Summary
Background
Estimates of the burden of disease in adults in sub-Saharan Africa largely rely on models of sparse data. We aimed to measure the burden of disease in adults living in a rural area of coastal Kenya with use of linked clinical and demographic surveillance data.
Methods
We used data from 18 712 adults admitted to Kilifi District Hospital (Kilifi, Kenya) between Jan 1, 2007, and Dec 31, 2012, linked to 790 635 person-years of observation within the Kilifi Health and Demographic Surveillance System, to establish the rates and major causes of admission to hospital. These data were also used to model disease-specific disability-adjusted life-years lost in the population. We used geographical mapping software to calculate admission rates stratified by distance from the hospital.
Findings
The main causes of admission to hospital in women living within 5 km of the hospital were infectious and parasitic diseases (303 per 100 000 person-years of observation), pregnancy-related disorders (239 per 100 000 person-years of observation), and circulatory illnesses (105 per 100 000 person-years of observation). Leading causes of hospital admission in men living within 5 km of the hospital were infectious and parasitic diseases (169 per 100 000 person-years of observation), injuries (135 per 100 000 person-years of observation), and digestive system disorders (112 per 100 000 person-years of observation). HIV-related diseases were the leading cause of disability-adjusted life-years lost (2050 per 100 000 person-years of observation), followed by non-communicable diseases (741 per 100 000 person-years of observation). For every 5 km increase in distance from the hospital, all-cause admission rates decreased by 11% (95% CI 7–14) in men and 20% (17–23) in women. The magnitude of this decline was highest for endocrine disorders in women (35%; 95% CI 22–46) and neoplasms in men (30%; 9–45).
Interpretation
Adults in rural Kenya face a combined burden of infectious diseases, pregnancy-related disorders, cardiovascular illnesses, and injuries. Disease burden estimates based on hospital data are affected by distance from the hospital, and the amount of underestimation of disease burden differs by both disease and sex.
Funding
The Wellcome Trust, GAVI Alliance.
Introduction
Although adults comprise more than half the total population of sub-Saharan Africa, they have been neglected in both the provision of health services and health-related research in Africa.1 Adults are crucial to ensure the survival of children2 and to drive economic development. The available published literature describing the burden of disease in adults in sub-Saharan Africa reports high amounts of uncertainty in the estimates, mainly as a result of weak or absent disease surveillance combined with the absence of vital registration in most of the region.3 Although focused surveys have estimated the burden of specific diseases, such as HIV and malaria,4, 5 these estimates do not provide a comprehensive description of the causes of poor health in the communities studied.
African populations are undergoing a demographic and epidemiological transition because of increased survival of children into adulthood,6 changes in the burden of infectious diseases such as malaria,7 and an increase in the risk factors for, and prevalence of, non-communicable diseases.8, 9 During this transition, data to guide public health policy on the continent are greatly needed.10 Available estimates of disease burden in sub-Saharan Africa rely heavily on modelling assumptions, which are questionable in the absence of adequate primary data.11 Although the use of verbal autopsy studies to establish community causes of death is increasing, and the techniques involved are improving,12 such studies do not have the diagnostic accuracy that hospitals can provide, and they do not include non-fatal causes of ill health.
Few published studies have used hospital-based data, and those that have done so have several limitations: they rarely use universally accepted coding systems and case definitions for the illnesses described;13 data capture is seldom complete because of various logistical challenges in the use of electronic medical systems;14 they do not usually report age-stratified incidence rates because of the absence of denominator data, which makes it impossible to distinguish changes in disease burden over time from changes in population structure;15 and they do not take into account the fact that admission rates fall with increasing distance from hospital, which leads to underestimation of the true disease burden in the population.16 Finally, although the disability-adjusted life-year (DALY) has been suggested as the appropriate metric for measurement of disease burden, few studies beyond the Global Burden of Disease (GBD) project have reported DALYs for sub-Saharan Africa.
In this analysis, we used linked hospital and population surveillance data to establish the inpatient burden and range of disease in adults in a rural coastal district in Kenya, and simultaneously describe the effect of distance from hospital on admission rates.
Methods
Study setting and participants
Kilifi District (now part of Kilifi County) is located on the Indian Ocean coast of Kenya. It is a poor, rural area and the population mainly relies on subsistence farming. The Kilifi Health and Demographic Surveillance System (KHDSS) covers roughly 40% of Kilifi District, along a coastal strip 35 km north and south of Kilifi town (figure 1). The KHDSS records births, deaths, and migration events in a population of 260 000 people, living in an area 891 km2 in size.17 The age and sex structure of the population differs substantially within the KHDSS area, with fewer adult men in the more rural regions than in the semi-urban regions because of selective outmigration of this group to nearby towns in search of paid employment. Since 2000, the KHDSS area has been mapped and updated with global positioning system receivers (Magellan Navigation Inc, Santa Clara, CA, USA) to define the coordinates of all homesteads and the vector paths of the roads in the area. Roughly 33% of all adult deaths in KHDSS occur at Kilifi District Hospital,18 and the overall mortality rates do not differ substantially with varying distance from the hospital (appendix p 3).
Figure 1.
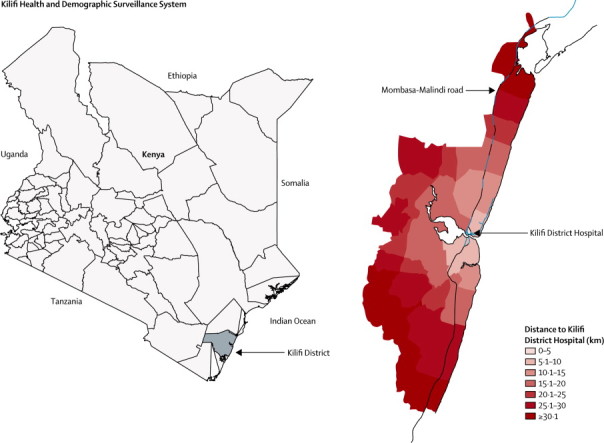
Location of the Kilifi Health and Demographic Surveillance System
Within the KHDSS area, the total fertility rate (4·73), crude birth rate (34·7 per 1000 person-years of observation), population growth rate (2·79% per year), and proportion of the population younger than 15 years (49%) are similar to the rates across Kenya. However, the under 5-mortality ratio (41 per 1000 livebirths) and HIV prevalence (4·9% in antenatal clinic attendees) are lower than the Kenyan national averages (74 per 1000 births and 8·0%, respectively).19
Kilifi District Hospital is situated at the centre of the KHDSS area and the main route of access to the hospital is the Mombasa-Malindi road (figure 1). The hospital serves as a first-level referral hospital that provides care to both adults and children in Kilifi District. The hospital provides an acute 24-h daily medical admission service run by a medical team of more than 40 staff that includes four consultants, ten medical and clinical officers, nurses, and support staff. The Kenya Ministry of Health classifies the hospital as a level IV hospital, with the highest level hospitals in Kenya classified as level VI.20
Medical care at Kilifi District Hospital is provided according to national and WHO guidelines.21 Laboratory facilities available at the hospital include basic haematological and biochemical tests and advanced microbiological culture facilities. Imaging facilities include ultrasound and radiographs. As a minimum, all admitted patients undergo a full blood count and an HIV antibody test in keeping with the Kenya Ministry of Health opt-out policy on HIV testing.22 Patients meeting criteria for possible invasive bacterial disease or meningitis are investigated with blood and cerebrospinal fluid cultures. Other tests, such as urea and electrolyte tests, liver function tests, arterial blood gas measurement, radiographs, and ultrasound imaging, are done depending on clinical indication. Patients who need more advanced testing, such as CT scans and histopathological examination, are transported to facilities in Mombasa, 60 km south of Kilifi.
A prospective surveillance of adult inpatients was initiated in January, 2007 to investigate the indirect effects on adult disease of the introduction of pneumococcal conjugate vaccine in children. This surveillance was started by the KEMRI-Wellcome Trust Research Programme, with funding from GAVI via the PneumoADIP (Pneumococcal vaccines Accelerated Development and Introduction Plan). The key elements of the surveillance were the standardisation of clinical investigations to follow Kenya Ministry of Health guidelines,21 the introduction of real-time electronic medical records, and the linking of hospital admissions to the population register of the KHDSS. On admission to hospital, patients are interviewed by ward clerks to establish whether they reside within the KHDSS and to find their unique identity within the population register. This unique personal identification is then linked to all clinical, laboratory, and demographic data. Admission, diagnostic, and discharge data are entered directly into a standardised electronic medical record with Filemaker version 11 software. At discharge, medical staff list up to two discharge diagnoses for each patient, on the basis of all clinical, radiological, and laboratory data available. Weekly clinical meetings for all cadres of staff are held as part of continuous medical education to standardise diagnostic procedures and medical care. Monthly audits are done to analyse the quality of data collected and to give feedback to the clinical team to maintain consistency and quality.
Procedures and statistical analysis
We used Stata version 11.2 to analyse data for all adult patients (aged ≥15 years) admitted to the hospital from Jan 1, 2007, to Dec 31, 2012. All discharge diagnoses were assigned three-character codes from the International Classification of Diseases (ICD) tenth revision (ICD-10). Because of the large number of individual diagnoses, we grouped them according to ICD chapters23 to compute admission rates. We calculated the proportions of admissions and of deaths due to all illnesses categorised by ICD chapter in adults presenting to Kilifi District Hospital. Admissions of pregnant women for labour and delivery were excluded; however admissions with pregnancy-related disorders, such as first-trimester abortion and post-partum sepsis, were included.
We divided the KHDSS area into seven strata with respect to distance from Kilifi District Hospital (figure 1). Each stratum represented an incremental distance of 5 km. We calculated distance to the hospital as the sum of the shortest distance from each homestead to the Mombasa-Malindi road and the distance along the road to the hospital. Rates of hospital admission, with allowance for several admissions, were calculated for KHDSS residents living within each distance stratum. We assumed that people living in the nearest distance stratum (within 5 km of the hospital) had perfect geographical access to the hospital (ie, the best access compared with everyone else in the surveillance area) and therefore the rates here would be least susceptible to distance-related bias. We used person-years based on exact periods of residence within the KHDSS as denominators to compute rates. All rates were directly age-standardised with use of the INDEPTH standard population structure for sub-Saharan Africa.24 To compare admission rates for ICD categories by distance, we used Poisson regression to calculate incidence rate ratios.
Measurement of disability-adjusted life-years
For each of the 15 leading discharge diagnoses of patients admitted to Kilifi District Hospital, we estimated the number of DALYs lost. We calculated DALYs with open-access templates from WHO.25 We used age-specific person-years of observation obtained from the KHDSS as denominators in incidence calculations. For the purposes of this analysis, we grouped all HIV-related illnesses (ICD-10 codes B20–B24), and diabetes-related illnesses (ICD-10 codes E10–E14) into single categories. We assumed that repeated admissions of patients with HIV or diabetes were attributable to the same underlying illness and therefore we limited the incidence calculation to the first admission episode for such patients. In calculations of the incidence of disorders that are viewed as part of the range of HIV disease (eg, gastroenteritis, meningitis, pneumonia, and cervical cancer), we excluded patients with HIV infection.
In keeping with the latest GBD methods, we did not discount future values (ie, life-years gained in the future) and no age weights were used.26 Life expectancy was set at 86 years for both men and women in agreement with new methods that use the lowest age-specific death rates recorded across countries in 2010.27 We used recently updated weights from the GBD project28 to apply disability weighting. We searched for published reports describing average survival of patients with the diseases of interest in similar settings in sub-Saharan Africa and entered these for the years lived with disability component of the DALY calculations.29, 30, 31, 32, 33, 34, 35, 36 For chronic illnesses (eg, asthma), for which no data were available for estimation of life expectancy after first diagnosis in similar settings, we used the difference between mean age at diagnosis and the overall KHDSS life expectancy figures17 to compute the years lived with disability.
All clinical data for this analysis were gathered as part of routine care; therefore, individual patient consent was not needed. Community consent was obtained for collection of demographic surveillance data. The analysis was approved by the Ethical Review Committee of the Kenya Medical Research Institute.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
After exclusion of pregnant women admitted for delivery, there were 18 712 adult patient admissions to Kilifi District Hospital between Jan 1, 2007, and Dec 31, 2012, of which 10 536 (56%) were women and 8176 (44%) were residents of the KHDSS. During the same period, there were 790 635 person-years of observation in adult residents of the KHDSS. Outcome data were available for 18 418 (98%) patients, of whom 1899 (10·3%) died. Median length of hospital stay was 73 h (IQR 38–178 h). In the patients who died in hospital, the median time to death was 112 h (IQR 40–279 h); 334 (17·6%) of these 1899 patients died within 24 h of admission.
Figure 2 shows the causes of admission and death by ICD category. Accidents and injuries accounted for the highest number (1625) of admissions in men, whereas infectious diseases (2597) were the most frequent cause of admission in women. Case-fatality rates were highest for nervous system disorders (26%) and circulatory system illnesses (23%). Infectious and parasitic diseases, dominated by HIV-related illnesses, accounted for 39% of all hospital deaths, most of which occurred in patients younger than 55 years of age. Circulatory system illnesses, mainly stroke and heart failure, were the second most common cause of death, and accounted for 16% of all deaths in hospital.
Figure 2.
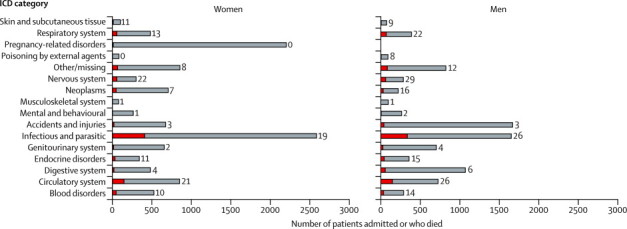
Number of admissions and inpatient deaths at Kilifi District Hospital by ICD category and sex, 2007–12
Length of bars indicate total number of admissions; red bars indicate number of deaths. Numerical labels on bars indicate case-fatality rate (%). The total number of admissions was 18 418, with 1899 deaths. ICD=International Classification of Diseases.
All reported rates of hospital admissions are age-standardised. Table 1 shows admission rates for each ICD category of disorders for people living in the stratum nearest to (within 5 km of) the hospital and for the entire KHDSS area. The all-cause hospital admission rates for people living within 5 km of Kilifi District Hospital were 1446 (95% CI 1297–1603) admissions per 100 000 person-years for women and 991 (861–1137) for men. Women were admitted to hospital at a significantly higher rate than were men (incidence rate ratio [IRR] 1·4, 95% CI 1·3–1·6; p<0·0001). Rates of hospital admission for women were highest for infectious and parasitic diseases, pregnancy-related disorders, and circulatory disorders (table 1), with the rate of admission for infectious diseases for women roughly double that for men (IRR 1·8, 95% CI 1·5–2·1; p<0·0001). In men, rates of hospital admission were highest for infectious and parasitic diseases, injuries, and digestive system illnesses (table 1). The rate of hospital admission for injuries was substantially higher for men than for women (IRR 1·9, 95% CI 1·4–2·5; p<0·0001). Rates of hospital admission for circulatory disorders did not vary significantly by sex (IRR 1·2, 95% CI 0·9–1·6; p=0·098). Table 2 shows age-specific admission rates for different disease categories for the distance stratum closest to the hospital, and appendix p 12 shows these rates for the entire KHDSS surveillance area. Appendix pp 13–17 shows rates of hospital admissions for each ICD code for the distance stratum closest to the hospital.
Table 1.
Age-standardised admission rates by ICD category of disorders and incidence rate ratios per 5 km increase in distance from Kilifi District Hospital
|
Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Rate in residents within 5 km of Kilifi District Hospital (95% CI) | Rate in all KHDSS residents (95% CI) | Incidence rate ratio(95% CI)* | Rate in residents within 5 km of Kilifi District Hospital (95% CI) | Rate in all KHDSS residents (95% CI) | Incidence rate ratio (95% CI)* | |
| Blood disorders | 42 (19–75) | 21 (19–23) | 0·90 (0·77–1·05) | 50 (23–88) | 13 (11–15) | 0·82 (0·66–1·01) |
| Circulatory system | 105 (69–155) | 28 (25–30) | 0·87 (0·78–0·97) | 87 (51–137) | 34 (30–37) | 0·93 (0·83–1·05) |
| Digestive system | 70 (40–111) | 16 (14–18) | 0·78 (0·65–0·93) | 112 (70–166) | 44 (40–48) | 0·88 (0·79–0·99) |
| Endocrine disorders | 103 (66–150) | 17 (15–19) | 0·65 (0·54–0·78) | 67 (37–113) | 18 (15–20) | 0·89 (0·75–1·04) |
| Genitourinary system | 83 (49–126) | 23 (20–25) | 0·79 (0·68–0·91) | 76 (44–125) | 39 (35–43) | 0·94 (0·83–1·07) |
| Infectious and parasitic | 303 (237–380) | 72 (67–76) | 0·76 (0·71–0·82) | 169 (117–235) | 52 (47–56) | 0·83 (0·75–0·93) |
| Accidents and injuries | 79 (63–98) | 24 (21–26) | 0·84 (0·73–0·96) | 135 (113–160) | 79 (73–84) | 0·97 (0·89–1·06) |
| Mental and behavioural disorders | 44 (22–80) | 10 (8–12) | 0·76 (0·60–0·95) | 37 (16–76) | 10 (8–11) | 0·76 (0·57–1·01) |
| Musculoskeletal system | 16 (4–41) | 3 (1–3) | 0·68 (0·44–1·06) | 11 (1–34) | 4 (2–4) | 0·76 (0·49–1·18) |
| Neoplasms | 111 (72–160) | 25 (21–27) | 0·72 (0·62–0·84) | 43 (19–82) | 11 (8–12) | 0·70 (0·55–0·91) |
| Nervous system | 24 (9–53) | 10 (8–11) | 0·91 (0·75–1·12) | 35 (13–69) | 13 (10–15) | 0·88 (0·70–1·10) |
| Poisoning | 10 (2–35) | 3 (2–4) | 0·81 (0·56–1·18) | 7 (1–34) | 3 (2–4) | 0·86 (0·59–1·25) |
| Pregnancy-related disorders | 239 (180–306) | 62 (57–65) | 0·81 (0·75–0·88) | NA | NA | NA |
| Respiratory system | 69 (40–111) | 20 (18–22) | 0·78 (0·67–0·91) | 52 (26–95) | 18 (15–20) | 0·83 (0·69–1·00) |
| Skin and subcutaneous tissue | 11 (2–35) | 4 (2–4) | 0·83 (0·59–1·18) | 14 (2–42) | 3 (2–4) | 0·77 (0·51–1·17) |
| Symptoms and signs not classified elsewhere | 93 (59–141) | 21 (18–23) | 0·77 (0·67–0·89) | 79 (44–125) | 25 (22–28) | 0·91 (0·80–1·05) |
| All hospital admissions† | 1446 (1297–1603) | 785 (770–799) | 0·80 (0·77–0·83) | 991 (861–1137) | 668 (652–683) | 0·89 (0·86–0·93) |
Rates and 95% CIs are per 100 000 person-years of observation among residents. ICD=International Classification of Diseases. KHDSS=Kilifi Health and Demographic Surveillance System. NA=not applicable.
Incidence rate ratios are per 5 km increase in distance from Kilifi District Hospital.
Admission rate attributable to all causes includes diagnoses that were not assigned an ICD code.
Table 2.
Age-specific rates of hospital admission for KHDSS residents living within 5 km of Kilifi District Hospital, by ICD category and age group
|
Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| 15–34 years | 35–54 years | 55–74 years | ≥75 years | 15–34 years | 35–54 years | 55–74 years | ≥75 years | |
| Blood disorders | 62 (41–90) | 32 (13–66) | 120 (48–248) | 0 (0) | 42 (24–69) | 46 (21–88) | 134 (49–292) | 382 (46–1379) |
| Circulatory system | 20 (9–38) | 96 (60–147) | 498 (333–715) | 827 (303–1800) | 16 (6–35) | 51 (25–94) | 312 (171–524) | 1337 (537–2754) |
| Digestive system | 115 (86–151) | 110 (71–164) | 69 (19–176) | 0 (0) | 61 (39–92) | 185 (130–256) | 379 (221–608) | 191 (5–1064) |
| Endocrine disorders | 35 (20–58) | 119 (78–175) | 429 (278–633) | 689 (224–1608) | 40 (22–66) | 62 (32–108) | 335 (187–552) | 0 (0) |
| Genitourinary system | 111 (82–146) | 170 (120–234) | 86 (28–200) | 138 (3–768) | 32 (16–56) | 62 (32–108) | 335 (187–552) | 573 (118–1674) |
| Infectious and parasitic | 297 (249–351) | 657 (554–774) | 532 (362–755) | 413 (85–1208) | 165 (126–211) | 334 (258–426) | 357 (204–580) | 191 (5–1064) |
| Accidents and injuries | 60 (39–87) | 133 (89–191) | 172 (82–316) | 0 (0) | 170 (131–217) | 206 (147–280) | 268 (138–468) | 0 (0) |
| Mental and behavioural disorders | 78 (54–108) | 74 (42–119) | 17 (0–96) | 0 (0) | 72 (47–104) | 57 (28–101) | 22 (1–124) | 0 (0) |
| Musculoskeletal system | 7 (1–19) | 55 (28–96) | 34 (4–124 | 0 (0) | 27 (13–49) | 0 (0) | 22 (1–124) | 0 (0) |
| Neoplasms | 82 (58–113) | 221 (163–292) | 275 (157–446) | 276 (33–996) | 16 (6–35) | 41 (18–81) | 156 (63–322) | 191 (5–1064) |
| Nervous system | 22 (11–41) | 37 (16–72) | 34 (4–124) | 276 (33–996) | 29 (15–52) | 41 (18–81) | 112 (36–260) | 0 (0) |
| Poisoning | 20 (9–38) | 14 (3–40) | 0 (0) | 0 (0) | 16 (6–35) | 10 (1–37) | 0 (0) | 0 (0) |
| Pregnancy-related disorders | 547 (481–620) | 303 (235–386) | 34 (4–124) | 0 (0) | NA | NA | NA | NA |
| Respiratory system | 58 (38–84) | 83 (49–131) | 223 (119–382) | 413 (85–1208) | 37 (20–62) | 57 (28–101) | 112 (36–260) | 382 (46–1379) |
| Skin and subcutaneous tissue | 58 (38–84) | 138 (93–197) | 257 (144–425) | 827 (303–1800) | 45 (26–72) | 92 (55–146) | 290 (155–496) | 382 (46–1379) |
| Symptoms and signs not classified elsewhere | 4 (1–16) | 28 (10–60) | 34 (4–124) | 928 (479–1621) | 5 (1–19) | 5 (0–29) | 89 (24–229) | 0 (0) |
Data are rate of hospital admission (95% CI). Rates and 95% CIs are per 100 000 person-years of observation in the KHDSS. Person-years of observation were as follows: 15–34 years: 45 152 (women). 37 668 (men); 35–54 years: 21 764 (women), 19 463 (men); 55–74 years: 5826 (women), 4480 (men); ≥75 years: 726 (women), 524 (men). KHDSS=Kilifi Health and Demographic Surveillance System. NA=not applicable.
Admission rates for KHDSS residents in all ICD categories decreased with increasing distance from their place of residence to the hospital (table 1 and figure 3). For men, the all-cause admission rate fell by 11% (95% CI 7–14) and for women it fell by 20% (17–23) for every 5 km increase in distance from the hospital. In men, admission rates decreased most sharply for neoplasms (30% decrease [95% CI 9–45] per 5 km increase in distance). In women, admission rates declined most sharply for endocrine disorders (35% per 5km increase in distance, 95% CI 22–46; table 1. Distance decay curves for each illness category are available in appendix pp 4–11.
Figure 3.
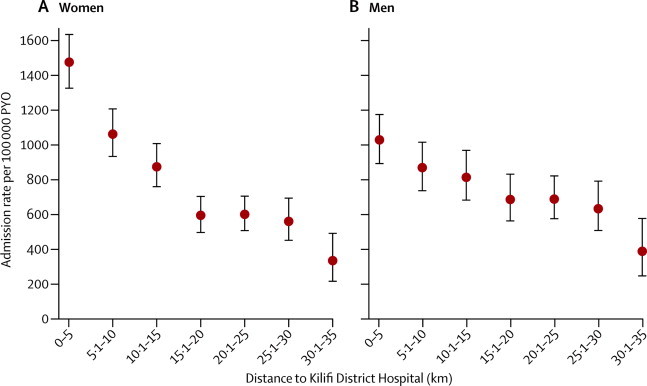
Age-standardised admission rates by distance from Kilifi District Hospital
(A) Women. (B) Men. PYO=person-years of observation
Table 3 shows the DALYs lost in the population living in the distance stratum closest to the hospital. The highest number of DALYs lost were attributable to HIV-related illnesses (2050 DALYs lost per 100 000 person-years of observation). Non-communicable diseases and injuries accounted for 741 DALYs lost per 100 000 person-years of observation. Although pregnancy-related disorders accounted for a high number of admissions in women (figure 2) and were the second most common cause of admission (table 1), they ranked 13th in the causes of DALYs lost. Meningitis not associated with HIV infection accounted for few hospital admissions, but because of its high case-fatality rate and high residual disability, it was a leading cause of DALYs lost. For all 15 illnesses listed in table 3, most of the DALYs lost were due to years of life lost rather than years of life lived with disability. DALYs lost for the whole KHDSS area are shown in appendix p 18.
Table 3.
Disability-adjusted life-years lost for the 15 leading causes of admission to Kilifi District Hospital in residents living within 5 km of the hospital
|
Women |
Men |
Disability-adjusted life-years lost | |||
|---|---|---|---|---|---|
| Years of life lost | Years lived with disability | Years of life lost | Years lived with disability | ||
| HIV | 2170 | 114 | 1713 | 60 | 2050 |
| Meningitis | 375 | 28 | 349 | 14 | 385 |
| Diabetes | 262 | 91 | 321 | 56 | 364 |
| Stroke | 130 | 13 | 183 | 11 | 166 |
| Pneumonia | 195 | 10 | 95 | 4 | 156 |
| Gastroenteritis | 141 | 1 | 183 | 1 | 161 |
| Heart failure | 97 | 6 | 20 | 8 | 68 |
| Benign prostatic hypertrophy | 0 | 0 | 63 | 0 | 63 |
| Asthma | 0 | 65 | 0 | 51 | 59 |
| Cervical cancer | 0 | 10 | 0 | 0 | 10 |
| Head injury | 0 | 3 | 0 | 8 | 5 |
| Spontaneous abortion | 0 | 4 | 0 | 0 | 4 |
| Peptic ulcer disease | 0 | 3 | 0 | 1 | 2 |
| Inguinal hernia | 0 | 0 | 0 | 1 | 0·3 |
| Fractures | 0 | 0 | 0 | 0 | 0·03 |
Years of life lost, years lived with disability, and disability-adjusted life-years lost are per 100 000 person-years of observation.
Discussion
Insufficient data about disease burden have been acknowledged as a major barrier to public health decision making in Africa.37 We have used linked clinical and demographic surveillance to comprehensively describe the burden of disease in adults admitted to hospital in Kilifi, Kenya, and we have investigated the extent to which access to hospital care can bias the use of hospital data to estimate the morbidity and mortality in the community. The population in Kilifi is undergoing an epidemiological transition38 that is typical of rural and semi-urban populations in sub-Saharan Africa.
A major strength of our analysis is the use of robust clinical and demographic data collected prospectively over a 6-year period by a dedicated and consistent team of clinical scientists. We improved the quality of both the care provided and the data collected by holding regular audits with feedback on performance. Linking of clinical surveillance to population data also enabled us to calculate the incidence of disease in adults, unlike most hospital-based studies from Africa, which do not have population denominator data.13
An absence of denominator data, and other deficiencies of hospital-based studies (panel), has led scientists to describe cause-specific deaths largely using verbal autopsy. In this technique, family members or caregivers of deceased patients are interviewed about the circumstances of death after the event with use of standardised questionnaires and the data are analysed by use of probabilistic models.12 The potential for misclassification in verbal-autopsy-based studies has contributed to controversies regarding the burden of adult mortality, such as that attributable to malaria.39 In settings where clinicians have access to high-quality clinical, laboratory, and imaging investigations, physician-coded hospital data are superior to verbal autopsy data.18
Panel. Research in context.
Systematic review
We have previously done a systematic review assessing data from studies of adults admitted to hospital in sub-Saharan Africa.13 The main finding was that the few studies available were done poorly, thus casting doubt as to the quality of burden of disease estimates for Africa. Additionally, in December, 2012, the publication of the latest findings from the Global Burden of Disease project by the Institute of Health Metrics stimulated substantial controversy, mainly as a result of insufficient primary data.11 The part of the world with the poorest quality of primary disease burden data is sub-Saharan Africa, where vital registration covers less than 5% of deaths.3
Interpretation
In this Article, we report a rich seam of primary data from adults in Kilifi, Kenya, and show how disease burden data could be obtained at many representative points in Africa through the integration of clinical surveillance into existing investments in demographic surveillance systems. In 2007, we linked adult hospital-based morbidity surveillance into the Kilifi Demographic Surveillance System to establish the major causes of hospital admission and death. We have calculated disease rates using accurate population denominators, and estimated disability-adjusted life-years lost because of leading causes of illness. The results show the dual burden of infectious and non-communicable disease faced by adults in sub-Saharan Africa. Additionally, we have characterised the effect of geographical access to care, which is the main bias in the use of hospital-based data to estimate disease incidence. The public health significance of our study is that it shows that a few additional investments in existing health and demographic surveillance systems can fill the information gap for cause-specific morbidity and mortality in African adults. Such data are essential to populate models of disease burden for the management of health planning in Africa in the next decade.
The computation of DALYs lost in this setting emphasises the importance of diseases, such as diabetes, which generate a high DALY burden despite the fact that they rank lower in the causes of hospital admission than do other illnesses (table 1). The GBD project has published summary figures of DALYs for the whole eastern region of sub-Saharan Africa.26 Our estimate of DALYs lost because of HIV is nearly double the GBD estimate for eastern sub-Saharan Africa, despite the fact that HIV prevalence in Kilifi (4·9% in antenatal clinic attendees40) is lower than the regional average, which ranges from 8 to 12%.41 By contrast, the number of DALYs lost to stroke and diabetes was slightly lower in our estimate than in the GBD regional estimate. The fact that our data are from one hospital means they have low generalisability, but the methods used here show the opportunity to extend the geographic representation of disease burden data by capitalising on existing centres of population surveillance. In the past two decades, substantial investment has been made in demographic surveillance systems in Africa within the INDEPTH network.42 What this study shows is the added value of the integration of data based on demographic surveillance systems with good-quality local morbidity surveillance.
In Kilifi, HIV-related diseases accounted for a large proportion of hospital admissions and of DALYs lost. The high case-fatality rates reported suggest that many patients presented with advanced disease. Late presentation to care has also been recorded in South Africa.43 Although progress has been made in Africa in reducing incident HIV infections,44 the people who do become infected in Kilifi are still not being enrolled sufficiently early into HIV care.45
Another important finding in this study is the comparatively high burden of non-communicable diseases and injuries in Kilifi. Injuries accounted for the highest number of admissions in men, and the rate of hospital admission for injuries was second only to that of infectious diseases. Complications of diabetes were the third leading cause of DALYs lost, followed by stroke—an unusual finding for a rural population in a developing nation. Studies of diabetes and stroke in Africa have generally shown a higher burden of these diseases in urban areas.46, 47 Women lost more DALYs to stroke than did men, largely because stroke occurred at an earlier age in women (mean 60 years of age) than in men (mean 65 years of age). As with HIV, most of the lost DALYs attributable to diabetes and stroke were because of premature death, rather than years of life lived with disability. Stroke, HIV-related illnesses, and diabetes all need well-functioning chronic care systems that are not yet available in most of sub-Saharan Africa.9
The data presented in our analysis are probably a substantial under-representation of the true burden of disease in the region because presentation rates decline notably with distance from hospital (figure 3). We minimised this bias by limiting incidence calculations to the distance stratum closest to the hospital. Although the analysis of distance decline by disease classification (appendix) has finite precision, in general, the effect of distance on presentation rates was greater for non-communicable than communicable diseases and was greatest for neoplastic and endocrine disorders. Apart from distance, other possible causes of disease underascertainment with use of hospital data are hospital user fees48 and presentation to traditional healers, which was beyond the scope of this study.
Because of insufficient diagnostic facilities at the hospital, some illness categories might have been underdiagnosed, especially rare malignancies. However, this would not have affected data for common cancers, such as cervical cancer, since adequate facilities were available to diagnose these diseases.
In conclusion, this analysis shows that the integration of clinical and demographic surveillance can be used to measure the burden of disease in adults at discrete centres in sub-Saharan Africa. The main problem with this form of surveillance is the degree of underascertainment attributable to living at a distance from hospital. We have described the magnitude and disease-specific pattern of this bias and the methods we used to minimise it.
We found that the main causes of morbidity in adults in coastal Kenya were HIV-related disease, pregnancy-related disorders, non-communicable diseases, and injuries. In view of the strong existing investment in demographic surveillance systems across Africa, our study depicts a model to investigate disease patterns in diverse settings across the continent that could be used to guide public health policy and stimulate appropriate research.
Acknowledgments
Acknowledgments
Clinical surveillance of adults at Kilifi District Hospital is supported by the GAVI Alliance. Demographic surveillance is supported by the Wellcome Trust. AS (098532), TW (076934), and LS (098504) are supported by the Wellcome Trust as Senior Research Fellows. This analysis is published with the permission of the director of the Kenya Medical Research Institute. We thank all staff involved in collection of demographical and clinical data.
Contributors
JAGS, EB, MK, TNW, JO, IK, BT, AOE, KM, EWB, CN, LM, AJB, SM, MOt, SK, and PK set up the clinical and demographic surveillance. AOE, JAGS, KM, EWB, LM, PK, BT, AJB, SM, IK, MOw, and SK supervised the clinical surveillance. AOE, JO, MOw, PA, CN, TNW, UKG, and JAGS did the statistical analyses. AOE wrote the draft report. AOE, JAGS, LS, MDK, KM, EB, AB, SM, TNW, and UKG revised the report. All authors read and approved the final version.
Declaration of interests
We declare that we have no competing interests.
Supplementary Material
References
- 1.Cohen B, Menken J, editors. Aging in sub-Saharan Africa: recommendation for furthering research. National Academy of Sciences; Washington, DC: 2006. [PubMed] [Google Scholar]
- 2.Clark SJ, Kahn K, Houle B. Young children's probability of dying before and after their mother's death: a rural South African population-based surveillance study. PLoS Med. 2013;10:e1001409. doi: 10.1371/journal.pmed.1001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Global health repository (database) 2013. http://apps.who.int/gho/data/ (accessed Dec 14, 2013).
- 4.National AIDS and STI Control Programme (NASCOP) 2007 Kenya AIDS Indicator Survey: final report. NASCOP; Nairobi, Kenya: 2009. http://nascop.or.ke/library/3d/Official_KAIS_Report_20091.pdf (accessed Aug 8, 2013). [Google Scholar]
- 5.Okiro EA, Kazembe LN, Kabaria CW. Childhood malaria admission rates to four hospitals in Malawi between 2000 and 2010. PLoS One. 2013;8:e62214. doi: 10.1371/journal.pone.0062214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajaratnam JK, Marcus JR, Flaxman AD. Neonatal, postnatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet. 2010;375:1988–2008. doi: 10.1016/S0140-6736(10)60703-9. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Rosenfeld LC, Lim SS. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 8.Daar AS, Singer Pa, Persad DL. Grand challenges in chronic non-communicable diseases. Nature. 2007;450:494–496. doi: 10.1038/450494a. [DOI] [PubMed] [Google Scholar]
- 9.Tollman SM, Kahn K, Sartorius B, Collinson MA, Clark SJ, Garenne ML. Implications of mortality transition for primary health care in rural South Africa: a population-based surveillance study. Lancet. 2008;372:893–901. doi: 10.1016/S0140-6736(08)61399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher D, Smeeth L, Sekajugo J. Health transition in Africa: practical policy proposals for primary care. Bull World Health Organ. 2010;88:943–948. doi: 10.2471/BLT.10.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byass P, de Courten M, Graham WJ. Reflections on the global burden of disease 2010 estimates. PLoS Med. 2013;10:e1001477. doi: 10.1371/journal.pmed.1001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byass P, Chandramohan D, Clark SJ. Strengthening standardised interpretation of verbal autopsy data: the new InterVA-4 tool. Glob Health Action. 2012;5:1–8. doi: 10.3402/gha.v5i0.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etyang AO, Scott JA. Medical causes of admissions to hospital among adults in Africa: a systematic review. Glob Health Action. 2013;6:1–14. doi: 10.3402/gha.v6i0.19090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SanJoaquin MA, Allain TJ, Molyneux ME. Surveillance Programme of IN-patients and Epidemiology (SPINE): implementation of an electronic data collection tool within a large hospital in Malawi. PLoS Med. 2013;10:e1001400. doi: 10.1371/journal.pmed.1001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setel PW, Macfarlane SB, Szreter S. A scandal of invisibility: making everyone count by counting everyone. Lancet. 2007;370:1569–1577. doi: 10.1016/S0140-6736(07)61307-5. [DOI] [PubMed] [Google Scholar]
- 16.Moisi JC, Nokes DJ, Gatakaa H. Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health Organ. 2011;89:102–111. doi: 10.2471/BLT.10.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JAG, Bauni E, Moisi JC. Profile: the Kilifi Health and Demographic Surveillance System (KHDSS) Int J Epidemiol. 2012;41:650–657. doi: 10.1093/ije/dys062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauni E, Ndila C, Mochamah G. Validating physician-certified verbal autopsy and probabilistic modeling (InterVA) approaches to verbal autopsy interpretation using hospital causes of adult deaths. Popul Health Metr. 2011;9:49. doi: 10.1186/1478-7954-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenya National Bureau of Statistics (KNBS) and ICF Macro . Kenya Demographic and Health Survey 2008–09. KNBS and ICF Macro; Calverton, MD: 2010. http://www.measuredhs.com/pubs/pdf/FR229/FR229.pdf (accessed Aug 17, 2013). [Google Scholar]
- 20.National Coordinating Agency for Population and Development. Ministry of Health. Central Bureau of Statistics. ORC Macro . Kenya Service Provision Assessment Survey 2004. National Coordinating Agency for Population and Development; Nairobi: 2005. [Google Scholar]
- 21.Kenya Ministry of Health Clinical guidelines for management and referral of common conditions at levels 4–6: hospitals. 2009. http://apps.who.int/medicinedocs/documents/s21000en/s21000en.pdf (accessed July 8, 2013).
- 22.WHO and UNAIDS . HIV/AIDS Programme. Guidance on provider-initiated HIV testing and counselling in health facilities. World Health Organisation; Geneva: 2007. http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf (accessed Aug 17, 2013). [Google Scholar]
- 23.WHO . International statistical classification of diseases and related health problems. World Health Organization; Geneva: 2004. [Google Scholar]
- 24.Sankoh OA, Ngom P, Clark SJ, de Savigny D, Binka F. In: Disease and mortality in Sub-Saharan Africa. 2nd edn. Jamison DT, Feachem RG, Makgoba MW, editors. World Bank; Washington, DC: 2006. Levels and patterns of mortality at indepth demographic surveillance systems; pp. 75–86. [PubMed] [Google Scholar]
- 25.WHO Global Burden of Disease templates. http://www.who.int/entity/healthinfo/bodreferencedalycalculationtemplate.xls?ua=1 (accessed July 5, 2013).
- 26.Murray CJ, Vos T, Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 27.Murray CJ, Ezzati M, Flaxman AD. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 28.Salomon JA, Vos T, Hogan DR. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walensky RP, Wolf LL, Wood R. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker RW, Rolfe M, Kelly PJ, George MO, James OF. Mortality and recovery after stroke in the Gambia. Stroke. 2003;34:1604–1609. doi: 10.1161/01.STR.0000077943.63718.67. [DOI] [PubMed] [Google Scholar]
- 31.Kengne AP, Ntyintyane LM, Mayosi BM. A systematic overview of prospective cohort studies of cardiovascular disease in sub-Saharan Africa. Cardiovasc J Afr. 2012;23:103–112. doi: 10.5830/CVJA-2011-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarborough M, Gordon SB, Whitty CJM. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007;357:2441–2450. doi: 10.1056/NEJMoa065711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill GV, Huddle KR, Monkoe G. Long-term (20 years) outcome and mortality of Type 1 diabetic patients in Soweto, South Africa. Diabet Med. 2005;22:1642–1646. doi: 10.1111/j.1464-5491.2005.01712.x. [DOI] [PubMed] [Google Scholar]
- 34.Bickler S, Ozgediz D, Gosselin R. Key concepts for estimating the burden of surgical conditions and the unmet need for surgical care. World J Surg. 2010;34:374–380. doi: 10.1007/s00268-009-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber MA, Aoki N, Scott BG, Beck JR. Determinants of mortality in patients with severe blunt head injury. Arch Surg. 2002;137:285–290. doi: 10.1001/archsurg.137.3.285. [DOI] [PubMed] [Google Scholar]
- 36.Sankaranarayanan R, Swaminathan R, Jayant K, Brenner H. An overview of cancer survival in Africa, Asia, the Caribbean and Central America: the case for investment in cancer health services. IARC Sci Publ. 2011;162:257–291. [PubMed] [Google Scholar]
- 37.Cooper RS, Osotimehin B, Kaufman JS, Forrester T. Disease burden in sub-Saharan Africa : what should we conclude in the absence of data ? Lancet. 1998;351:208–210. doi: 10.1016/S0140-6736(97)06512-4. [DOI] [PubMed] [Google Scholar]
- 38.O'Meara WP, Bejon P, Mwangi TW. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White NJ, Dondorp AM, Faiz A, Mishra S, Hien TT. New global estimates of malaria deaths. Lancet. 2012;380:559–560. doi: 10.1016/S0140-6736(12)61321-X. [DOI] [PubMed] [Google Scholar]
- 40.Scott JAG, Berkley JA, Mwangi I. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montana LS, Mishra V, Hong R. Comparison of HIV prevalence estimates from antenatal care surveillance and population-based surveys in sub-Saharan Africa. Sex Transm Infect. 2008;84(suppl 1):i78–i84. doi: 10.1136/sti.2008.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sankoh O, Byass P. The INDEPTH Network: filling vital gaps in global epidemiology. Int J Epidemiol. 2012;41:579–588. doi: 10.1093/ije/dys081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kigozi IM, Dobkin LM, Martin JN. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52:280–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joint United Nations Programme on HIV/AIDS . Global report : UNAIDS report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2010. http://www.unaids.org/globalreport/Global_report.htm (accessed Aug 19, 2013). [Google Scholar]
- 45.Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS (Auckl) 2013;5:1–17. doi: 10.2147/HIV.S28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker RW, McLarty DG, Kitange HM. Stroke mortality in urban and rural Tanzania. Adult Morbidity and Mortality Project. Lancet. 2000;355:1684–1687. doi: 10.1016/s0140-6736(00)02240-6. [DOI] [PubMed] [Google Scholar]
- 47.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254–2266. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 48.Chuma J, Musimbi J, Okungu V, Goodman C, Molyneux C. Reducing user fees for primary health care in Kenya: policy on paper or policy in practice? Int J Equity Health. 2009;8:15. doi: 10.1186/1475-9276-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


