Abstract
Objectives
We investigated directed therapy based on TFAP2C-regulated pathways to inform new therapeutic approaches for treatment of luminal breast cancer.
Background
TFAP2C regulates the expression of genes characterizing the luminal phenotype including ESR1 and RET, but pathway cross talk and potential for distinct elements have not been characterized.
Methods
Activation of extracellular signal-regulated kinases (ERK) and AKT was assessed using phosphorylation-specific Western blot. Cell proliferation was measured with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] after siRNA (small interfering RNA) gene knockdown or drug treatment. Cell cycle, Ki-67, and cleaved caspase 3 were measured by fluorescence-activated cell sorting. Tumorigenesis was assessed in mice xenografts.
Results
Knockdown of TFAP2C or RET inhibited GDNF (glial cell line–derived neurotrophic factor)–mediated activation of ERK and AKT in MCF-7 cells. Similarly, sunitinib, a small-molecule inhibitor of RET, blocked GDNF-mediated activation of ERK and AKT. Inhibition of RET either by gene knockdown or by treatment with sunitinib or vandetanib reduced RET-dependent growth of luminal breast cancer cells. Interestingly, knockdown of TFAP2C, which controls both ER (estrogen receptor) and RET, demonstrated a greater effect on cell growth than either RET or ER alone. Parallel experiments using treatment with tamoxifen and sunitinib confirmed the increased effectiveness of dual inhibition of the ER and RET pathways in regulating cell growth. Whereas targeting the ER pathway altered cell proliferation, as measured by Ki-67 and S-phase, anti-RET primarily increased apoptosis, as demonstrated by cleaved caspase 3 and increased TUNEL (terminal deoxyneucleotidyl transferase dUTP nick end labeling) expression in xenografts.
Conclusions
ER and RET primarily function through distinct pathways regulating proliferation and cell survival, respectively. The findings inform a therapeutic approach based on combination therapy with antiestrogen and anti-RET in luminal breast cancer.
Keywords: breast cancer, estrogen therapy, RET, sunitinib, vandetanib
Breast cancer is the most common cancer in women in the United States and is the second leading cause of cancer death.1 Tumor subtypes have been defined by expression of estrogen receptor (ER) and progesterone receptor and overexpression of HER2. Each phenotype represents a distinct clinical entity with different responses to chemotherapy and patterns of recurrence and survival.2 Better understanding of the mechanisms of gene regulation that account for the different expression profiles between phenotypes and the pathways that drive tumor growth survival, and invasion will identify new targets for therapy.
TFAP2C is a member of the AP2 family, which regulates transcriptional activity of target genes by direct binding to a consensus binding sequence.3,4 In breast cancer, TFAP2C targets a number of key genes in the ER-associated gene cluster. In addition, TFAP2C does not show the same regulatory activity in triple-negative breast cancer cell lines, indicating a potentially alternate role in the basal phenotype.5 Knockout of TFAP2C in ER-expressing MCF-7 luminal breast carcinoma cells has been shown to block hormone response and slow tumor formation in a mouse xenograft.6 In addition, detailed analysis of TFAP2C target genes in luminal breast cancer has identified numerous target genes whose expression is characteristic of the luminal breast phenotype.5 On the basis of these findings, we hypothesize that TFAP2C is a key regulator of multiple genes in the ER-associated cluster that contribute to pathways of proliferation, growth, and survival and that TFAP2C is a key determinant of tumor behavior in hormone-responsive breast cancer.
One mechanism by which TFAP2C regulates cell proliferation is through ER-dependent pathways,6 which functionally interface with the RET receptor.7 Expression of RET has been discovered in breast cancers with an association with ER expression,8–10 and we have shown that TFAP2C is capable of regulating expression of RET independent of ER transcriptional regulation.11 Expression of RET has been identified as a negative prognostic indicator in breast cancer,7 and RET activation could play a role in estrogen independent activation of estrogen response elements and contribute to hormone therapy resistance.12 However, the effects of RET on proliferation in breast cancer and the intermediary pathways involved are not well understood. In addition, the functional significance of RET-ER interactions and the potential of targeting these distinct pathways as a novel treatment strategy in luminal breast cancer were investigated.
METHODS
Cell Lines
The MCF-7, BT-474, and MDA-MB-231 cell lines were obtained from the American Type Culture Collection. Cells were grown using DMEM (Dulbecco’s Modified Eagle Medium) or DMEM/F12 medium with 10% fetal bovine serum, penicillin 100 units/mL, and streptomycin 100 μg/mL at 37°C and 5% CO2.
Chemicals and Treatment
Sunitinib for in vitro studies was obtained from Sigma-Aldrich (St Louis, MO) and dissolved in dimethyl sulfoxide to a final concentration of 500 nM. Sunitinib for mouse gavage was obtained from Selleck Chemicals (Munich, Germany) and dissolved in olive oil. Vandetanib was obtained from AstraZeneca Pharmaceuticals (Macclesfield, United Kingdom) and dissolved in dimethyl sulfoxide to a final concentration of 10 μM. z-4-Hydroxytamoxifen was obtained from Sigma-Aldrich and dissolved in ethanol to a final concentration of 10 μM. Glial cell line–derived neurotrophic factor (GDNF) was purchased from Sigma-Aldrich and dissolved in phosphate-buffered saline and to a final concentration of 2.5 ng/mL.
Small Interfering RNA Transfections
Small interfering RNAs (siRNAs) were obtained for RET (sc-156121; Santa Cruz Biotechnologies, Santa Cruz, CA), TFAP2C (D-005238-01; Dharmacon, Lafayette, CO), ER (Ambion, Austin, TX), and NT (nontargeting; D-001210-01-05; Dharmacon). Transfection was performed according to the manufacturer’s protocol using the Lipofectamine RNAImax reagent (Invitrogen, Carlsbad, CA). All siRNA transfections were performed for 72 hours before analysis.
Phosphorylation-Specific Western Blot
Samples were treated with GDNF (25 ng/mL) or vehicle in OPTI-MEM media for 30 minutes. Total protein was isolated using RIPA buffer with Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL) and PhosStop phosphatase inhibitor (Roche, In-dianapolis, IN). The antibodies used for Western blot were as follows: ERK (Cell Signaling Technologies, Danvers, MA), p-ERK (Cell Signaling), AKT (Cell Signaling), and p-AKT (phosphorylated AKT; Cell Signaling), RET (Cell Signaling), and TFAP2C (Santa Cruz Biotechnologies). Protein levels were quantified using ImageJ (http://rsb.info.nih.gov/ij/), and statistical calculations were performed using 3 biological replicates.
MTT Proliferation Assay
Cells were plated on a 24-well plate at 10,000 cells per well in technical triplicate. Cells were allowed to adhere overnight, and the appropriate hormone- and/or drug-containing media were added. Samples were incubated with MTT (1 mg/mL) for 3 hours at 37°C and crystals solubilized in dimethyl sulfoxide and read on an Infinite 200 Pro plate reader (Tecan, Switzerland) at an absorbance wavelength of 590 nm with a reference wavelength of 630 nm. Samples were averaged over 3 biological replicates.
Cell Staining and Flow Cytometry
Cells were grown for 72 hours in the presence of siRNA or for 36 hours with pharmacological treatments. Samples were prepared with staining for Ki-67 using anti-human Ki-67 Alexa Fluor 647 (eBioscience, San Diego, CA) and for activated cleaved caspase 3 (CC3) using the CaspGlow Active Casp 3 kit (eBioscience) according to the manufacture’s protocol. Cell-cycle phase was measured using the Click-iT EdU assay (Invitrogen) according to the manufacture’s protocol. All samples were control stained for live cells with FxCycle Violet (Life Technologies). Flow cytometry was performed using a FACS (fluorescence-activated cell sorting) DiVa software (Becton Dickinson, Franklin Lakes, NJ). Cells were first gated around the FxCycle Violet, followed by side- and forward-scatter profiles to eliminate adherent cells. Analysis was performed to determine the percentage of cells staining positive for Ki-67 or activated CC3 and to determine the percentage of sample cells in each cell-cycle phase.
Tumor Xenografts
Female Nu/J mice 6 weeks old (Jackson Laboratory, Bar Harbor, MN) were implanted with a 1.7-mg estrogen pellet (Innovative Research, Sarasota, FL), and 5 × 106 MCF-7 cells were injected subcutaneously into the right flank. Five mice were randomized to daily oral gavage of sunitinib 50 mg/kg in olive oil and 10 mice to the control group. Two mice in the treatment group died of gavage complication and were not included in the analysis. Tumors were harvested at 3 weeks and formalin-fixed, paraffin-embedded sections were evaluated by hematoxylin-eosin staining, and immunohistochemistry was performed for Ki-67 (Dako, Denmark) and TUNEL (Millipore, Bilerica, MA). Ki-67 was scored according to the percentage of positive cells, with 200 cells counted per sample. TUNEL was quantified using positive cells per high-powered field, with 10 fields counted per sample.
Statistical Analysis
Statistical analysis was performed using the 2-sided Student t test for continuous variables. Frequency association of categorical variables was performed using the Fisher exact test. All statistical calculations were performed using R (www.r-project.org). Statistical significance was defined as P < 0.05.
RESULTS
Activation of ERK1/2 and AKT Is Regulated by TFAP2C Through the RET Receptor in MCF-7 Breast Cancer Cells
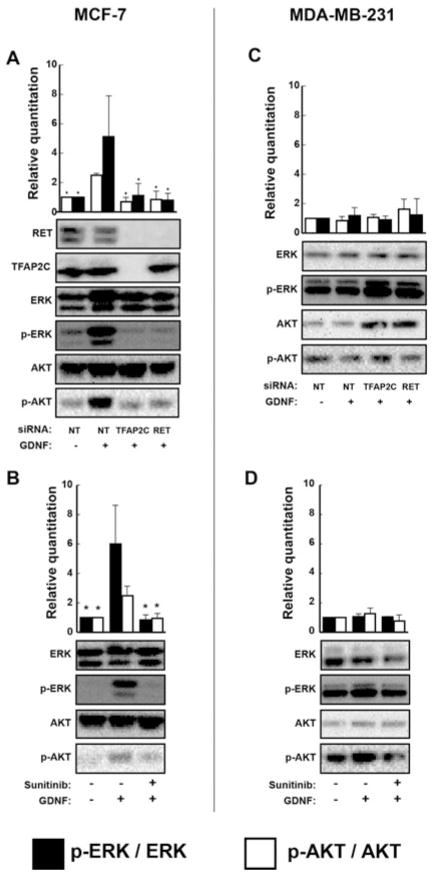
We sought to evaluate the functional effects of RET in luminal breast cancer by examining effects of ERK1/2 and AKT activation in MCF-7 breast carcinoma cells. Stimulation of MCF-7 cells by GDNF resulted in a 5-fold increase in phosphorylated ERK1/2 and a 2-fold increase in p-AKT (Fig. 1A; P < 0.001). Knockout of TFAP2C abrogated ERK1/2 and AKT activation by GDNF (Fig. 1A; P < 0.001). Knockout of the TFAP2C target gene, RET, had similar effects on blocking GDNF-mediated ERK1/2 and AKT activation indicating that the effects of knockdown of TFAP2C were mediated through RET (Figure 1C, D). To further demonstrate the role of RET, we used sunitinib, a small-molecule tyrosine kinase inhibitor (TKI) with significant activity against RET. Treatment with sunitinib similarly prevented ERK1/2 and AKT activation in response to GDNF (Fig. 1B; P < 0.001). Treatment with GDNF could not stimulate phosphorylation of ERK1/2 or AKT in MDA-MB-231 breast cancer cells, and no differences were observed with knockout of RET and TFAP2C or treatment with sunitinib (Fig. 1). These findings are consistent with the lack of RET and TFAP2C expression in the ER-negative MDA-MB-231 cell line.
FIGURE 1.
TFAP2C regulates ERK and AKT activation via RET. (A), Treatment of MCF-7 with GDNF results in induction of ERK and AKT phosphorylation. This effect is prevented by gene knockdown of TFAP2C, with a similar effect noted with direct RET knockdown showing that GDNF-mediated activation of ERK and AKT is dependent on TFAP2C regulation of RET in MCF-7. (B), GDNF-mediated activation of ERK and AKT is prevented by treatment with the RET inhibitor sunitinib in MCF-7. (C), There is no effect of GDNF treatment or RET or TFAP2C knockout in MDA-MB-231, which do not express RET or TFAP2C. (D), There is no effect of sunitinib treatment on ERK or AKT activation in MDA-MB-231. *P < 0.001.
MCF-7 Proliferation Is Regulated by TFAP2C Through Multiple Pathways
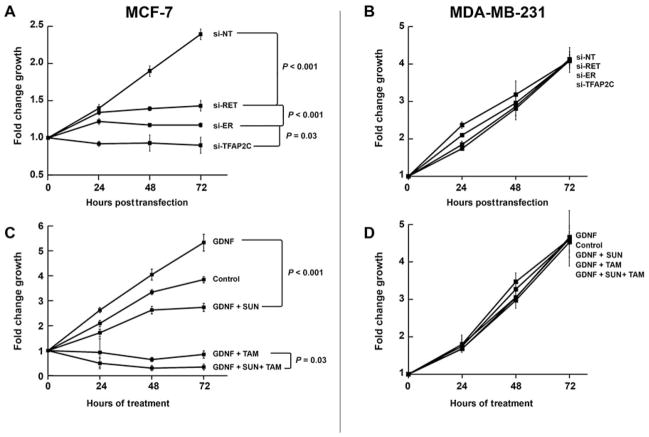
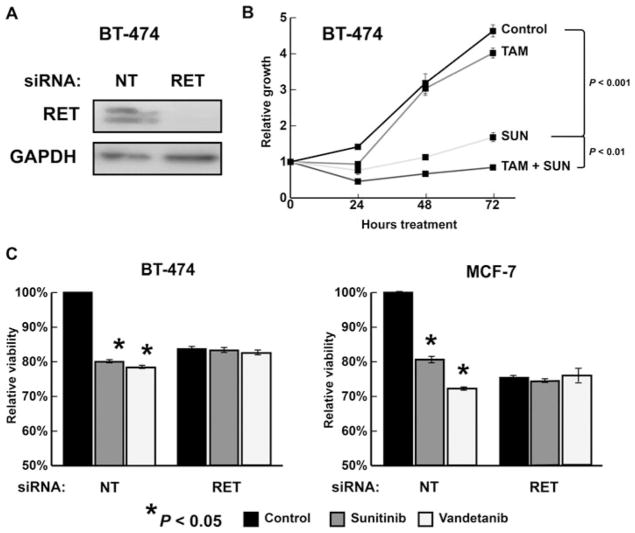
The effects on ERK1/2 and AKT activation suggested that the TFAP2C-RET-ERK/AKT pathways might have effects on cell growth. Knockdown of RET with siRNA in MCF-7 cells caused a significant reduction in growth starting at 48 hours, with increasing effects noted at 72 hours compared with nontargeting siRNA (Fig. 2A; P < 0.001). As expected, knockout of ER similarly reduced proliferation in MCF-7 cells. Interestingly, knockdown of TFAP2C, which eliminated expression of both RET and ER, caused a significant reduction in cell numbers over a 72-hour period compared with knockdown of either RET or ER individually. In MDA-MB-231 cells, knockdown of RET, ER, or TFAP2C had no effect on growth curves (Fig. 2B). These results suggest that growth-mediated effects driven by RET and ER may function independently, thus providing the rationale for targeting multiple pathways. To test this hypothesis, proliferation assay was performed in MCF-7 cells using pharmacological inhibition of RET, ER, or both. Stimulation of MCF-7 cells with GDNF led to a significant increase in cell growth, which further demonstrated the ability of RET to increase proliferation in luminal breast cancer. Cell growth was significantly reduced by either sunitinib or tamoxifen (P < 0.01). In addition, combination treatment with sunitinib and tamoxifen resulted in a significant reduction in proliferation when compared with treatment with either drug alone (Fig. 2C; P = 0.025), thus confirming the ability to target the RET and ER signaling pathways independently. Parallel experiments with treatment of MDA-MB-231 cells with GDNF, tamoxifen, sunitinib, or a combination of drugs demonstrated no alterations in cell growth (Fig. 2D). To confirm the effects in another luminal breast cancer cell line, we examined the BT-474 breast carcinoma cell line, which is a cell line model for luminal B subtype of breast cancer. The BT-474 cell line expresses ER, has amplified expression of HER2, and is relatively hormone insensitive. BT-474 cells demonstrate RET expression, which can be knocked down with siRNA to RET (Fig. 3A). BT-474 showed minimal reduction of growth with tamoxifen treatment (Fig. 3B), whereas treatment with sunitinib resulted in a significant reduction in growth (P < 0.001). Similar to MCF-7, combination treatment of sunitinibtamoxifen resulted in a reduction in proliferation greater than either compound alone (Fig. 3B; P = 0.01).
FIGURE 2.
TFAP2C regulates cell growth through the ER and RET pathways. (A), Knockdown of RET or ER results in significantly reduced growth of MCF-7 cells. Knockout of TFAP2C results in a larger reduction in cell number than either RET or ER knockdown alone. (B), Knockdown of RET, ER, or TFAP2C does not affect growth of MDA-MB-231 cells. (C), Treatment with GDNF increases growth of MCF-7 cells and treatment with tamoxifen (TAM) or sunitinib (SUN) reduce cell growth, whereas treatment with both tamoxifen and sunitinib has a more significant effect on growth compared with either drug alone. (D), Treatment with GDNF, sunitinib, tamoxifen, or sunitinib and tamoxifen did not alter proliferation in MDA-MB-231 cells.
FIGURE 3.
The effects of sunitinib (SUN) in luminal breast cancer are mediated by the RET receptor. (A), Western blot demonstrating RET expression in the luminal breast cancer cell line BT-474 and confirming effectiveness of RET siRNA to eliminate RET expression. (B), Treatment with sunitinib reduces cell growth in BT-474 with additive results when combined with tamoxifen (TAM) treatment. (C), Treatment with sunitinib or vandetanib reduced viability in MCF-7 and BT-474. Knockdown of RET expression by siRNA eliminated the antiproliferative effects of treatment of both sunitinib and vandetanib in both cell lines. For both cell lines, data were normalized to NT transfection without drug treatment.
Sunitinib is known to have TKI activity against several RKTs and is not specific for RET. In comparison, vandetanib is a TKI with greater specificity for the tyrosine kinase activity of RET.13 Similar to sunitinib, vandetanib treatment resulted in a significant reduction of viability in MCF-7 (P < 0.001) and BT-474 (P = 0.01) (Fig. 3C). To demonstrate that the growth-repressive effects of sunitinib and vandetanib are due to RET, siRNA was used to knockdown RET expression in MCF-7 and BT-474 and effects of TKI treatment were compared with knockdown with NT siRNA. Gene knockdown of RET using siRNA eliminated the antiproliferative effects of TKI treatment in both cell lines, demonstrating that sunitinib and vandetanib are acting through RET in luminal breast cancer (Fig. 3C). Furthermore, no additional growth-repressive effects of TKI treatment were found after knockdown of RET, suggesting no physiological role for additional RTKs in these cell line models.
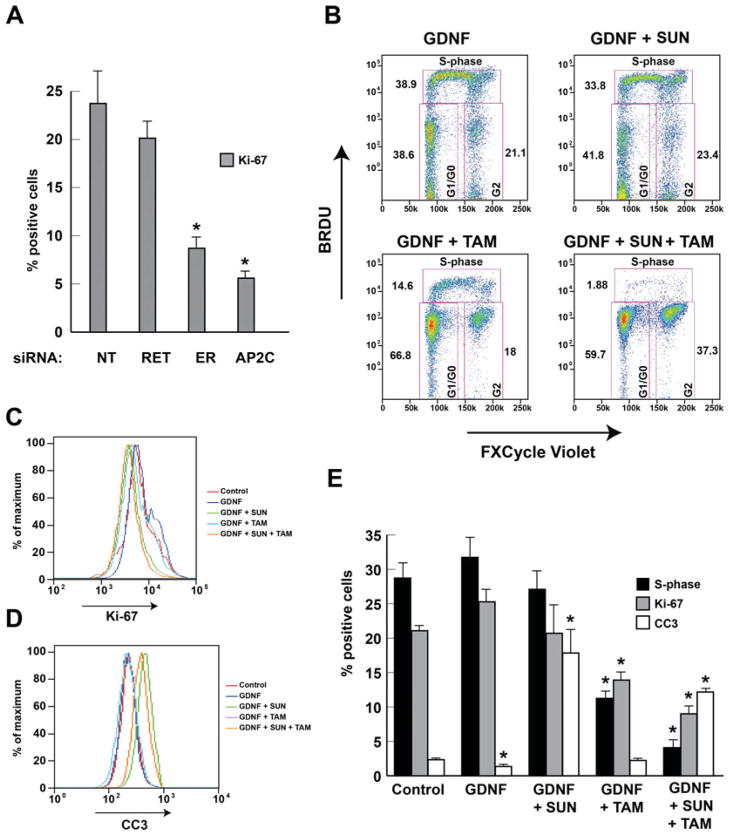
Tamoxifen Blocks Proliferation and Sunitinib Increases Apoptosis in Hormone-Responsive Breast Cancer
To investigate the mechanism by which RET activation increases cell growth, we used FACS to measure cell division rate measured by S-phase and Ki-67 expression, and apoptotic rate demonstrated by cleaved CC3 activation. Gene knockdown of RET resulted in a nonsignificant reduction in Ki-67 positivity, whereas knockdown of both ER or TFAP2C resulted in a significant reduction (Fig. 4A). Treatment of MCF-7 cells with GDNF resulted in a slight but not statistically significant increase in Ki-67 proliferative index and an increase in the percentage of cells in S-phase (Fig. 4). However, GDNF resulted in a significant reduction of CC3-positive cells (Fig. 4B–E). Treatment with sunitinib abrogated the GDNF-mediated increase in Ki-67 and S-phase and, interestingly, caused a highly significant increase in CC3-positive apoptotic cells. Treatment with tamoxifen alone caused a significant reduction in Ki-67 and S-phase without a change in CC3-positive cells. In concordance with the proliferation assay, combination treatment with both sunitinib and tamoxifen caused an additive reduction in Ki-67 and S-phase while producing a significant increase in CC3-positive apoptotic cells compared with controls. These results provide further evidence that both RET and ER modulate cell growth through distinct pathways. The data further support a model where ER signaling primarily alters cell proliferation (as noted by effects on S-phase and Ki-67), whereas RET primarily alters cell survival/apoptosis (as noted by effects on CC3 activation).
FIGURE 4.
ER controls proliferation and RET controls cell survival. (A), FACS showed a significant reduction in Ki-67 proliferative index with knockdown of ER or TFAP2C with nonsignificant effect with knockdown of RET in MCF-7. (B), Example of FACS of MCF-7 cells with GDNF stimulation and treatment with tamoxifen (TAM) and/or sunitinib (SUN). Histogram representation of the effect on Ki-67 positivity (C) and expression of CC3 (D) after GDNF stimulation with tamoxifen and/or sunitinib treatment. (E), Experimental data from FACS showing summary of S-phase, Ki-67, and CC3 with GDNF stimulation and treatment with tamoxifen and/or sunitinib treatment. *P < 0.05.
Sunitinib Reduces MCF-7 Xenograft Tumorigenesis
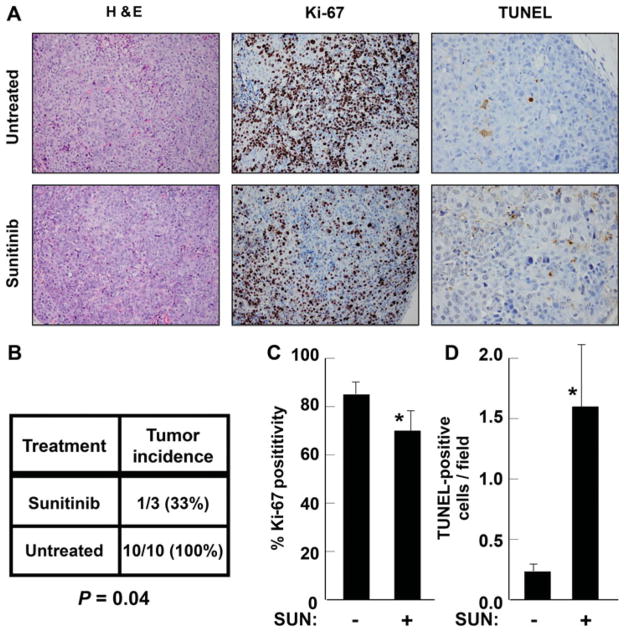
To test the observed effects of sunitinib in vivo, tumorigenesis was studied. Athymic mice injected with MCF-7 cells had a decreased rate of tumor formation when treated with daily gavage of sunitinib, with only 33% of tumors in the treatment group reaching 0.5 cm in greatest dimension at 3 weeks postinjection compared with 100% in the control group (Figure 5B; P = 0.04). Immunohistochemistry of tumors (Fig. 5A) demonstrated reduced mean Ki-67 proliferative index in the treatment group compared with the control group (Figure 5C; 70% vs 85%; P = 0.02). In addition, staining for TUNEL showed a significantly higher mean apoptotic rate in the sunitinib-treated group than that in the nontreated group (Figure 5D; 1.6 vs 0.23 cells/high-powered field; P = 0.01). These results indicate that RET signaling primarily is involved in increasing tumor growth by altering cell survival/apoptosis in luminal breast cancer.
FIGURE 5.
Treatment of mice with tumors confirms apoptotic effects of sunitinib (SUN). (A), Micrographs from MCF-7 xenograft tumors showing hematoxylin-eosin (H&E) staining and immunohistochemistry for Ki-67 and TUNEL. (B), Animals treated with sunitinib were significantly less likely to develop tumors than control animals. (C), Ki-67 positivity was significantly reduced by sunitinib treatment. (D), TUNEL-positive apoptotic cells were significantly increased in sunitinib-treated animals. *P < 0.05.
DISCUSSION
We have previously established that TFAP2C plays a key role in the regulation of the gene expression cluster that defines the ER-positive luminal breast cancer phenotype5 and that TFAP2C regulates estrogen-dependent growth through several pathways including ER.6 More recently, we have shown that TFAP2C is a necessary cofactor for RET expression, which binds to the RET gene promoter in multiple locations that are ER dependent and independent.11 There is an established element of cross talk between the ER and RET pathways, including transcriptional regulation of RET by ER, and a mechanism whereby RET may be involved in hormone resistance.7,10,12 The data presented herein establish that RET and ER, which are both regulated by TFAP2C, affect distinct downstream pathways that alter tumor growth, further establishing a key role for TFAP2C in luminal breast cancer.
RET expression has been characterized in breast cancer, with increased expression linked to poorer prognosis7; however, the physiological effect of RET activation in primary breast cancer is not well understood. Our data provide evidence that activation of RET by its ligand, GDNF, causes induction of cell survival, reducing the number of apoptotic cells, which confirms that RET influences tumor growth through survival/apoptosis in hormone-responsive breast cancer. One question relates to the durability of drug effects. The in vivo responses were examined by studying growth of xenografts in animals treated with sunitinib over a 3-week course, suggesting that the treatment response is durable and has effects on tumor growth. Although sunitinib treatment of mice with xenografts induced a statistically significant decrease in Ki-67 expression in tumors, the predominant effect noted involved an increase in apoptosis. In comparison, the ER pathway primarily affected tumor growth through cell proliferation, as evaluated by decreases in S-phase and Ki-67 expression when ER is inhibited with tamoxifen. The combination therapy with antiestrogen (eg, tamoxifen) and anti-RET (eg, sunitinib) may offer a therapeutic advantage by decreasing proliferation via inhibition of the ER pathway and increasing apoptosis via inhibition of the RET pathway. These data provide the critical biological basis for the potential of combination therapy based on targeting distinct mechanisms of cancer cell proliferation and survival.
Although luminal breast cancers have the best prognosis, they have the potential to develop hormone resistance. Furthermore, neoadjuvant chemotherapy for ER-positive luminal breast cancers is often disappointing and rarely results in a pathological complete response.14–17 Thus, the development of targeted therapy to RET could be important for patients with luminal tumors to prevent or treat hormone-resistant tumors and improve response to neoadjuvant chemotherapy. A potential neoadjuvant protocol using a combination of antiestrogen and anti-RET therapy might increase response, allowing increased use of breast conservation in women with locally advanced ER-positive tumors.
RET expression has also been described in a subset of ER-negative breast cancers. These tumors have more aggressive clinical characteristics than ER-positive tumors.18,19 Further work is needed to characterize the physiological significance of RET expression in ER-negative breast cancer. However, targeting RET and downstream pathways in the subset of ER-negative, RET-expressing tumors could provide an additional treatment option for these patients.
Expression of RET in luminal breast cancer is transcriptionally regulated by ER, and in vitro treatment of luminal breast cancer cells with tamoxifen reduces RET expression.20 Previously, we have shown that regulation of RET by TFAP2C occurs through dependent and independent binding locations of estrogen on the RET gene promoter such that RET protein is reduced but not eliminated in the absence of activated ER. Two studies have shown that although RET protein levels are decreased in the absence of activated ER (either with hormone-depleted media or tamoxifen treatment), GDNF stimulation of RET still leads to increased activation of ERK and AKT and increased soft agar colony formation.7,10 In addition, the effect of ER stimulation by estrogen is reduced in luminal breast cancer if RET expression has been eliminated with siRNA. These results demonstrate not only significant RET-ER cross talk but also that RET and ER can activate pathways independent of each other. Our findings further characterize the distinct pathways, with ER activating primarily pathways of proliferation and RET activating primarily pathways of survival.
Sunitinib is a multi-TKI, and it is possible that the effects of sunitinib are mediated in part by 1 or more additional RTKs. In addition, TFAP2C is known to control expression of other RTKs such as FGFR2 and FGFR4.5 However, treatment with vandetanib, which has greater specificity for RET, produced similar antiproliferative effects. Furthermore, the antiproliferative effect of both TKIs was shown to be dependent on RET expression, as evidenced by a lack of antiproliferative effect with knockdown of RET in 2 luminal breast cancer cell lines. Further study is needed to characterize expression of other tyrosine kinases, including EGFR (epidermal growth factor receptor), VEGFR (vascular endothelial growth factor receptor), PDGFR (platelet derived growth factor receptor), and FGFR (fibroblast growth factor receptor), in breast cancer and the role of these receptors to determine proliferation, survival, and response to tyrosine kinase inhibition in breast cancer. For example, antagonism of EGFR has been shown to induce apoptosis in breast cancer and EGFR is expressed in MCF-7.21 Finally, the effectiveness of TKIs in inhibiting proliferation across individual breast tumors requires further investigation. Furthermore, the Food and Drug Administration has recently approved the use of an additional TKI, carbozantinib, which offers the potential of testing additional drugs with anti-RET activity.22
CONCLUSIONS
Our preclinical data indicate that TKI treatment could be effective in increasing apoptosis, which can be combined with the effects of tamoxifen to reduce cell proliferation, indicating that anti-RET and anti-ER dual-receptor therapy should be investigated as a novel treatment strategy for patients with hormone-responsive breast cancer.
Footnotes
Disclosure: Supported by the National Institutes of Health (NIH) grants R01CA109294 (PI: R.J. Weigel) and T32CA148062 (PI: R.J. Weigel) and by a generous gift from the Kristen Olewine Milke Breast Cancer Research Fund. P.M.S. was supported by the NIH grant T32CA148062. The authors declare no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams T, Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991;5:670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- 4.McPherson LA, Weigel RJ. AP2alpha and AP2gamma: a comparison of binding site specificity and trans-activation of the estrogen receptor promoter and single site promoter constructs. Nucleic Acids Res. 1999;27:4040–4049. doi: 10.1093/nar/27.20.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodfield GW, Chen Y, Bair TB, et al. Identification of primary gene targets of TFAP2C in hormone responsive breast carcinoma cells. Genes Chromosomes Cancer. 2010;49:948–962. doi: 10.1002/gcc.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodfield GW, Horan AD, Chen Y, et al. TFAP2C controls hormone response in breast cancer cells through multiple pathways of estrogen signaling. Cancer Res. 2007;67:8439–8443. doi: 10.1158/0008-5472.CAN-07-2293. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Mayer JA, Mazumdar A, et al. The rearranged during transfection/papillary thyroid carcinoma tyrosine kinase is an estrogen-dependent gene required for the growth of estrogen receptor positive breast cancer cells. Breast Cancer Res Treat. 2012;133:487–500. doi: 10.1007/s10549-011-1775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tozlu S, Girault I, Vacher S, et al. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr Relat Cancer. 2006;13:1109–1120. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 9.Kao J, Salari K, Bocanegra M, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaza-Menacho I, Morandi A, Robertson D, et al. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene. 2010;29:4648–4657. doi: 10.1038/onc.2010.209. [DOI] [PubMed] [Google Scholar]
- 11.Spanheimer PM, Woodfield GW, Cyr AR, et al. Expression of the RET proto-oncogene is regulated by TFAP2C in breast cancer independent of the estrogen receptor. Ann Surg Oncol. 2013;20:2204–2212. doi: 10.1245/s10434-012-2570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulay A, Breuleux M, Stephan C, et al. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res. 2008;68:3743–3751. doi: 10.1158/0008-5472.CAN-07-5100. [DOI] [PubMed] [Google Scholar]
- 13.Morandi A, Plaza-Menacho I, Isacke CM. RET in breast cancer: functional and therapeutic implications. Trends Mol Med. 2011;17:149–157. doi: 10.1016/j.molmed.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham JD, Weiss SE, Ahmed S, et al. The efficacy of neoadjuvant chemotherapy compared to postoperative therapy in the treatment of locally advanced breast cancer. Cancer Invest. 1998;16:80–86. doi: 10.3109/07357909809039761. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 16.Spanheimer PM, Carr JC, Thomas A, et al. The response to neoadjuvant chemotherapy predicts clinical outcome and increases breast conservation in advanced breast cancer. Am J Surg. 2013;206:2–7. doi: 10.1016/j.amjsurg.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236:295–302. doi: 10.1097/01.SLA.0000027526.67560.64. discussion 302–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powles TJ, Hickish TF, Makris A, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol. 1995;13:547–552. doi: 10.1200/JCO.1995.13.3.547. [DOI] [PubMed] [Google Scholar]
- 20.Stine ZE, McGaughey DM, Bessling SL, et al. Steroid hormone modulation of RET through two estrogen responsive enhancers in breast cancer. Hum Mol Genet. 2011;20:3746–3756. doi: 10.1093/hmg/ddr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar S, Mazumdar A, Dash R, et al. ZD6474, a dual tyrosine kinase inhibitor of EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/PI3-K and induces apoptosis in breast cancer cells. Cancer Biol Ther. 2010;9:592–603. doi: 10.4161/cbt.9.8.11103. [DOI] [PubMed] [Google Scholar]
- 22.Nixon IJ, Shaha AR, Tuttle MR. Targeted therapy in thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2013;21:130–134. doi: 10.1097/MOO.0b013e32835aa2c2. [DOI] [PubMed] [Google Scholar]