256. In the accelerated EPI scan the 48 most central lines were acquired at full sampling density, and the remaining upper half of k-space was acquired with an ORF of 4, yielding a total of 48 + 26 ky lines. GRAPPA weights were estimated from the central lines and applied to the outer part of k-space to synthesize the missing lines.
Second, the impact of using GRAPPA weights and coil sensitivity maps derived from data in which the off-resonance sensitivity differs from that of the accelerated iEPI scan, but in which both acquisitions have a constant k-space velocity, was investigated. For SENSE, it is clear that significant geometric distortions will misplace coil sensitivity information that is relevant for the pixelwise unfolding operation. However, this is not so intuitive for k-space-based methods such as GRAPPA. The phase accrual due to various amounts of off-resonances will alter the relationship between neighboring lines in k-space, but the question is to which extent this will alter GRAPPA weights and how well the calibration scan and the accelerated scan must match. To enhance the effects of susceptibility distortions, this second experiment was performed on the 3T system and this time a volunteer was used to obtain relevant local susceptibility gradients. Three calibration scans were acquired (with increasing distortion levels): conventional SE, three-interleave EPI, and ss-EPI. A three-interleave (R = 3) DW-EPI scan was then acquired with a tetrahedral gradient-encoding scheme (b = 1000, NEX = 2). Coil sensitivity maps and GRAPPA weights were estimated from each of the three calibration data sets and applied to the three-interleave DW-EPI scan to generate DWIs using either SENSE or hybrid GRAPPA reconstructions.
On Perturbations Induced Through Motion
In DWI, motion artifacts can be categorized into two groups. The first category results from bulk physiologic motion between the excitation and the readout, leading to signal loss and often dark “worm-like” structures in the image. This artifact occurs primarily in the lower part of the brain and is effectively avoided by the use of peripheral or cardiac gating (32), or is reduced via multiple scan averages. However, each method will add scan time, which is why this artifact is often accepted in an acute-stroke setting for the sake of rapid scan completion.
The second category of motion artifacts involves rigid head motion that occurs between the acquisitions of different interleaves and different DW component images. These misalignments lead to blurred images, erroneous computation of ADC maps, or even ghosting artifacts in the case of interleaved acquisitions. With the proposed approach of attaining GRAPPA weights and coil sensitivity maps from the b0 image data, motion can also affect the PI calibration. For SENSE, such ghost artifacts may put the coil sensitivity at wrong positions, yielding SENSE reconstruction errors. However, it is less obvious what happens to the GRAPPA weights.
To investigate the interaction between motion and PI reconstructions, final reconstruction results with and without rigid motion correction under two different experimental conditions were compared. First, we assumed a simple head misregistration between the calibration scan and the PI scan. For the case of our PI-accelerated DW iEPI pulse sequence design, this corresponds to movement between the b0 and subsequent diffusion-encoded images without any motion between interleaves. In this case, the PI calibration derived from the b0 image should be unaffected. Second, we investigated the effects of persistent (mostly in-plane) motion during the entire scan (i.e., motion between shots, slices, and volumes). Because of the inconsistencies in the formation of the b0 image caused by this motion, one should also expect some degradation of the PI calibration. Applying the same imaging parameters used during the off-resonance experiments, a three-interleave tetrahedral DWI sequence was run three times as follows: 1) without motion, 2) with constant head motion, and 3) without motion but with the head rotated 40° from condition 1. Motion between the calibration data and the PI scan was simulated by estimating calibration information from the first b0 image in the rotated head in condition 3 and applying it to the motion-free scan in condition 1. For the persistent motion experiment, only data from condition 2 were used for estimation and application of the PI information.
After the coil sensitivity maps and GRAPPA weights were extracted from the b0 image, each interleaf of the scan was used to reconstruct an individual image using SENSE or GRAPPA. Thereafter, in-plane motion correction was performed by minimizing the sum-of-squares (SoS) differences between the b0 images (one image is formed from each shot in the scan as PI yields a full image for each interleaf). Similarly, SENSE and GRAPPA were used to reconstruct each interleaf of all the DW images, using the reconstruction of the first interleaf of the first diffusion-encoding direction as image template to register the remaining DW images. In order to capture large motion, the images were smoothed with a Gaussian filter of 15 mm for an initial coarse estimation, followed by a second iteration using a 3-mm smoothing filter to capture fine motion.
SNR
While it can be assumed that the iEPI approach presented here for DWI is preferred over phase navigation in terms of avoiding motion-induced ghosting, the use of PI normally comes with an SNR penalty as the total number of samples is reduced. A further worsening of the SNR results from g-factor noise amplification from the PI reconstruction. However, in this implementation all SENSE- or GRAPPA-reconstructed interleaves will be magnitude averaged, thereby incorporating an identical number of samples as in the ssDWI-EPI case, leaving only the g-factor noise penalty.
Another benefit of interleaved sequences is the shortened EPI trains. To some extent this reduces the TE, but more importantly reduces signal loss during the course of the EPI readout. To study the impact of certain acceleration factors for various resolutions and to investigate the SNR/scan time efficiency, brain DWI data at 1.5T using Ninter = 1–4 and image resolutions of 128 × 128 and 192 × 192 were acquired. Five repetitions (Nrep = 5) of the tetrahedral encoding scheme were acquired continuously after each other for each resolution and Ninter. Isotropic DWI maps were calculated using GRAPPA and SENSE for each repetition. From these maps, noise maps were calculated via the voxelwise relative standard deviation (SD), σrel, from the Nrep isoDWI signal intensities:
| [1] |
Because scan time increases with Ninter, the noise maps were normalized to the same effective scan time by multiplying them by , via the fact that . Hence, the noise maps presented here will actually reflect SNR/scan time efficiency. For a fair comparison of normalized scan time, the one-, two-, and four-interleave scans were magnitude-averaged using four, two, and one repetition(s), respectively (the three-shot scans were excluded because they were not divisible with five repetitions). Moreover, with twice-refocused diffusion sequences and half-Fourier imaging, the TE (and hence the SNR) becomes very sensitive to the number of overscans (i.e., the number of extra lines beyond half k-space) per interleave. Therefore, to acquire the single-shot scans in an SNR-optimal manner, the overscans were reduced to 16. In this way the TE was reduced by ~20 ms for the single-shot scans.
Clinical MR Protocol
Clinical DWI acquisitions were performed in a consecutive series of 153 patients (78 men and 75 women) who had been admitted with stroke-like symptoms. The existing product version of DW-ssEPI was run in parallel with our PI-driven interleaved DW-EPI sequence using similar parameters as for the volunteers scans above, with the exception of 23 5-mm slices and an initial TR of 4.8 s. For the first 88 patients, the interleaved DW-EPI sequence was run twice with four-shot/128 × 128 resolution and with three-shot/192 × 192 resolution. A comparative evaluation of improvements in the diagnostic image merits was performed on this initial patient set. The evaluation criteria included the level of EPI artifacts, level of PI artifacts, SNR, diagnostic details, and diagnostic confidence. The evaluation resulted in confidence that the higher spatial resolution was supportable, and the protocol was modified to use only the three-interleave 192 × 192 scan. By increasing NEX to 3 and reducing TR to 3 s, this yielded a scan time of 2:21 min for the new sequence. Conversely, the total scan time of the product 128 × 128 ssDW-EPI version was 0:40 min (+ 0:10 min reference scan). These two clinical scans were compared only with respect to stroke diagnosis, and not as a comparison of SNR.
RESULTS
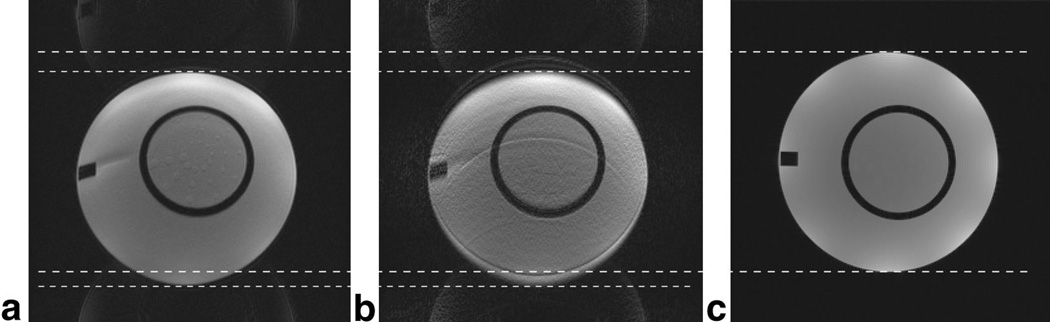
Figure 1a and c show phantom data obtained using ss-EPI and a conventional SE sequence, respectively. The dashed lines delineate the upper and lower edges of the phantom defined by these images. Figure 1b shows the self-calibrated PI scan with a variable sampling density scheme (48 ACS lines, ORF = 4). By comparing Fig. 1b and c, one can see that only the high-spatial-frequency components of the image are approaching the true geometry, while the low-spatial-frequency components of the image are still equally as distorted as in Fig. 1a. This renders the image self-inconsistent and hence not useable in practice. In other words, self-calibrating k-space sampling trajectories, as used in conventional GRAPPA or mSENSE accelerated sequences, interact badly with the off-resonance sensitivity in EPI. This may be due to 1) the two different off-resonance sensitivities in k-space per se, and/or 2) the fact that PI calibration information is perturbed by the off-resonances so much that it cannot be applied to the outer parts of k-space. The next figure isolates and tests the latter explanation.
FIG. 1.
Conventional self-calibrated GRAPPA in combination with EPI. (a) ss-EPI and (c) conventional SE show the phantom with and without geometric distortions. Using a variable k-space sampling scheme for EPI with an ORF of 4 results in a GRAPPA-reconstructed image (b). Here the low-resolution portion of the image remains shifted, whereas the higher spatial frequencies (edges) are four times less distorted than the low-spatial-frequency components, yielding this double-image appearance. The white dashed lines are guides for the true phantom edges defined in c and the distorted edges in a. This is evidence that conventional self-calibration may not be used with EPI.
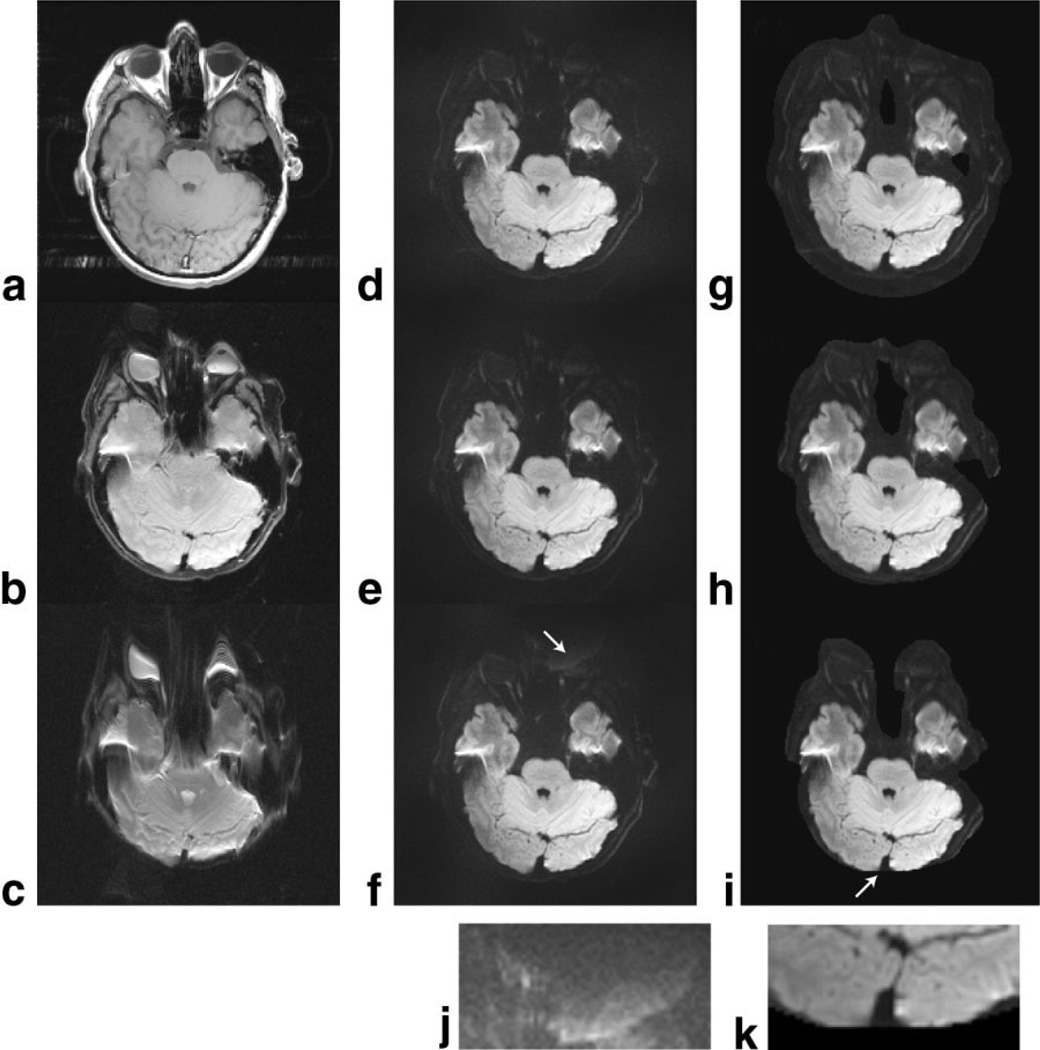
In Fig. 2 the three-interleave DW-EPI data were reconstructed using three different sets of GRAPPA weights and coil sensitivity calibration data derived from the SE (Fig. 2a, no off-resonances), three-interleave SE-EPI (Fig. 2b, off-resonances matched to the scan), or ss-SE-EPI scans (Fig. 2c, three times the off-resonances). By comparing the images for both GRAPPA (d–f) and SENSE (g–i) reconstructions with the three sets of calibration data, it is found that susceptibility differences between the calibration and the PI-accelerated scans are of lower importance than expected. Only moderate reconstruction errors occurred when the one-shot calibration scan was used, in which case the mismatch between the coil sensitivity maps derived from Fig. 2c and the three-shot scan was enough to make the SENSE data fall outside the supported region defined by the calibration scan (see arrow and enlarged inset in Fig. 2k). The corresponding GRAPPA image (Fig. 2f) shows instead a slight noise enhancement and a vague ghost (see arrow and enlarged inset in Fig. 2j). Nevertheless, neither GRAPPA nor SENSE are prone to reconstruction artifacts near the temporal lobes (Fig. 2e and h), despite the susceptibility-induced distortions in those areas (Fig. 2c). This implies that there is in fact a high degree of flexibility in the choice of calibration scans (i.e., EPI vs. conventional images), although highly distorted calibration scans, such as the one-shot scan, are less adequate. It also leads to the conclusion that the poor image quality in Fig. 1 is due solely to the different k-space velocities (explanation 1) introduced through the variable k-space sampling density scheme, and is not due to the GRAPPA weights or coil sensitivity information calculated from data with unmatched off-resonance sensitivity.
FIG. 2.
3T volunteer data showing that external calibration data (a–c) with different amounts of distortion affect the image quality for GRAPPA (d–f) and SENSE (g–i) reconstructions of three-shot DWI data. (a) Conventional SE, (b) three-shot b0 EPI, (c) one-shot b0 EPI, (d–f) GRAPPA-reconstructed, and (g–i) SENSE-reconstructed three-shot isotropic DW images using calibration data are shown on the same row. Overall, both GRAPPA and SENSE show little dependence on the choice of calibration data, except for the missing data for SENSE in i and slight noise enhancement in f (see insets for f and i). This gives flexibility in the choice of calibration data, and shows that the proposed use of a multishot b0 image for calibration purposes is not suboptimal compared to, e.g., a time-consuming SE calibration scan (a).
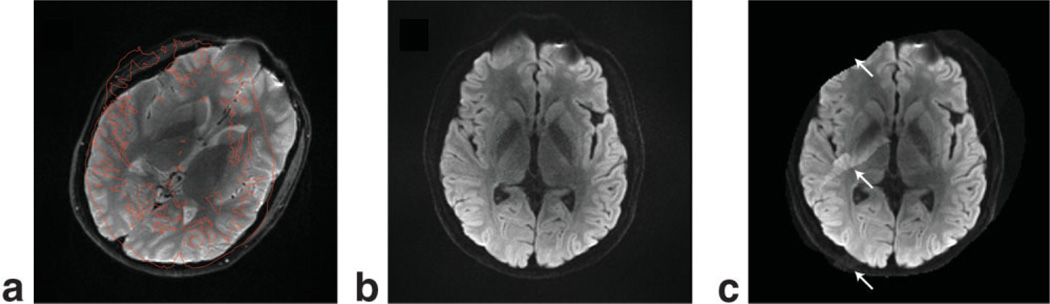
In Fig. 3a the image used as calibration is shown with the edge features of the PI DWI image superimposed in red to illustrate the degree of reorientation between the two scans. The extreme rotation was picked to exaggerate the following effects for reasons of better visualization. Figure 3b and c show the isotropic DW images reconstructed with GRAPPA and SENSE, respectively. For GRAPPA, no residual aliasing can be seen and the image quality is comparable to that in Fig. 2d–f. However, since the SENSE algorithm operates on a pixel-by-pixel basis in the image domain, misalignments between the calibration and PI scans are clearly more problematic, leaving significant residual aliasing from areas where proper coil sensitivity information does not exist.
FIG. 3.
a: Three-shot b0 EPI data acquired with the head rotated 40°. The contours of the following three-shot isotropic DWI are outlined in red. The data in a are reconstructed with (b) GRAPPA and (c) SENSE. GRAPPA is shown to be immune to motion that may occur between the acquisition of the data used for calibration and the subsequent DWI. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
While these results are encouraging for GRAPPA, there is still the matter of motion that may occur throughout the DW-EPI scan. Figure 4 shows data acquired while the volunteer performed a significant and continuous oscillatory head rotation. The top row (Fig. 4a) shows the first b0 image used to estimate the GRAPPA weights and coil sensitivity maps. The image suffers from prominent ghosting due to the head motion. In the second row (Fig. 4b and e), the same b0 image as in Fig. 4a is shown after the following four steps were carried out: 1) GRAPPA weights and coil sensitivity information calculated from image in Fig. 4a, 2) GRAPPA and SENSE reconstruction of each interleaf, 3) motion correction between the individually reconstructed “shot-images,” and 4) magnitude averaging across the shot-images. Using GRAPPA (Fig. 4b) in combination with motion correction, no residual ghosts and no superimposed structures remain. From the ghost-free b0 image in Fig. 4b, we can draw three conclusions: 1) our image entropy-based ghost minimization routine that corrects only for intrashot ghosting was not deceived by the motion-induced intershot ghosting, 2) the accuracy of the GRAPPA weights is not reduced by the intershot ghosting (i.e., no GRAPPA-related aliasing was introduced), and 3) the 2D motion correction by coregistration of shots 2 and 3 onto shot 1 is an acceptable approach. Similar GRAPPA reconstructions of the isotropic DW images are shown without (Fig. 4c) and with (Fig. 4d) motion correction of the 24 component images (3 shots × 4 directions × 2 repetitions) applied. However, due to some through-plane motion, the image in Fig. 4d is not quite as sharp as those presented in Figs. 2 and 3. For the SENSE case, the motion-induced ghosting in Fig. 4a also affected the coil sensitivity maps and in turn the shot-images, leading to patches of residual ghosts that prevented intensity-based realignment on the b0 images from working satisfactorily (Fig. 4e). With regard to the SENSE-reconstructed DW images, the ghosting from the coil sensitivity map is even more apparent (Fig. 4f), in which case the intensity of the ghosts is so bright that the motion correction does not pick up any true motion (Fig. 4g). On this note, please recall that EPI ghosts do not follow the head as it rotates, since they occur in the phase-encoding direction or y-axis in the laboratory space. This explains why the motion correction is not “supposed” to find the motion if the ghost intensity is high in relation to the true object, as in Fig. 4f.
FIG. 4.
Sustained motion during the entire diffusion scan (i.e., also during the acquisition of the first b0 image used for PI calibration). a: b0 image reconstructed without PI and used for the estimation of coil sensitivity maps and GRAPPA weights. b: Same b0 image, where each of the three shots has been reconstructed using the GRAPPA weights obtained from the image itself, followed by motion correction between the “shot-images” and magnitude averaging. Isotropic DWI using the same GRAPPA weights, (c) without and (d) with motion correction between the shots, is shown. The corresponding SENSE reconstructions are shown in e–g. Unlike GRAPPA, the SENSE reconstruction is impaired by the intershot ghosts in a, which on top of residual aliasing in the brain also deceives the intensity-based motion correction.
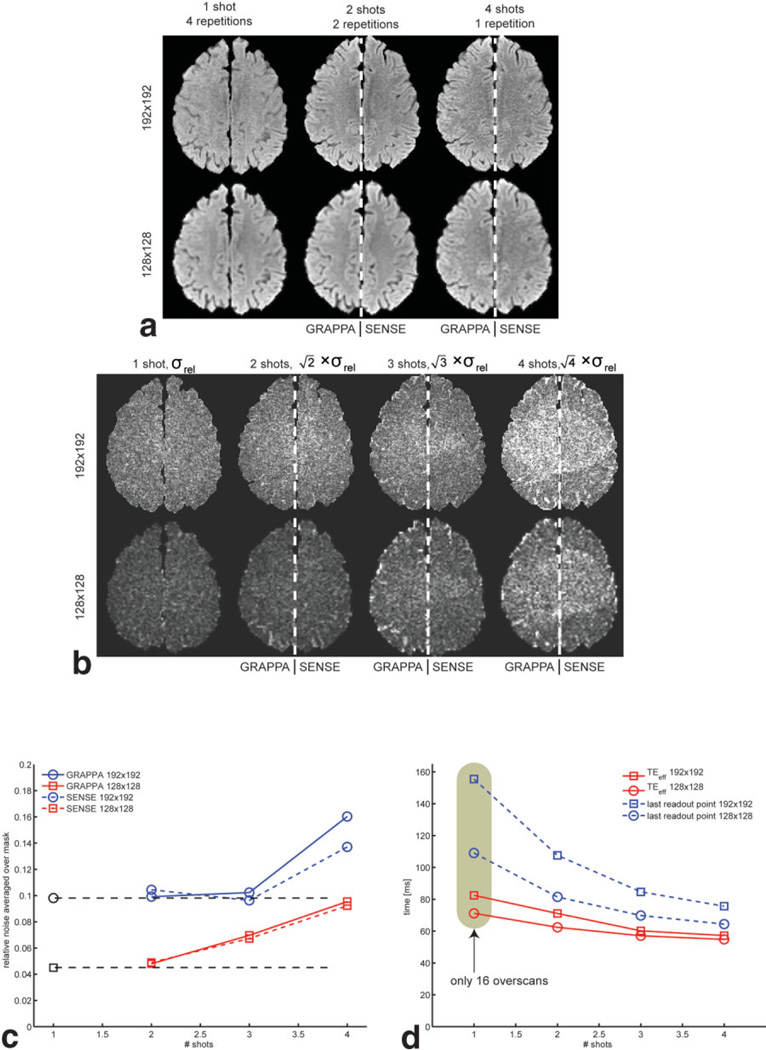
The results from the noise experiment for the isotropic DWI data are shown in Fig. 5. In Fig. 5a, isoDWI maps averaged over different number of repetitions (to normalize to the same scan time) are presented with 192 × 192 (top row) and 128 × 128 (bottom row) resolution. Columns (left-to-right) correspond to one-shot (four averages), two-shot (two averages), and four-shot (one average) scans. Except for the one-shot column, which was reconstructed without PI, the remaining images were concatenated from both GRAPPA and SENSE reconstructions (the left side (patient’s right) was reconstructed using GRAPPA, and the right side was reconstructed with SENSE). This greatly aids comparison of the two methods. A closer look at the single- and two-shot data suggests that they have a similar noise appearance. This is explained by the fact that at R = 2 the g-factor is very small, and the single-shot methods acquire mostly noise for the outer k-space lines due to the long EPI readout train (despite reducing the overscans from 24 to 16). For the four-shot scan, the g-factor, for both GRAPPA and SENSE, makes the image prone to more spatially varying noise. Also, unlike the significant shortening of the EPI train by going from one to two shots, there are diminishing returns and less SNR to gain by increasing the reduction factor ever further. Figure 5b contains σrel-maps with a similar arrangement as in Fig. 5a, but now also with the three-shot data. As mentioned earlier, σrel is scaled by to allow one to visually compare the noise/scan time penalty. For the higher resolution (top), is almost constant across one to three shots, with comparable noise levels between GRAPPA and SENSE. For the lower 128 × 128 resolution (bottom), σrel is naturally lower throughout the image when compared to the 192 × 192 resolution, but in this case the noise is more steadily increasing from two shots (Fig. 5c). The reason for this difference is that there is not the same penalty of long EPI trains for single-shot 128 × 128 matrices as for 192 × 192 matrices. To illustrate the SNR gains from using multishot (ms)-EPI, TEeff and the time for the end of the readout train are plotted in Fig. 5d.
FIG. 5.
SNR efficiency of volunteer brain data. a: Split view with both GRAPPA and SENSE reconstructions in each subpanel for an easy noise comparison between the two for different image resolutions and shots. All data have been averaged over different numbers of repetitions to normalize by scan time. One and two shots are comparable, while the structured noise is disturbing for the four-shot case. b: Relative SD maps, normalized to the same scan time (, Eq. [1]). For 192 × 192, there is no apparent noise difference between one to three shots. In c the mean of over all brain pixels in b is given. The black dots and dashed lines indicate the noise level of the ss-EPI, which is constant for one- to three-shot GRAPPA with a 192 × 192 matrix. In d the TEs and time for the end of the EPI train are given. Overall, SENSE and GRAPPA demonstrate very similar noise characteristics, except for 192 × 192 at R = 4, where SENSE has a small advantage.
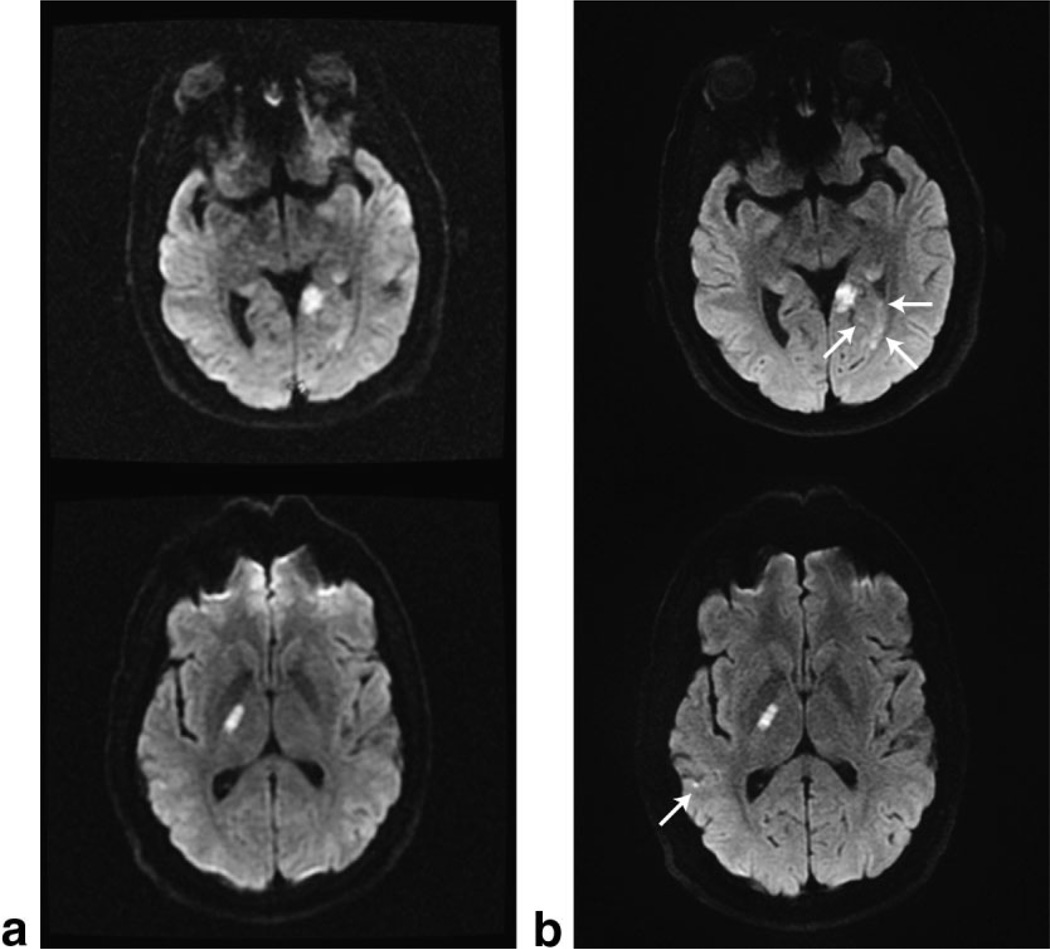
Finally, four clinical stroke cases acquired with the standard 128 × 128, ss-DW-EPI sequence and our 192 × 192, three-shot DW-EPI sequence reconstructed with GRAPPA are presented in Fig. 6 (left and right columns, respectively). For the upper two cases, the arrows indicate small lesions that are very hard to delineate on conventional ssEPI series, but are easily picked up on the high-resolution three-shot GRAPPA DWI scans. Despite the increased spatial resolution, the three-shot scans also have less geometric distortions. This is important when trying to find or rule out lesions in areas of increased susceptibility gradients. Moreover, cortical lesions could also be delineated much better with the new approach. In none of our clinical cases did motion cause residual artifacts, despite the prolonged scan time.
FIG. 6.
Clinical examples of the standard diffusion sequence using (a) 128 × 128 ss-EPI data acquired in less than 1 min, and (b) our three-shot 192 × 192 DW-EPI data reconstructed with GRAPPA using a tetrahedral diffusion scheme repeated three times. The top and bottom rows were acquired with the eight-channel neurovascular array coil and the eight-channel brain coil, respectively.
DISCUSSION
A new DW-EPI variant that combines PI with DW iEPI has been introduced. This method shares the benefits of navigated iEPI in terms of reduced distortions, without the risk of generating the bothersome ghosting artifacts that are otherwise typical for ms-DW-EPI. Navigated iEPI has, under the assumption of ideal navigator correction, one benefit over our method in the low-SNR regime. The Ninter times more acquired data fed to the Fourier transform will result in a less pronounced Rician distribution in the magnitude images than our approach, since the magnitude averaging of the “shot-images” does not alter the Rician distribution—it only reduces the variance. For a reliable image reconstruction in an acute-stroke setting, we have found it advantageous to be able to extract the PI calibration information from the scan itself rather than relying on a previous calibration scan. However, in this study we have also shown that conventional self-calibrating techniques, such as those proposed for GRAPPA and mSENSE using a variable k-space sampling pattern, are incompatible with EPI (see Fig. 1). This is due to two different sampling bandwidths along the phase-encode dimension that cause the low spatial frequencies to be distorted more than the higher spatial frequencies. Despite these facts, it was demonstrated that neither the GRAPPA weights nor the SENSE coil sensitivity maps were particularly sensitive to off-resonances. The different external images (SE, three-shot EPI, and one-shot EPI) used for calibration produced ghost- and alias-free reconstructions of the three-shot DW-EPI data (Fig. 2). Only for more extreme cases of mismatch (e.g., geometric distortions of ssEPI at 3T) will reconstruction artifacts occur for SENSE, and slight noise enhancement for GRAPPA. It is worthwhile to note that the use of the three-shot b0 EPI image (as a part of the scan) for PI calibration does not produce a worse reconstruction than a time-consuming but high-SNR and distortion-free SE image. This finding renders interleaved self-calibration fairly optimal in this regard, as we neither need to scan nor carry around a separate PI calibration data set.
With regard to head motion, the image quality of the PI reconstructions performed was found to be more favorable with GRAPPA than with SENSE. This can be appreciated best from the reconstructions of experiments in which sustained motion was performed (Fig. 4). From Fig. 4 we conclude that GRAPPA will perform well even for very uncooperative patients, and compared to SENSE (Fig. 3c), GRAPPA appears to be more robust to misalignments between the calibration scans (in our case the b0 images) and the DW images. SENSE artifacts remain despite the 20 × 20 voxel smoothing kernel applied on the coil sensitivity maps, which acts like a dilation filter that bridges local signal voids and increases the extent of the coil sensitivity maps around the brain. For misalignments larger than in Fig. 3, the GRAPPA weights will also eventually fail. Such large motion was not possible given the limited room between the head and the eight-channel RF coil used throughout this study, but it may be possible for smaller objects or larger RF coils. These findings add to other advantages of GRAPPA over SENSE that have already been reported in the literature, such as proper reconstruction even when a part of the object is outside the FOV (20).
At this point it should be noted that augmented SENSE methods exist that could overcome the artifacts shown in Figs. 3 and 4 for SENSE and make it comparable to GRAPPA. The use of adaptive regularization schemes (33), background elimination, further extrapolation (in addition to the implicit extrapolation due to smoothing performed here) via, e.g., local polynomial filtering (17) or global fitting schemes are examples of SENSE enhancements, none of which have been explored in this paper. Using these techniques, larger misalignments between the calibration scan and the accelerated scans should be tolerable. Furthermore, the coil sensitivity field should be less prone to motion artifacts, leading to less image ghosting, which in turn would allow motion correction on that data to function similarly to the GRAPPA reconstructions shown here.
To date, this sequence has been tested for reduction factors of up to 4 at matrix sizes of 128 and 192. For these acquisition matrices, the eight-channel head array used for this study currently poses a fundamental limit to acceleration factors of 4 and beyond. Nevertheless, with dedicated “many”-channel head arrays, it can be anticipated that acceleration factors can be increased ever further, and thus geometric distortions and blurring can be further reduced. Of course, the point at which PI reconstruction artifacts and g-factor-related noise enhancement outweighs the distortion and blurring reduction of EPI depends on several factors, such as the coil type, number of coils, acquisition matrix size, and slice thickness. As shown in Fig. 5, there is no penalty in SNR/scan time moving from a single-shot acquisition to two to three shots at high image resolution, thanks to the shorter EPI train of the latter. Hence the distortion benefits of a two- to three-shot technique come without penalty at 192 × 192 resolution, and even more so for higher resolutions. However, even with an, e.g., 64-channel array, and in the absence of g-factors, the SNR per scan time ratio improvement will offer diminishing returns for a higher number of shots.
Further strides in terms of SNR improvement were also made in this study by using a tetrahedral diffusion-encoding scheme, which reduces the TE and increases the number of slices/TR. Despite this SNR gain, we found it necessary to repeat the tetrahedral encoding scheme three times to compensate for the SNR loss (by a factor of >2) at 192 × 192 resolution from the increased spatial resolution. This averaging was especially needed when the eight-element neurovascular coil was used, which is less optimal for PI, and could barely support the reduction factor of 3. If very short scan times are required (probably at lower image resolutions), a single repetition for all of the interleaves for the (first) b0 image and one interleave for each diffusion direction may be acquired. This would still make the sequence a self-calibrated R = 3 acquisition that, on our clinical system, could acquire over 28 slices in less than 30 s.
For the ongoing clinical study, GRAPPA was used solely for the reconstructions, as no need to use SENSE was found, given the results presented in this paper. To further increase robustness with respect to motion for our clinical scans, three sets of GRAPPA weights from all three repetitions of the b0 images are calculated. The set of weights that has the smallest fit error to the data is used for the reconstruction. In numerous test cases, we found that the fit-error of the GRAPPA weights was in perfect agreement with the amount of ghosting in the image. This allows us to preclude the scenario in which the patient moves substantially between the interleaves that form the first b0 image, which would produce inferior GRAPPA weights and hamper the entire data set.
CONCLUSIONS
A new PI-driven ms-DW-EPI sequence has been developed that allows the use of high-resolution DWI with reduced geometric distortions and equivalent SNR efficiency. It was found that the variable k-space sampling density normally used in GRAPPA and mSENSE led to considerable reconstruction artifacts for ss-EPI. On the contrary, even quite substantial perturbations in the calibration scans due to off-resonances led only to slight noise enhancement for GRAPPA and limited artifacts for SENSE. However, obtaining the PI calibration information from the b0 of the data itself, as proposed in this work, increased the SNR slightly. For both the case of motion during the acquisition of b0 images (used for PI calibration), and motion between the b0 and the rest of the DWI data, GRAPPA was superior to SENSE, whereas with respect to the SNR/scan time ratio, both PI methods were comparable. The advances in DWI presented in this study lead to spatial resolutions that approach those of conventional images. From that finding, in conjunction with a decrease in geometric distortions, we anticipate both an increase in lesion detectability and confidence in the diagnostic workup of acute-stroke patients.
Acknowledgments
Grant sponsor: National Institutes of Health; 1R01EB002711; Grant sponsor: Center of Advanced MR Technology at Stanford; Grant number: P41RR09784; Grant sponsors: Lucas Foundation; Oak Foundation.
REFERENCES
- 1.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, Asgari H, Norman D. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 2.Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T, Ueda S, Sato H. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996;14:1113–1116. doi: 10.1016/s0730-725x(96)00237-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Chang KH, Song IC, Kim HD, Seong SO, Kim YH, Han MH. Brain abscess and necrotic or cystic brain tumor: discrimination with signal intensity on diffusion-weighted MR imaging. AJR Am J Roentgenol. 1998;171:1487–1490. doi: 10.2214/ajr.171.6.9843275. [DOI] [PubMed] [Google Scholar]
- 4.Lai PH, Ho JT, Chen WL, Hsu SS, Wang JS, Pan HB, Yang CF. Brain abscess and necrotic brain tumor: discrimination with proton MR spectroscopy and diffusion-weighted imaging. AJNR Am J Neuroradiol. 2002;23:1369–1377. [PMC free article] [PubMed] [Google Scholar]
- 5.Farzaneh F, Riederer SJ, Pelc NJ. Analysis of T2 limitations and off-resonance effects on spatial resolution and artifacts in echo-planar imaging. Magn Reson Med. 1990;14:123–139. doi: 10.1002/mrm.1910140112. [DOI] [PubMed] [Google Scholar]
- 6.Bammer R, Stollberger R, Augustin M, Simbrunner J, Offenbacher H, Kooijman H, Ropele S, Kapeller P, Wach P, Ebner F, Fazekas F Magnetic Resonance Institute UoGA. Diffusion-weighted imaging with navigated interleaved echo-planar imaging and a conventional gradient system. Radiology. 1999;211:799–806. doi: 10.1148/radiology.211.3.r99jn15799. [DOI] [PubMed] [Google Scholar]
- 7.Butts K, Riederer SJ, Ehman RL, Thompson RM, Jack CR Magnetic Resonance Laboratory MCRM. Interleaved echo planar imaging on a standard MRI system. Magn Reson Med. 1994;31:67–72. doi: 10.1002/mrm.1910310111. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AW, Gore JC. Analysis and correction of motion artifacts in diffusion weighted imaging. Magn Reson Med. 1994;32:379–387. doi: 10.1002/mrm.1910320313. [DOI] [PubMed] [Google Scholar]
- 9.Butts K, de Crespigny A, Pauly JM, Moseley M. Diffusion-weighted interleaved echo-planar imaging with a pair of orthogonal navigator echoes. Magn Reson Med. 1996;35:763–770. doi: 10.1002/mrm.1910350518. [DOI] [PubMed] [Google Scholar]
- 10.Butts K, Pauly J, de Crespigny A, Moseley M. Isotropic diffusion-weighted and spiral-navigated interleaved EPI for routine imaging of acute stroke. Magn Reson Med. 1997;38:741–749. doi: 10.1002/mrm.1910380510. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Bammer R, Kim DH, Moseley ME. Self-navigated interleaved spiral (SNAILS): application to high-resolution diffusion tensor imaging. Magn Reson Med. 2004;52:1388–1396. doi: 10.1002/mrm.20288. [DOI] [PubMed] [Google Scholar]
- 12.Bammer R, Auer M, Keeling SL, Augustin M, Stables LA, Prokesch RW, Stollberger R, Moseley ME, Fazekas F. Diffusion tensor imaging using single-shot SENSE-EPI. Magn Reson Med. 2002;48:128–136. doi: 10.1002/mrm.10184. [DOI] [PubMed] [Google Scholar]
- 13.Bammer R, Keeling SL, Augustin M, Pruessmann KP, Wolf R, Stollberger R, Hartung HP, Fazekas F. Improved diffusion-weighted single-shot echo-planar imaging (EPI) in stroke using sensitivity encoding (SENSE) Magn Reson Med. 2001;46:548–554. doi: 10.1002/mrm.1226. [DOI] [PubMed] [Google Scholar]
- 14.Cercignani M, Horsfield MA, Agosta F, Filippi M. Sensitivity-encoded diffusion tensor MR imaging of the cervical cord. AJNR Am J Neuroradiol. 2003;24:1254–1256. [PMC free article] [PubMed] [Google Scholar]
- 15.Jaermann T, Crelier G, Pruessmann KP, Golay X, Netsch T, van Muiswinkel AM, Mori S, van Zijl PC, Valavanis A, Kollias S, Boesiger P. SENSE-DTI at 3 T. Magn Reson Med. 2004;51:230–236. doi: 10.1002/mrm.10707. [DOI] [PubMed] [Google Scholar]
- 16.Jaermann T, Pruessmann KP, Valavanis A, Kollias S, Boesiger P. Influence of SENSE on image properties in high-resolution single-shot echo-planar DTI. Magn Reson Med. 2006;55:335–342. doi: 10.1002/mrm.20769. [DOI] [PubMed] [Google Scholar]
- 17.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 18.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 19.Beatty PJ, Brau ACS. Understanding the GRAPPA paradox. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, WA, USA. 2006. (Abstract 2467). [Google Scholar]
- 20.Griswold MA, Kannengiesser S, Heidemann RM, Wang J, Jakob PM. Field-of-view limitations in parallel imaging. Magn Reson Med. 2004;52:1118–1126. doi: 10.1002/mrm.20249. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Kluge T, Nittka M, Jellus V, Kuehn B, Kiefer B. Parallel acquisition techniques with modified SENSE reconstruction (mSENSE). Proceedings of the 1st Wurzburg Workshop on Parallel Imaging; Wurzburg. 2001. p. 92. [Google Scholar]
- 22.Heidemann RM, Griswold MA, Haase A, Jakob PM. VD-AUTO-SMASH imaging. Magn Reson Med. 2001;45:1066–1074. doi: 10.1002/mrm.1141. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakos WE, Panych LP, Kacher DF, Westin CF, Bao SM, Mulkern RV, Jolesz FA. Sensitivity profiles from an array of coils for encoding and reconstruction in parallel (SPACE RIP) Magn Reson Med. 2000;44:301–308. doi: 10.1002/1522-2594(200008)44:2<301::aid-mrm18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Samsonov AA, Block WF, Arunachalam A, Field AS. Advances in locally constrained k-space-based parallel MRI. Magn Reson Med. 2006;55:431–438. doi: 10.1002/mrm.20757. [DOI] [PubMed] [Google Scholar]
- 25.Yeh EN, McKenzie CA, Ohliger MA, Sodickson DK. Parallel magnetic resonance imaging with adaptive radius in k-space (PARS): constrained image reconstruction using k-space locality in radiofrequency coil encoded data. Magn Reson Med. 2005;53:1383–1392. doi: 10.1002/mrm.20490. [DOI] [PubMed] [Google Scholar]
- 26.Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA) Magn Reson Med. 2005;53:981–985. doi: 10.1002/mrm.20430. [DOI] [PubMed] [Google Scholar]
- 27.Conturo TE, McKinstry RC, Akbudak E, Robinson BH. Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magn Reson Med. 1996;35:399–412. doi: 10.1002/mrm.1910350319. [DOI] [PubMed] [Google Scholar]
- 28.Skare S, Clayton D, Newbould R, Moseley ME, Bammer R. A fast and robust minimum entropy based non-interactive Nyquist ghost correction algorithm. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, WA, USA. 2006. (Abstract 2349). [Google Scholar]
- 29.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 30.Griswold MA. Advanced k-space techniques. Second International Workshop on Parallel MRI; 2004; Zurich. pp. 16–18. [Google Scholar]
- 31.Qu P, Shen GX, Wang C, Wu B, Yuan J. Tailored utilization of acquired k-space points for GRAPPA reconstruction. J Magn Reson. 2005;174:60–67. doi: 10.1016/j.jmr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Skare S, Andersson JL. On the effects of gating in diffusion imaging of the brain using single shot EPI. Magn Reson Imaging. 2001;19:1125–1128. doi: 10.1016/s0730-725x(01)00415-5. [DOI] [PubMed] [Google Scholar]
- 33.Tsao J, Pruessmann KP, Boesiger P. Feedback regularization for SENSE reconstruction. Proceedings of the 10th Annual Meeting of ISMRM; Honolulu, HI, USA. 2002. (Abstract 739). [Google Scholar]