Abstract
Background
Uveal melanoma exhibits a high incidence of metastases and no systemic therapy clearly improves outcomes. The anti-CTLA-4 antibody ipilimumab is a standard of care for metastatic melanoma; however, the clinical activity of CTLA-4 inhibition in patients with metastatic uveal melanoma is poorly defined.
Methods
To assess ipilimumab in this setting, we performed a multicenter, retrospective analysis of four hospitals in the United States and Europe. Clinical characteristics, toxicities and radiographic disease burden as determined by central, blinded radiology review were determined.
Results
Thirty-nine patients were identified (34 treated with 3 mg/kg and 5 treated with 10 mg/kg). Using the immune-related response criteria and modified WHO criteria, response rate (RR) and combined response plus stable disease (SD) rate were assessed after 12 weeks, 23 weeks and total (median follow-up 50.4 weeks (12.6 months)). At week 12, the RR and response plus SD rate were 2.6% and 46.0%, at week 23: 2.6% and 28.2%. There was one complete response and one late partial response (at 100 weeks after initial SD) for irRR of 5.1%. Immune-related adverse events (irAE) were observed in 28 (71.8%) patients, with seven (17.9%) grade 3-4 events. irAEs were more frequent in patients receiving 10 mg/kg versus 3 mg/kg. The median overall survival from first dose of ipilimumab was 9.6 months (confidence interval 6.3-13.4 months, range: 1.6-41.6 months). Performance status, LDH and absolute lymphocyte count ≥1000 cells/μL at week 7 were significantly associated with survival.
Conclusions
In uveal melanoma, durable responses to ipilimumab and manageable toxicity were observed.
Keywords: uveal melanoma, ipilimumab, CTLA-4, immunotherapy, absolute lymphocyte count
Introduction
Uveal melanoma is a rare form of melanoma that represents about 3-5% of the incidence of cutaneous melanomas.1 Metastasis in uveal melanoma is common with approximately 50% of patients developing distant cancer within 15 years of initial diagnosis.2 Uveal melanoma harbors a unique set of genetic alterations compared with cutaneous melanoma. Whereas cutaneous melanoma often harbors activating mutations in BRAF and NRAS, mutations in the heterotrimeric G protein alpha-subunits, GNAQ and GNA11, have been reported in approximately 80% of uveal melanomas.3 GNAQ and GNA11 mutations are not, however, correlated with disease free survival or the development of metastasis.4
The outcome for patients with metastatic uveal melanoma is dismal, with a median survival of approximately 12 months,5 and no systemic therapy has improved survival.6 Drugs commonly used to treat advanced cutaneous melanoma rarely achieve durable responses in patients with uveal melanoma. Treatment with dacarbazine (DTIC), carmustine (BCNU), cisplatin, and tamoxifen (Dartmouth regimen) was reported to show a response rate of 6% and a phase II study of carboplatin, paclitaxel and sorafenib described no objective responses7, 8. A retrospective review of 143 patients treated with chemotherapy at MD Anderson Cancer Center reported a single objective response and other reviews of the Eastern Cooperative Oncology Group (ECOG) and Southwestern Oncology Group described similar findings.9, 10
Immunotherapy for the treatment of metastatic uveal melanoma has also conceptually been of interest. It is hypothesized that uveal melanoma may be more immunogenic than other tumors since it arises in the immunologically privileged site of the eye. Further, uveal melanoma has high expression of multiple cancer antigens known to be immunogenic, including gp100, MAGE, MART-1, tyrosinase and TRP-1.11, 12 Clinical experience with immunotherapy in uveal melanoma is limited with case reports describing success; however, larger series showed equivocal benefit.13, 14
Ipilimumab (Bristol-Myers Squibb, Princeton, NJ) is a fully human monoclonal antibody that augments anti-tumor immunity through blockade of cytotoxic T lymphocyte antigen-4. Ipilimumab has become a standard of care for the treatment of patients with metastatic melanoma after an overall survival benefit was demonstrated.15 The activity of ipilimumab in uveal melanoma, however, has not been well described. A retrospective series of 13 patients with metastatic uveal melanoma treated with ipilimumab reported three patients with stable disease as the best response,16 and a smaller review described two out of five patients with stable disease at 11 months.17 Only preliminary data describing patients with uveal melanoma treated with ipilimumab in expanded access programs have been presented.18 Given the limited therapeutic options available to patients with uveal melanoma, determining the efficacy of ipilimumab in uveal melanoma is essential.
We conducted a multicenter, retrospective analysis of 39 patients with metastatic uveal melanoma treated with ipilimumab under an expanded access clinical program or using commercial drug. We report the clinical activity and toxicity observed from four academic hospitals in the United States and Europe.
Methods
Patients and Clinical Characteristics
After obtaining institutional review board approval at each site, patients with metastatic uveal melanoma treated with ipilimumab were identified from the databases of four institutions (Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, United States and University Hospital of Lausanne, Switzerland). Patients treated on clinical protocols and with commercial drug were included. Patients receiving ipilimumab in combination with other agents or as re-induction therapy were excluded. Relevant clinical parameters were collected including age, gender, ECOG performance status, site(s) of metastatic disease, lines of prior therapy as well as dose of ipilimumab received. Laboratory parameters were collected including lactate dehydrogenase (LDH) at time of first ipilimumab infusion, and absolute lymphocyte count (ALC) before treatment as well as at approximately seven weeks after initiation of therapy. Treatment response and safety data were also determined. All data was aggregated following patient de-identification.
Efficacy and Toxicity Assessment
Efficacy outcomes were determined by a radiologist at each site who was blinded to outcome. Beneficial effects of ipilimumab were categorized as complete response (CR), partial response (PR) or stable disease (SD). Response plus SD rate was calculated as the percentage of patients achieving a CR, PR or SD at 12 weeks, 23 weeks or longer after starting ipilimumab treatment. The immune-related response criteria (irRC) and modified World Health Organization (mWHO) criteria were applied to determine each patient's response.19 Overall survival (OS) was calculated by Kaplan-Meier methodology from first dose of ipilimumab to date of death by any cause. Toxicity was assessed through chart review and graded using Common Terminology Criteria for Adverse Events (version 4.0). Special attention was given to events of special interest or immune-related adverse events (irAE) including rash, colitis, hepatitis, thyroiditis and hypophysitis.
Univariate comparisons of OS for ipilimumab dose (10 mg/kg or 3 mg/kg), baseline LDH, ECOG performance status, and ALC were conducted using Kaplan-Meier estimates; differences were assessed using the log-rank test. LDH was divided as above or below the institutional upper limit of normal; ALC was divided into low (<1000 cells/μL) or normal (≥1000 cells/μL). ECOG performance status was classified as fully active versus any restriction (0 versus 1-2). Statistically significant predictors in the univariate comparisons were then included in a multivariable Cox proportional hazards regression model. The Cox regression was stratified by number of prior therapies (treatment-naive versus other) to allow for underlying differences in the baseline hazards of death between these two groups. Landmark analyses were conducted to compare OS between 7-week ALC levels (low versus normal) as this has been suggested as a biomarker of ipilimumab efficacy in cutaneous melanoma.20, 21 Comparisons of rates of adverse events were based on Fisher's exact test. Statistical significance was defined as p < 0.05.
Results
Patient and Clinical Characteristics
The clinical characteristics of the thirty-nine patients included in the analysis are shown in Table 1. Patients were predominately male with a median age of 61 years and median ECOG status of 0. The median number of prior therapies was one with approximately half of patients being treated as part of the Bristol-Myers Squibb expanded access clinical protocol (CA184045). Five patients (13%) received ipilimumab at 10 mg/kg while others received 3 mg/kg. The median number of ipilimumab doses was four (range 1-16). Three patients received maintenance dosing.
Table 1. Patient Characteristics.
| Total n | 39 | ||
| Age, median (Range), years | 61 | (39-84) | |
| Sex | Male | 23 | 59% |
| Female | 16 | 41% | |
| ECOG PS pretreatment | 0 | 22 | 56% |
| (median ECOG 0, range 0-2) | 1 | 8 | 21% |
| 2 | 4 | 10% | |
| Unknown | 5 | 13% | |
| Sites of Metastatic Disease (≥5% only) | Liver | 32 | 82% |
| Lung | 15 | 38% | |
| Bone | 9 | 23% | |
| Soft Tissue | 7 | 18% | |
| Skin | 3 | 8% | |
| Lymph Node | 3 | 8% | |
| Pancreas | 2 | 5% | |
| Brain | 2 | 5% | |
| Pretreatment median LDH (range) | 300 | (171-5132) | |
| Patients with elevated LDH (%) | 30 | 63% | |
| Pretreatment median ALC | 1.2 | (0.5-2.66) | |
| Prior lines of therapy, n (%) | 0 | 4 | 10% |
| 1 | 22 | 56% | |
| 2 | 9 | 23% | |
| ≥3 | 4 | 10% | |
| Median prior lines of therapy | 1 | (0-5) | |
| Ipilimumab dose | 3 mg/kg | 34 | 87% |
| 10mg/kg | 5 | 13% | |
| Median dose of ipilimumab | 3 mg/kg | ||
| Median doses ipilimumab, n (range) | 4 | (1-16) | |
| Protocol | 21 | 54% | |
| Commercial | 18 | 46% |
LDH = lactate dehydrogenase in units/L
ALC = absolute lymphocyte count (Kcells/μL)
Response Analysis
Of the 39 total patients identified, 35 were evaluable for radiographic assessment of changes in tumor burden following ipilimumab. The four patients who were not assessable either died prior to assessment of change in tumor burden or were transitioned to palliative care only without subsequent imaging. These patients were assumed to have had progressive disease and were included in all analyses.
Given that tumor assessments were performed both on and off protocol, the timing of restaging radiography was somewhat variable in the study population. With that caveat, it was observed that the first through fourth scans took place at medians of 10.7, 16.1, 23.0 and 31.3 weeks. When adjusting for the twelve patients who were restaged prior to completing ipilimumab induction (prior to 10 weeks), the median time to first scan was 12.0 weeks. In the twelve patients who had early restaging performed, the median time to first scan was 6.2 weeks.
By irRC, at the time of the first radiographic assessment (approximately week 12) there were one irCR and 17 irSD. The irCR was confirmed on subsequent scans, and, notably, one patient who had irSD at week 12 and week 23 developed a late response, achieving irPR at approximately week 100. Prior to week 23, six irSD had progressive disease (PD). One irSD had a confirmatory scan for SD but was not yet evaluable at 23 weeks. Additionally, three patients who had irSD at week 12 and 23 later had PD (at weeks 28, 31 and 33). Data on response and response plus SD rate are listed in Table 2A. In evaluation of the total study cohort (median follow-up of 50.4 weeks (12.6 months)), there was one irCR, one irPR and six irSD. Seventeen patients had PD at the time of the first scan; however, only seven of these patients had the required follow-up scan documenting progression, as required by irRC. The response rate in total follow up by irRC was 5.1% (2/39).
Table 2. Clinical outcomes for benefiting patients (A) and details for those benefiting at 6 months (B).
| A | |||
|---|---|---|---|
| At Last Follow-up |
12 weeks |
23 weeks |
|
|
| |||
| CR | 1 | 1 | 1 |
| PR | 1 | 0 | 0 |
| SD | 6 | 17 | 10 |
| RR | 5.1% | 2.6% | 2.6% |
| CR+PR+SD | 20.5% | 46.1% | 28.2% |
| B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study # | Age | Pre LDH | Pre ALC | ALC 7 weeks | Change in ALC | Study vs. Standard Practice | Dose | Sites of metastatic disease at baseline | irAE | Prior Rx | Time from first dose to death or follow up (months) | Current Status | Alive |
| 8 | 51 | 376 | 1.22 | 1.88 | 0.66 | Standard | 3 | Liver, Lung, Soft Tissue | None | Ganetespib | 10.8 | PD at week 31 | No |
| 19 | 44 | 176 | 1.92 | 3.07 | 1.15 | Standard | 3 | Liver, Lung | G1 Rash | None | 6.6 | SD at week 26+ | Yes |
| 20 | 49 | 203 | 1.52 | 1.93 | 0.41 | Standard | 3 | Lung | G2 Rash | None | 11.7 | SD at week 39+ | Yes |
| 22 | 66 | 2363 | 0.84 | 1.2 | 0.36 | Standard | 3 | Liver, Pancreas, Lymph nodes | None | Temozolomide | 8.1 | SD at week 32+ | Yes |
| 25 | 76 | 312 | 1.3 | 1.4 | 0.1 | Study | 10 | Liver | G3 Hepatitis | Pegylated arginine deiminase | 19.0 | PD at week 33 | No |
| 28 | 60 | 133 | 1.5 | 1.8 | 0.3 | Study | 10 | Lung | G3 Hypophysitis | None | 19.3 | SD at week 24+ | Yes |
| 29 | 63 | 202 | 1.8 | 2.4 | 0.6 | Study | 10 | Liver | None | Interleukin-2 | 9.6 | SD at week 38+ | Yes |
| 32 | 60 | 240 | 2.4 | 2.1 | -0.3 | Standard | 3 | Liver, Brain | None | None | 8.9 | PD at week 28 | Yes |
| 37 | 55 | 296 | 1.4 | 2.7 | 1.3 | Standard | 3 | Brain, Soft Tissue | None | Temozolomide, Everolimus | 7.6 | SD at week 30+ | Yes |
|
| |||||||||||||
| 27 | 54 | 169 | 1.3 | 1.6 | 0.3 | Study | 10 | Liver, Lung | None | Selumetinib, Pegylated arginine deiminase | 41.6 | PR at week 140+ | Yes |
|
| |||||||||||||
| 21 | 68 | 185 | 1.6 | 2.62 | 1.02 | Standard | 3 | Liver, Skin, Soft Tissue | G1 rash | Temozolomide | 15.5 | CR at week 50+ | Yes |
CR=complete response, PR=partial response, SD=stable disease, RR=response rate, irAE=immune-related Adverse Event, G=Grade
By the mWHO criteria, at first radiographic assessment (week 12), one CR and fifteen SD were observed. Seventeen patients had PD at the time of the first scan. Two patients classified as SD by the irRC were reclassified as having PD by mWHO given the appearance of new lesions. One patient was initially classified as having SD but entered greater than 50% tumor reduction at approximately week 100. In evaluation of the total study cohort at last follow-up, the overall response rate by mWHO was identical to the response rate by the irRC (5.1%, 2/39 patients).
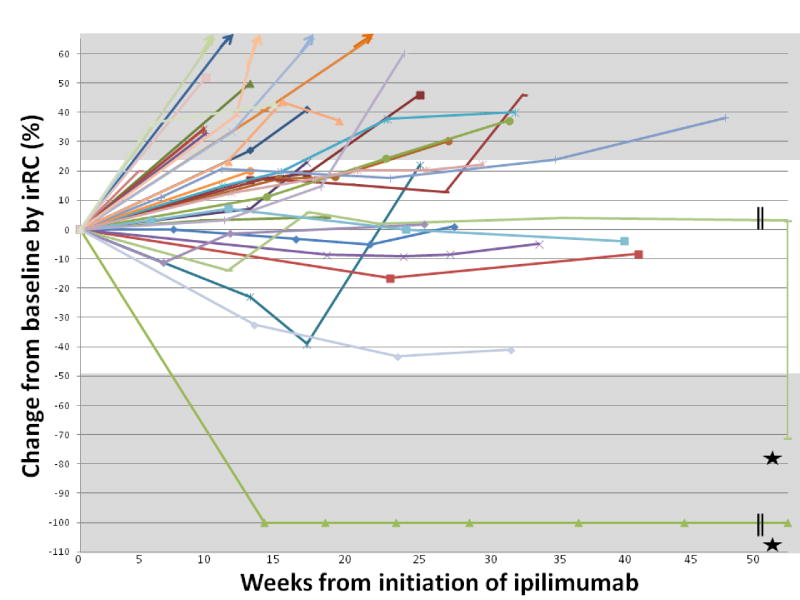
The changes in disease burden from baseline by individual patient are shown in Figure 1. The patient who achieved a CR by both irRC and mWHO was treated with ipilimumab at 3 mg/kg and has had a durable response currently ongoing at 62 weeks. This patient had received treatment with temozolomide chemotherapy prior to ipilimumab and experienced grade 1 rash during treatment. Sites of responding disease in this patient included liver, soft tissue and skin metastases. The patient who achieved mWHO SD but late irPR by irRC was previously treated with selumetinib and pegylated arginine deiminase. This response is on-going at 140 weeks. This patient had late toxicity with the development of grade 4 uveitis approximately 2.5 years following the initiation of ipilimumab. Sites of responding disease included brain and soft tissue metastases. Another notable patient achieved irSD though had significant tumor shrinkage through both 12 and 23 weeks. This patient was previously treated with temozolomide and everolimus and the response to ipilimumab is on-going at 30.4 weeks. Sites of responding disease in this patient included liver and lung metastases. Further clinical details for patients with response or stable disease at 23 weeks are described in Table 2B.
Figure 1. Change in disease burden for each patient over time.
Star indicates that patient 27 experienced initial irSD, entering irPR at approximately week 100 with on-going response at week 140 and patient 21 experienced irCR that is on-going at week 62.
The biochemical parameters of patients experiencing response or SD at at last follow up included LDH level that was within normal limits in all but three patients, with two of those being just slightly out of range. All patients experiencing experiencing response or SD at last follow up had a rise in ALC from baseline to week 7 (median increase of 600 cells/μL), except for one patient who had a very slight decrease of 300 cells/μL. Half of patients obtaining response or SD at last follow up experienced irAE including two patients with irSD who experienced grade 3 hepatitis and grade 4 hypophysitis, respectively.
Overall Survival Analysis
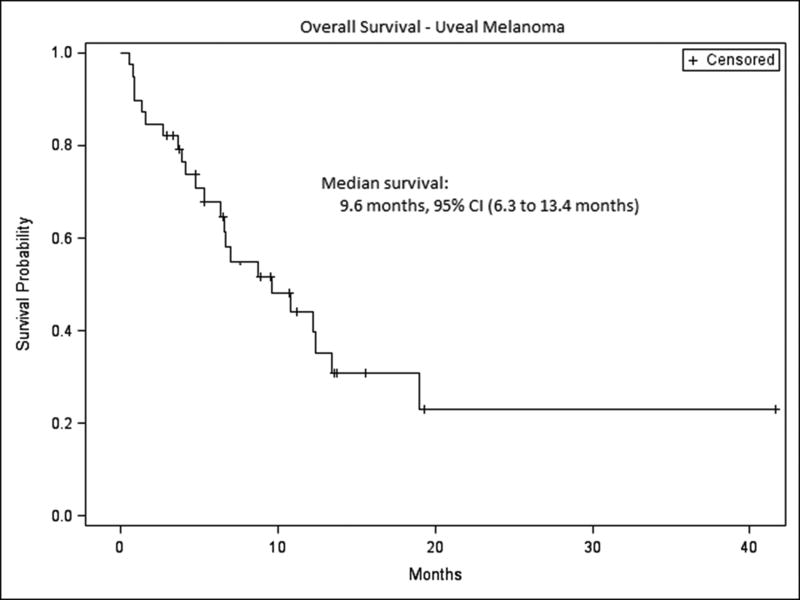
After a median follow-up of 12.6 months for survivors, the median overall survival by Kaplan-Meier methodology for the entire cohort was 9.6 months, with a 95% confidence interval (CI) of 6.3 to 13.4 months (Figure 2). In univariate analysis, neither dose of ipilimumab (3 mg/kg vs. 10 mg/kg) nor baseline ALC (low vs. normal) was significantly associated with survival (log-rank p=0.41 and 0.10, respectively). However, ECOG performance status (0 vs 1-2, log-rank p < 0.0001), lines of prior treatment (0 vs any, log-rank p = 0.04) and LDH within normal limits (normal vs. elevated, log-rank p=0.005) were associated with improved survival. The median length of time between diagnosis of metastatic cancer and first dose of ipilimumab was 12.1 months (range 0.6 to 47 months). Based on a multivariable Cox proportional hazard model of overall survival with ECOG status and LDH class as covariates, ECOG status of 0 demonstrated an 87% reduction in the hazard of death compared with ECOG 1-2 (hazard ratio (HR): 0.13, 95% CI (0.04 to 0.44), p=0.001) and LDH within institutional normal limits showed an 82% reduction in the hazard of death (HR: 0.18, 95% CI (0.05 to 0.73), p=0.02).
Figure 2. Overall survival for entire cohort.
The median overall survival for the total cohort was 9.6 months, 95% CI (6.3 to 13.4 months).
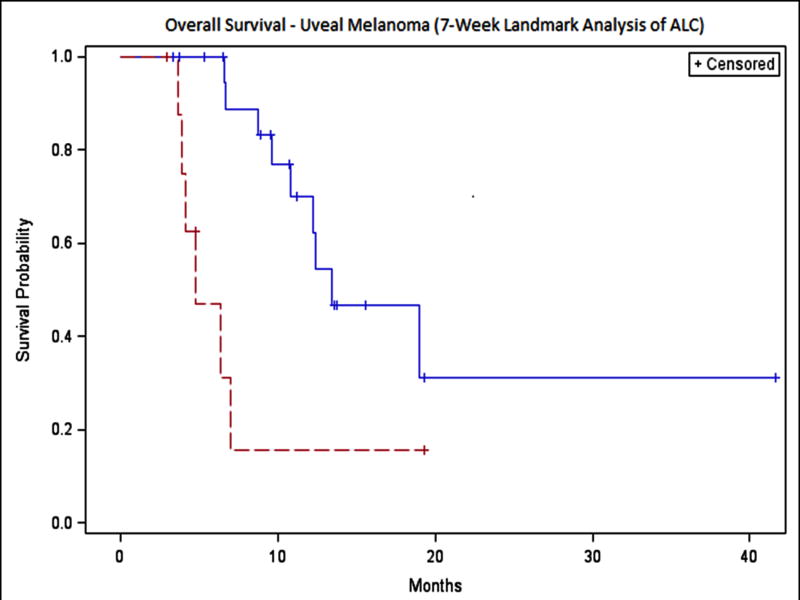
A landmark analysis of week 7 ALC indicated statistically significant differences in OS between normal and low ALC levels (Figure 3). For the 31 patients who were alive and had ALC measurements at week 7, ALC ≥1000 cells/μL (n = 22) was associated with a median OS of 13.4 months, 95% CI (9.6 to ∞) compared with a median of 4.8 months, 95% CI (3.6 to 7.0) in patients with ALC < 1000 cells/μL (n=9) (log-rank p=0.004). All patients with SD, PR or CR at 23 weeks had ALC ≥1000 cells/μL at week 7. The landmark analysis excluded six patients with survival times less than seven weeks and two additional patients with ALC missing at week 7.
Figure 3. Overall survival stratified by ALC at seven weeks.
ALC ≥ 1 (Solid Line): median overall survival of 13.4 months, 95% CI (9.6 to ∞), ALC < 1 (Dotted Line): median overall survival of 4.8 months, 95% CI (3.6 to 7.0).
Toxicity Analysis
The overall incidence of irAE was 71.8% (Table 3). Rash was the most common irAE, affecting eleven patients. Two patients had diarrhea and in both cases, grade 3 colitis was confirmed via colonoscopy. These patients were treated with intravenous corticosteroids followed by slow steroid taper with rapid resolution of diarrhea. There were four patients reported to have thyroiditis (grade 1), one patient each with hepatitis and pancreatitis (both grade 3) and two patients reported to have hypophysitis (both grade 3). One patient experienced grade 4 uveitis. Patients with grade 3-4 liver and pancreatic toxicities received prompt corticosteroid intervention with resolution of symptoms in all cases without recurrence after steroid taper. Some patients required systemic steroids for management of irAEs. Two patients with thyroiditis and both patients with hypophysitis required on-going hormone replacement after ipilimumab treatment. The patient with uveitis had significant vision loss impacting her activities of daily living that has persisted despite attempted corticosteroid pulse and taper. Though numbers were small, the overall incidence of immune-related toxicities was greater in patients receiving 10 mg/kg vs. 3 mg/kg: 67.6% (23 of 34) of patients receiving 3 mg/kg had irAEs of any grade compared with 100% of patients at 10 mg/kg (5 of 5). However, the comparison was not statistically significant (Fisher's exact p-value = 0.30). The rates of grade 3-4 events were higher, though not statistically significant, in the 10 mg/kg group (2 of 5, 40%) compared with the 3 mg/kg group (5 of 34, 14.7%) (Fisher's exact p-value = 0.21).
Table 3. Toxicities.
| Ipilimumab 3 mg/kg | Ipilimumab 10 mg/kg | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| irAE | Any G | % | G3-4 | % | Any G | % | G3-4 | % | Any G | % | G3-4 | % |
| Dermatitis | 14 | 41.2% | 0 | 0.0% | 2 | 40.0% | 0 | 0.0% | 16 | 41.0% | 0 | 0.0% |
| Colitis | 3 | 8.8% | 2 | 5.9% | 0 | 0.0% | 0 | 0.0% | 3 | 7.7% | 2 | 5.1% |
| Thyroiditis | 3 | 8.8% | 0 | 0.0% | 1 | 20.0% | 0 | 0.0% | 4 | 10.3% | 0 | 0.0% |
| Uveitis | 1 | 2.9% | 1 | 2.9% | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | 1 | 2.6% |
| Pancreatitis | 1 | 2.9% | 1 | 2.9% | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | 1 | 2.6% |
| Hepatitis | 0 | 0.0% | 0 | 0.0% | 1 | 20.0% | 1 | 20.0% | 1 | 2.6% | 1 | 2.6% |
| Hypophysitis | 1 | 2.9% | 1 | 2.9% | 1 | 20.0% | 1 | 20.0% | 2 | 5.1% | 2 | 5.1% |
| Total | 23 | 67.6% | 5 | 14.7% | 5 | 100.0% | 2 | 40.0% | 28 | 71.8% | 7 | 17.9% |
Discussion
Our retrospective study evaluating the activity of ipilimumab in 39 patients from four academic centers in the United States and Europe is the largest report to date of the activity of ipilimumab in uveal melanoma and provides the first evidence that ipilimumab can generate mWHO and irRC responses, as well as stable disease, in patients with metastatic uveal melanoma. The response rate at last follow up in our study was 5.1%. This is similar to the reported response rates for ipilimumab in cutaneous melanoma of 4.2-10.9% and is higher than any published therapy specifically for uveal melanoma.15, 22, 23 The median overall survival by Kaplan-Meier methodology in our study was 9.6 months. Several patients had on-going on-going stable disease at the time of the analysis, with two patients having durable responses of >60 weeks. These durable responses underscore the potential utility of immune-check point blockade for the treatment of patients with metastatic uveal melanoma.
The toxicity of ipilimumab in our series also suggests that it can be given safely in this patient population. Previous descriptions of grade 3-4 toxicities with ipilimumab have reported rates around 10%.18 The grade 3-4 toxicity rate in this study is higher, at 17.9%; however this appears to have been weighted by those patients who received 10 mg/kg of ipilimumab. Importantly, all grade 3-4 irAEs, with the exception of uveitis in a patient who had been treated for over two years, resolved with prompt initiation of high-dose corticosteroids followed by a slow steroid taper. This is similar to what has been described in the management of irAE in cutaneous melanoma and underscores the need for vigilance by the treating physician regarding these potential events.
There have been many studies published on various treatment regimens for metastatic uveal melanoma though no clear standard therapy exists. Multiple reviews of chemotherapy have described response rates in the single digits.9, 10 Biochemotherapy, generally combining cisplatin, vinblastine, dacarbazine with interleukin-2 and interferon-α, has been advocated in retrospective analysis to be associated with improved survival however the treatment is associated with significant toxicity which many patients will be unable to tolerate.24
More recently, there has been a focus on the emerging molecular biology of uveal melanoma. Preliminary efficacy has been reported for an ongoing randomized phase II study of selumetinib versus temozolomide, with responses observed in 4 of 21 (19%) patients treated with the MEK inhibitor.25 However, in the phase I study of the MEK inhibitor trametinib, eight of 16 patients with uveal melanoma met criteria for SD though no responses were seen and the median progression free survival was only 1.8 months.26 In a phase II trial of the VEGF-trap, aflibercept, five of 10 patients with uveal melanoma were noted to be progression free at 4 months, though there were no responses seen.27
While several of these molecularly targeted approaches are promising, durable benefit remains elusive. In our study of ipilimumab, two patients (5.1%) had responses and nine (23.1%) had SD lasting beyond 33 weeks by irRC and mWHO. This is similar to what has been described for ipilimumab in cutaneous melanoma where a “tail on the curve” phenomenon indicates that some patients have durable clinical benefit over years.28 Even removing the potential for long term benefit however, we observed three and six month stable disease rates of 46.1% and 28.2%. Prior experiences with DTIC and temozolomide in metastatic uveal melanoma have reported median progression times of approximately 1.5 months, and a progression-free survival of four months has been proposed as a significant improvement in stable disease for uveal melanoma in clinical trials (ClinicalTrials.gov Identifier: NCT01143402). Though our data is limited by its retrospective nature, ipilimumab seems to compare favorably to other published systemic therapies for patients with metastatic uveal melanoma.
Our data also suggest that the timing for use of ipilimumab may be important as well. Though sample sizes are small, we observed significant associations between both ECOG performance status and LDH with survival from the time of ipilimumab treatment. This suggests that the maximum benefit from ipilimumab is likely to come early in the treatment course of these patients, prior to acceleration of cancer growth and decline in functional status. Early treatment is also reinforced by the possibility for late immune induction with anti-tumor effect. Though we did not observe a patient who met criteria for progression with subsequent response, we did observe a patient with SD who eventually developed a PR. Allowing time for late induction of an immune-related anti-tumor effect can be difficult and this emphasizes the importance of performance status in consideration of ipilimumab treatment.
It has been suggested that rise in ALC during ipilimumab treatment may associated with clinical benefit and overall survival.20 We noted ten of eleven patients obtaining response or SD at last follow up had a rise in ALC and that ALC ≥1000 cells/μL at week 7 significantly stratified patients by median overall survival (13.4 versus 4.8 months, log-rank p = 0.004). All patients experiencing response or SD at last follow up had ALC ≥1200 cells/μL at 7 weeks. These data recommend ALC at week 7 for further study as a biomarker of activity for ipilimumab in uveal melanoma. Given the aggressiveness of this disease, the potential for an early biomarker of treatment efficacy would be very useful in the clinical management uveal melanoma.
Our investigation is limited by several factors. Toxicities were captured by chart review and thus may have led to a bias toward under-reporting, though the fact that just over half of the patients were treated on a clinical protocol may help to alleviate this to some degree. Additionally, though the databases of four melanoma referral centers were utilized, the study sample size of 39 patients is relatively small. As such, the data may be subject to variability with obfuscation of significant differences that might exist among different patient sub-sets. Finally, our cohort was heterogeneous with patients treated on protocol and off, in various lines of therapy and with different doses of ipilimumab.
Despite these limitations, this investigation represents a robust evaluation of the clinical experience with ipilimumab in patients with metastatic uveal melanoma and is the first to report responses to ipilimumab in patients with uveal melanoma. A further strength of this report is that approximately half of our patients were treated off protocol in a standard clinical practice setting, as compared with prior reports where patients were restricted to an expanded access program (ClinicalTrials.gov Identifier: NCT00495066). Further, by drawing on the experience from four academic centers, we may have minimized institutional biases that might be present within a single center.
It appears that further exploration of the molecular biology of uveal melanoma will lead to novel targeted therapeutic strategies; however, our data suggest that ipilimumab is a treatment that can induce responses or stable disease for some patients and is already available in standard practice. The toxicity we observed was manageable and not significantly different than that seen in the treatment of metastatic cutaneous melanoma. Prospective studies of ipilimumab in metastatic uveal melanoma are on-going to better delineate the exact clinical utility of this agent. Based on our data, we would suggest that ipilimumab be considered a reasonable therapeutic choice for this patient population. Patients with uveal melanoma should be included in future clinical studies evaluating novel immunotherapeutic approaches.
Acknowledgments
Funding: This work had no specific funding
Footnotes
Disclosure: Dr's Wolchok and Hodi have served as consults to Bristol-Myers Squibb
References
- 1.Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology. 2003;110(5):956–61. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 2.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–9. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 3.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer J, Kilic E, Vaarwater J, Bastian BC, Garbe C, de Klein A. Oncogenic GNAQ mutations are not correlated with disease-free survival in uveal melanoma. Br J Cancer. 2009;101(5):813–5. doi: 10.1038/sj.bjc.6605226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23(31):8076–80. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 6.Salmon RJ, Levy C, Plancher C, et al. Treatment of liver metastases from uveal melanoma by combined surgery-chemotherapy. Eur J Surg Oncol. 1998;24(2):127–30. doi: 10.1016/s0748-7983(98)91485-8. [DOI] [PubMed] [Google Scholar]
- 7.Nathan F, Sato T, Hart E. Response to combination chemotherapy of liver metastasis from chorhoidal melanoma compared with cutaneous melanoma. Proc Am Soc Clin Oncol. 13(394):1994. [Google Scholar]
- 8.Bhatia S, Moon J, Margolin KA, et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS One. 2012;7(11):e48787. doi: 10.1371/journal.pone.0048787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. DAnderson Cancer Center experience and prognostic factors. Cancer. 1995;76(9):1665–70. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Albert DM, Ryan LM, Borden EC. Metastatic ocular and cutaneous melanoma: a comparison of patient characteristics and prognosis. Arch Ophthalmol. 1996;114(1):107–8. [PubMed] [Google Scholar]
- 11.de Vries TJ, Trancikova D, Ruiter DJ, van Muijen GN. High expression of immunotherapy candidate proteins gp100, MART-1, tyrosinase and TRP-1 in uveal melanoma. Br J Cancer. 1998;78(9):1156–61. doi: 10.1038/bjc.1998.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luyten GP, van der Spek CW, Brand I, et al. Expression of MAGE, gp100 and tyrosinase genes in uveal melanoma cell lines. Melanoma Res. 1998;8(1):11–6. doi: 10.1097/00008390-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Becker JC, Terheyden P, Kampgen E, et al. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br J Cancer. 2002;87(8):840–5. doi: 10.1038/sj.bjc.6600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soni S, Lee DS, DiVito J, Jr, et al. Treatment of pediatric ocular melanoma with high-dose interleukin-2 and thalidomide. J Pediatr Hematol Oncol. 2002;24(6):488–91. doi: 10.1097/00043426-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielli R, Ridolfi R, Chiarion-Sileni V, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61(1):41–8. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khattak MA, Fisher R, Hughes P, Gore M, Larkin J. Ipilimumab activity in advanced uveal melanoma. Melanoma Res. 2013;23(1):79–81. doi: 10.1097/CMR.0b013e32835b554f. [DOI] [PubMed] [Google Scholar]
- 18.D Lawrence DM, Hamid O, et al. Treatment of patients (pts) with stage III or IV melanoma on an ipilimumab (Ipi) expanded access program (EAP): results for 3mg/kg cohort. Society for Melanoma Research (abstract) 2012 [Google Scholar]
- 19.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 20.Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013 doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 21.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 23.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 24.Bedikian AY, Johnson MM, Warneke CL, et al. Systemic therapy for unresectable metastatic melanoma: impact of biochemotherapy on long-term survival. J Immunotoxicol. 2008;5(2):201–7. doi: 10.1080/15476910802131519. [DOI] [PubMed] [Google Scholar]
- 25.Carvajal R, Ambrosini G, Wolchok J, et al. Pharmacodynamic activity of selumetinib to predict radiographic response in advanced uveal melanoma. J Clin Oncol. 2012;30 suppl; abstr 8598. [Google Scholar]
- 26.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarhini AA, Frankel P, Margolin KA, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage iv melanoma of cutaneous or uveal origin. Clin Cancer Res. 2011;17(20):6574–81. doi: 10.1158/1078-0432.CCR-11-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ascierto PA, Marincola FM, Ribas A. Anti-CTLA4 monoclonal antibodies: the past and the future in clinical application. J Transl Med. 2011;9:196. doi: 10.1186/1479-5876-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]