Abstract
Background
With the recent implementation of bundling reimbursement policy the use of intravenous (IV) iron preparations for the management of anemia in the ESRD population has dramatically increased. Iron overload increases the risk of infections in individuals with or without kidney disease. IV iron administration in ESRD patients impairs bacteriocidal capacity of PMNs against Escherichia Coli. These preparations consist of an elemental iron core and a carbohydrate shell. In addition to the iron core the carbohydrate shell may affect PMNs. We therefore examined the effect of iron sucrose, a commonly used preparation, on phagocytic capacity of PMNs from a group of normal individuals against Gram positive (Staphylococcus Aureus) and Gram negative (E. Coli) bacteria.
Methods
Iron sucrose was added to heparinized blood samples at pharmacologically-relevant concentrations and incubated for 4 and 24 hours at 37° C to simulate in vivo condition. Blood samples mixed with equal volume of saline solution served as controls. To isolate the effects of the carbohydrate shell, blood samples were co-treated with the iron chelator, desferrioxamine.
Results
Iron sucrose caused significant PMN apoptosis and dose-dependent suppression of phagocytic function against both Gram positive and negative bacteria. These abnormalities were prevented by desferrioxamine which precluded contribution of the carbohydrate shell to the PMN dysfunction.
Conclusions
At pharmacologically-relevant concentrations iron sucrose promotes apoptosis and inhibits phagocytic activities of PMNs. The deleterious effect of iron sucrose is mediated by its elemental iron core, not its carbohydrate shell, and as such may be shared by other IV iron preparations.
Keywords: Iron, Infection, Inflammation, Immune deficiency, Anemia, End-stage renal disease, Dialysis
Introduction
Bacterial infections are the second most common cause of death in patients with end-stage renal disease (ESRD)[1] [2] [3]. This is largely due to the deficient immune response in uremia [1] [4] which is caused by: dysfunction of the antigen presenting cells [4] [5] [6], depletion of the dendritic cells [7], diminished numbers and reduced antibody producing capacity of B lymphocyte [4] [8] [9] [10], increased T cell turnover and apoptosis culminating in depletion of naïve and central memory CD 4+ and CD8+ T lymphocyte [11] [12], impaired cell-mediated immunity [4] [13] and reduced granulocyte and monocyte/macrophage phagocytic function [4] [14] [15]. Iron overload has been shown to be associated with increased incidence of bacterial infections in patients with and without ESRD [8] [16] [17] and polymorphonuclear leukocytes (PMN) phagocytic functions appear to be depressed in patients with iron overload [18] [19] [20] [21] [22].With recent implementation of bundling reimbursement policy, the use of intravenous iron (IV Fe) preparations in ESRD patients has dramatically increased [23]. This approach has been adopted by dialysis facilities in an attempt to reduce the cost of anemia treatment by limiting the use of erythropoiesis stimulating agents (ESA). In many instances IV iron preparations are administered on a routine basis with insufficient attention to the total body iron stores. In fact excessive use of intravenous iron compounds has lead to an epidemic of iron overload in the dialysis population [24] [25]. In an elegant study, Deicher et al. [18] evaluated phagocytosis and killing of Escherichia coli before, during, one hour, and two days after IV administration of 300 mg of iron sucrose in a group of 10 ESRD patients maintained on peritoneal dialysis. The results were compared to those obtained in 10 placebo-treated patients. They found a significant reduction in the absolute count and percentage of E. coli killed by PMNs of the iron sucrose–treated peritoneal dialysis patients over time as compared to values found in the placebo-treated patients. These observations provided compelling evidence for the inhibitory action of iron sucrose on neutrophilic granulocytes in this population.
IV iron preparations consist of a core of elemental iron covered by a carbohydrate envelope. In addition to the elemental iron contained in the core of the parenteral iron products, their carbohydrate shell may potentially affect PMNs and monocytes. For instance it is conceivable that the carbohydrate shell of these products may be detected by the pattern recognition receptors such as toll-like receptors (TLR) 2 and 4 expressed on the PMN and monocyte plasma membrane. If true binding of the complex carbohydrate shell of IV iron products to these receptors can result in activation of PMNs and monocytes and thereby limit or preclude their participation in the phagocytosis and killing of the invading bacteria in vivo or in the in vitro laboratory setting. The present study was designed to examine the effect of one of the commonly used IV iron preparations, iron sucrose, on the phagocytic capacity of PMNs from a group of normal individuals against Gram positive (Staphylococcus Aureus) and Gram negative (Escherichia Coli) bacteria. To this end iron sucrose was added to heparinized blood samples from each subject at pharmacologically-relevant concentrations and incubated for 4 hr and 24 hr at 37° C to simulate in vivo condition. Blood samples mixed with equal volume of saline solution served as controls. To isolate the possible effect of the complex carbohydrate shell from that of the elemental iron core of the drug, blood samples were simultaneously treated with iron sucrose and the iron chelator, desferrioxamine. In addition to IV iron preparations and iron overload, dialysis procedure, uremic toxins, dialyzer bio-incompatibility, underlying illnesses, and the prevailing systemic inflammation can contribute to the PMN dysfunction [26]. We elected to use blood samples from normal individuals to isolate the effect of iron sucrose per se from those of uremic toxins, dialysis related factors and systemic inflammation. The study showed that iron sucrose causes a significant dose-dependent suppression of phagocytic activities of PMNs against both Gram positive and Gram negative bacteria. Iron sucrose induced suppression of PMN phagocytic activity was prevented by co-treatment with iron chelator, desferrioxamine, thus precluding the contribution of the carbohydrate shell of iron sucrose to the PMN dysfunction. In addition, we compared the extent of apoptosis in blood samples incubated with or without iron sucrose. Iron sucrose significantly increased PMN apoptosis in a dose-dependent manner.
Materials and Methods
Blood Collection and Study Participants
Ten healthy individuals, 3 women and 7 men, aged 21-43 years participated in this study. The study protocol was approved by the Institutional Review Board of the University of California Irvine (HS# 2007-5572) and completed with the assistance of the University of California, Irvine General Clinical Research Center. Written informed consent was obtained from all subjects. Blood samples were obtained by venepuncture and gently placed into the heparinized tubes and processed as described below. Each blood sample was divided into multiple aliquots to which iron sucrose (American reagent, INC. Shirley, NY) was added to reach the final concentrations of 20, 100 and 200 mg/L. The rationale for the choice of these concentrations was based on the anticipated plasma levels following administration of the approved doses of IV iron products considering the blood volume of about 5 liters of which 60–70% is plasma where the drug is distributed. Given the large size of the iron complex, the distribution of the product is largely limited to plasma. Aliquots mixed with the vehicle served as controls. The samples were then incubated for 4 and 24 hrs at 37°C in a humidified incubator prior to flow cytometric analysis.
Assessment of Phagocytosis by Flow Cytomery
The detection of phagocytosis in PMNs was carried out with pHrodo™ E. coli and pHrodo™ S. aureus BioParticles Phagocytosis Kits (Invitrogen, Calsbad, CA). The pHrodoTM dye becomes fluorescent upon acidification in endocytic compartments, which directly relates to phagocytosis of the labeled particles. PHrodo-labeled E. coli and S. aureus BioParticles® (20 μL) were incubated with aliquots of whole peripheral blood that was pretreated with different concentration of iron. After 15-minute incubation at 37°C, erythrocytes were lysed, washed with PBS and the samples were analyzed by flow cytometry (FACSCalibur, BD Biosciences). Forward and side scatter was used to gate the PMN and phagocytic uptake was determined by measuring fluorescence signal emitted by endocytosed bacteria. Results are expressed as % phagocytosis of the control group. Cell Quest software was used for analysis (Becton-Dickinson, San Jose, CA, USA).
Detection of Toll Like Receptor 2 or 4 Expression by Flow Cytomery
After the incubation for 24 hours at 37°C with iron, TLRs were stained using the following antibodies: Alexa Fluor® 488 conjugated Anti-human TLR2 (eBioscience, San Diego, CA) and Alexa Fluor® 488 conjugated Anti-human TLR4 (eBioscience). Mouse IgG2a K Isotype Control Alexa Fluor® 488 (eBioscience) was used as a negative control. The samples were incubated with each antibody in the dark room at 4°C for 20 min, and were washed twice with PBS before FACS analysis.
Viability Assessment by Flow Cytomery
Apoptosis was measured using Annexin V-FITC Apoptosis Detection Kit (Bio Vision). After incubation for 24 hours at 37°C, the red blood cell contents of the test samples were lysed by ACK lysing, washed, resuspend in Annexin binding buffer and stained with FITC labeled Annexin V and propidium iodide (PI), 50,000 cells from each sample were assessed by flow cytometry (FACSCalibur, Becton-Dickinson, San Jose, CA, USA). Cell Quest software was used for analysis (Becton-Dickinson, San Jose, CA, USA).
Statistical Analysis
Student's t test and one-way analysis of variance (ANOVA) were used in statistical analysis of the data using Excel for Windows software (Microsoft, Redmond, WA). P values equal to or less than 0.05 were considered significant. Data are expressed as mean ± SD.
Results
The effects of iron on phagocytic function of PMNs
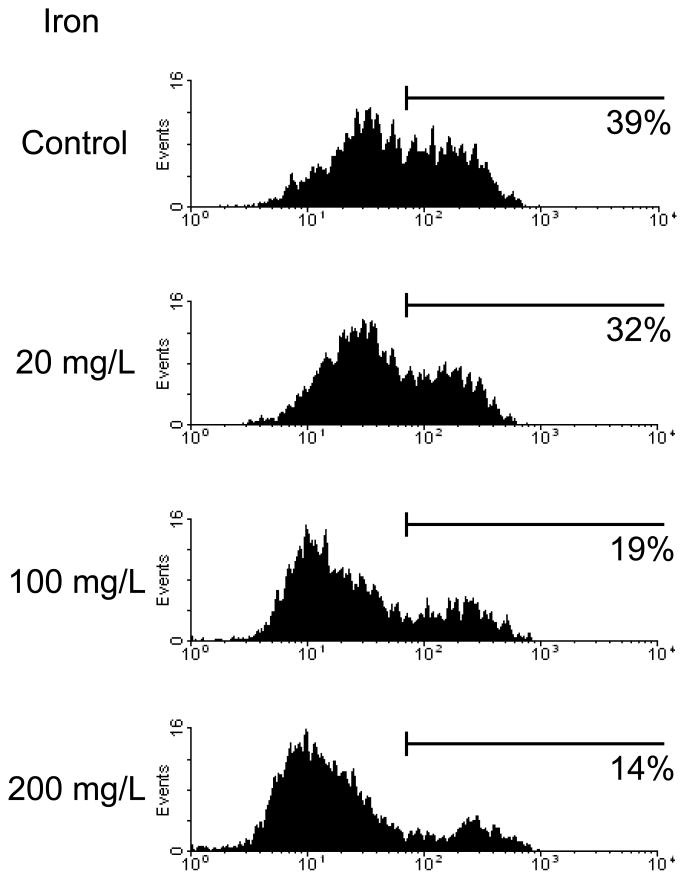
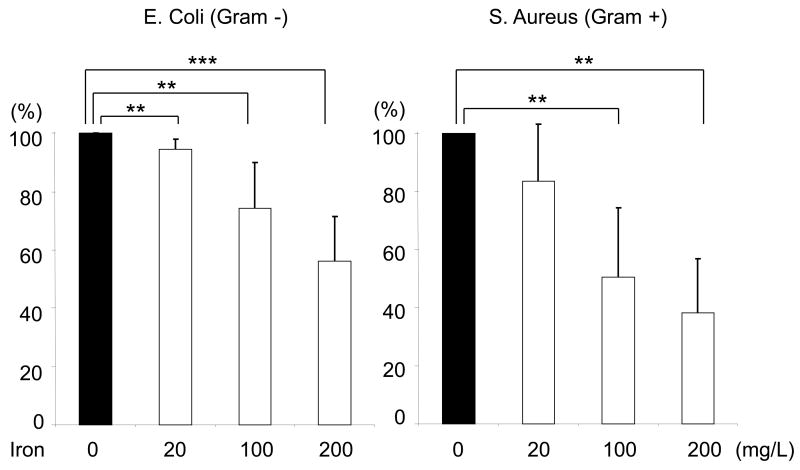
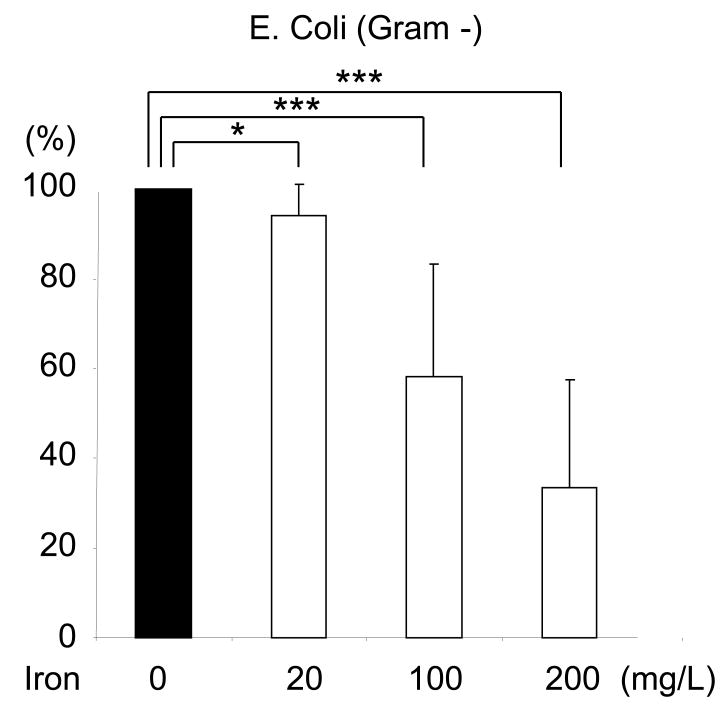
Incubation with iron sucrose for 4 hours caused a significant concentration-dependent suppression of phagocytic activity of PMNs against S. Aureus, (Data are depicted in Figure1A). Likewise 4-hour incubation with iron sucrose caused a significant concentration-dependent inhibition of PMN phagocytic activity against E. coli, (Data are shown in Figure 1B). Similarly, incubation with iron sucrose for 24 hours resulted in significant reduction of phagocytic function of PMNs against negative bacteria (Data are shown in Figure 1C).
Figure 1. Effect of Iron Sucrose on phagocytic function in PMNs.
The iron sucrose was added to heparinized blood samples from each healthy subject at various concentrations, 0, 20, 100, 200 mg/L and incubated for 4 hr and 24 hr at 37° C. Gram positive (Staphylococcus Aureus) and Gram negative (Escherichia Coli) bacteria were used for the evaluation of phagocytic function in PMNs. The phagocytic function against Gram positive and negative bacteria was evaluated by flow cytometry. A: Representive FACS data for phagocytic function against gram positive bacteria. B: Phagocytic function against Gram negative and positive bacteria after 4 hours incubation with iron sucrose. C: Phagocytic function after 24 hours incubation with iron sucrose. * P<0.05, ** P< 0.01, *** P<0.005.
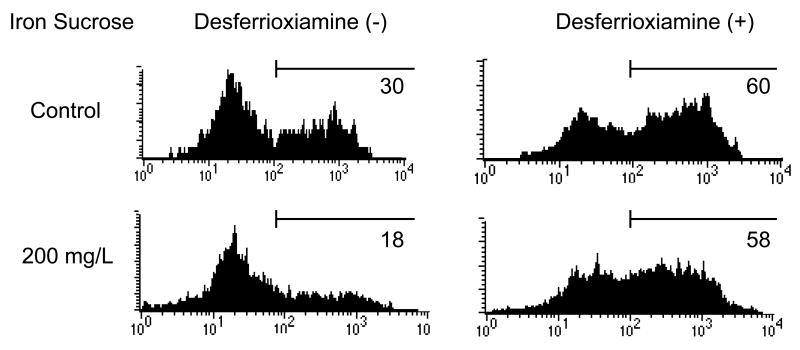
To exclude the possible effect of the complex carbohydrate shell from that of the elemental iron core of the drug, blood samples were simultaneously treated with iron sucrose and the iron chelator, desferrioxamine (50μmol/L). The Iron sucrose induced suppression of PMN phagocytotic activity was completely prevented by desferrioxamine (Figure2). These findings provide irrefutable evidence for the role of iron core and preclude the contribution of the carbohydrate shell of iron sucrose to the observed PMN dysfunction.
Figure 2. Prevention of Iron Sucrose induced phagocytic dysfunction with desferrioxiamine in PMNs.
To exclude the possible effect of the complex carbohydrate shell from that of the elemental iron core of the drug, blood samples were simultaneously treated with iron sucrose and the iron chelator, desferrioxamine.
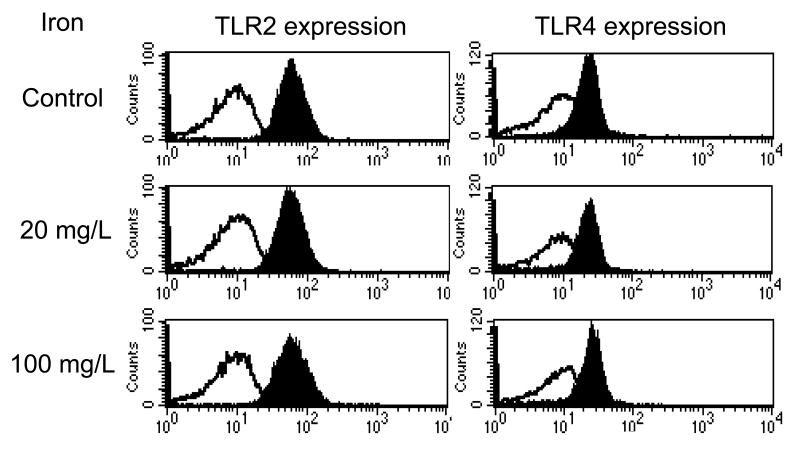
Effects of iron on toll-like receptor (TLR) 2and 4 expression in PMNs
TLRs play an essential role in the detection of invading pathogens. TLR2 and TLR4 are expressed on PMNs and serve a critical role in host defenses against Gram positive and Gram negative bacteria respectively. TLR2 recognizes the peptidoglycans on Gram positive organism whereas TLR-4 recognizes the conserved lipopolysaccharide (LPS) pattern of Gram-negative bacteria. Exposure to these ligands results in up-regulation and activation of these receptors which, in turn, lead to leukocyte activation, cytokine production, and phagocytosis of the given bacteria. We, therefore, asked if the observed suppression of PMN phagocytic function by iron sucrose is associated with and, in part, mediated by changes in TLR 2 and 4 expressions. The study revealed no significant effect on either TLR 2 or TLR 4 expression on PMN in response to iron sucrose at either 4 hours or 24 hours (Mean Fluorescence Intensity: Control, 20, 100mg/L = TLR2; 61.1 ± 3.1, 58.3 ± 2.4, 62.8 ± 2.8, TLR4; 22.5 ± 2.5, 23.7 ± 3.1, 25.2 ± 2.9, respectively, p= NS, Figure 3).
Figure 3. Effect of Iron Sucrose on Toll-like eceptor expression in PMNs.
The iron sucrose was added to heparinized blood samples from each healthy subject at various concentrations. The toll-like receptor 2 and 4 expressions in PMNs were examined using flow cytometry after 4 or 24 hours incubation with Iron Sucrose. Black histogram: anti- human TLR2 or 4 (+). White histogram: negative control.
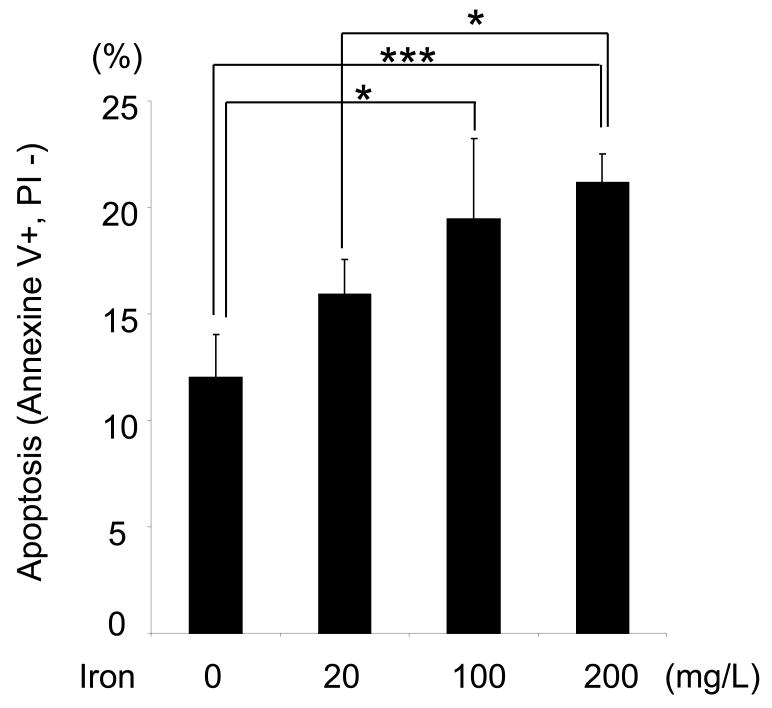
Effects of iron sucrose on PMN viability and apoptosis
Annexin V and propidium iodide (PI) staining was used to investigate the PMN viability in blood samples incubated with or without iron sucrose for 4 or 24 hours. There was no significant difference in Annexin V and propidium iodide (PI) staining at 4hours. However, iron sucrose resulted in a significantly concentration-dependent increase in the apoptotic PMNs [annexine V (+) PI (-)] (Figure 4). The presence of significant impairment of phagocytic activity of PMNs despite preservation of their viability within the 4-hr exposure to iron sucrose suggests that the inhibitory effect of this compound is independent of its cytocidal action which requires longer exposure.
Figure 4. Effect of Iron Sucrose on PMNs' viability.
The iron sucrose was added to heparinized blood samples from each healthy subject at various concentrations. Annexine-V (+) PI (-) population was evaluated as apoptotic PMNs using flow cytometry after 24 hours incubation with iron sucrose. * P<0.05, *** P<0.005.
Discussion
The present study showed that pharmacologically-relevant concentrations of iron sucrose significantly suppressed the phagocytic function of PMNs from healthy donors in a dose-dependent manner against both Gram positive (S. Aureus) and Gram negative bacteria (Escherichia Coli). The molecules expressed on the wall of Gram positive bacterial (peptidoglycans) and Gram negative bacteria (lipopolysaccharides) are recognized by pattern recognition receptors, TLR2 and TLR4 respectively. These receptors are expressed on PMNs, monocytes, and macrophages. Exposure to these ligands results in up-regulation and activation of these receptors which, in turn, lead to leukocyte activation, cytokine production, and phagocytosis of the given bacteria. To explore the possibility that iron sucrose-induced suppression of phagocytic activity of PMNs against Gram positive and Gram negative bacteria is mediated by modulation of TLR2 or TLR4 expression, we examined the expression of these receptors on PMNs in blood samples incubated with iron sucrose. The experiments revealed no significant change in either TLR2 or TLR4 expression in PMNs with this iron preparation.
To dissect the contribution of iron core from the carbohydrate shell of iron sucrose to the observed suppression of PMNs' phagocytic activity, we examined the effect of co-incubation of blood samples with iron sucrose and iron chelator, desferrioxamine. Co-treatment with iron chelator prevented the suppression of phagocytic function in PMNs by iron sucrose. This observation excluded the possible contribution of the carbohydrate shell of iron sucrose to the PMN dysfunction. We next asked if iron sucrose affects the viability of PMNs. To this end we compared the extent of apoptosis in blood samples incubated with or without iron sucrose. The experiments showed a significant dose-dependent increase in PMN apoptosis with iron sucrose. This observation points to the injurious effect of iron sucrose on PMNs which may, in part, account for the observed impairment of their phagocytic activity.
Phagocytosis is a major component of the host defense against microbial infection. Consequently inhibition of phagocytic activity of PMNs by iron sucrose can contribute to increased incidence and severity and poor outcome of bacterial infections in patients treated with excessive does of this and other IV iron preparations. Besides suppression of phagocytic function of PMNs, iron overload can increase susceptibility to and severity of bacterial infections by promoting growth and enhancing virulence of bacteria [27] [28]. In order to acquire iron, bacteria compete with the host for the available iron by secreting low molecular weight iron chelators known as siderophores. These siderophores avidly bind free iron to form iron complexes which are taken up by microbe via receptor-mediated endocytosis [29] [30]. Under normal condition nearly all of the plasma iron content is bound to transferrin which make it inaccessible to the bacteria [27]. Administration of IV iron preparations results in rapid saturation of plasma transferrin and the rise in catalytically active non-transferrin-bound iron in the plasma, thereby providing readily accessible iron for bacteria. Thus, IV iron preparations may not only suppress the host's innate immune response but also increase the potential risk of infection. Interestingly S. aureus possesses a transferrin receptor that allows it to acquire iron available in the blood stream [29]. The deleterious effect of iron sucrose on phagocytic activity in PMNs was compounded by the increased PMN apoptosis. This is most likely due to iron induced oxidative stress in these cells. It should be noted that when compared to the intestinal absorption of 1-2 mg of iron in the course of 3 meals per day, rapid IV infusion of large quantities of iron (100-1000 mg) within a few minutes represents an enormous load with which the body is not accustomed. In fact administration of these products has been shown to result in appearance of catalytically-active non-transferrin bound iron and a rise in markers of oxidative stress and inflammation in dialysis patients [31] [32] [33] [34] [35]. This is compounded by elevation of plasma hepcidin [36] which renders the ESRD patients particularly susceptible to the adverse effects of intravenously administered iron preparations especially when used indiscriminately. This is because by blocking ferroportin which is the main pathway of iron export in the macrophages and other reticuloendothelial cells, elevation of hepcidin leads to trapping and rapid expansion of iron pool in these cells. IV sucrose consists of a core of elemental iron covered by a carbohydrate envelope. Following their IV administration, these products are taken up by the reticuloendothelial cells which remove and release their carbohydrate moiety for clearance by the liver. The iron core is then used for storage in ferritin and release for binding to transferrin [37]. However due to their massive load and rapid introduction, administration of these products lead to elevated levels of catalytically active iron within the cells and non-transferrin-bound iron in the plasma [38]. This leads to formation of iron-catalyzed hydroxyl radical and oxidative stress which can account for the PMN apoptosis observed in the present study [39] [40] [41] [42] [43]. The results of the present in vitro study which demonstrated a concentration-dependent suppression of PMN phagocytic capacity and viability by iron sucrose, parallel the findings of an earlier cross-sectional investigation of ESA- and IV iron-treated ESRD patients reported by Patruta et al [22]. These investigators examined the phagocytic and bacteriocidal properties of PMN from subgroups of patients with a wide range of serum ferritin levels (<60 to > 650 micrograms/L) against E. Coli and found significant association between ferritin level and impairment of PMN functions in hemodialysis patients. Based on these observations they cautioned against excessive use of i.v. iron in hemodialysis patients with elevated ferritin levels.
Conclusions
The results showed that iron sucrose at pharmacologically-relevant concentrations significantly inhibits phagocytic function in PMNs against both Gram positive and negative bacteria, and that the deleterious effect of iron sucrose was mediated by its elemental iron core but not its carbohydrate shell and as such may be shared by other IV iron preparations. Furthermore, iron sucrose significantly increased PMN apoptosis which may, in part, account for the observed impairment of their phagocytic activity. These findings build upon previous studies and provide compelling evidence for the potential adverse effects of the indiscriminate use of the IV iron preparations in the highly vulnerable patients with advanced CKD.
Acknowledgments
Funding: This work was supported in part by NIH-NCRR UL1 TR000153, KL2 TR000147 and the Juvenile Diabetes Research Foundation International 17-2011-609.
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest associated with the work presented in this manuscript.
References
- 1.Girndt M, Sester U, Sester M, et al. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant. 1999;14:2807–2810. doi: 10.1093/ndt/14.12.2807. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System: USRDS. Annual Data Report National Institutes of Health Diabetes and Digestive and Kidney Diseases. Bethesda. MD: 1998. [Google Scholar]
- 3.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 4.Girndt M, Sester M, Sester U, et al. Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl. 2001;78:S206–211. doi: 10.1046/j.1523-1755.2001.59780206.x. [DOI] [PubMed] [Google Scholar]
- 5.Sester U, Sester M, Hauk M, et al. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol Dial Transplant. 2000;15:1217–1223. doi: 10.1093/ndt/15.8.1217. [DOI] [PubMed] [Google Scholar]
- 6.Meuer SC, Hauer M, Kurz P, et al. Selective blockade of the antigen-receptor-mediated pathway of T cell activation in patients with impaired primary immune responses. J Clin Invest. 1987;80:743–749. doi: 10.1172/JCI113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal S, Gollapudi P, Elahimehr R, et al. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant. 2010;25:737–746. doi: 10.1093/ndt/gfp580. [DOI] [PubMed] [Google Scholar]
- 8.Boelaert JR, Daneels RF, Schurgers ML, et al. Iron overload in haemodialysis patients increases the risk of bacteraemia: a prospective study. Nephrol Dial Transplant. 1990;5:130–134. doi: 10.1093/ndt/5.2.130. [DOI] [PubMed] [Google Scholar]
- 9.Smogorzewski M, Massry SG. Defects in B-cell function and metabolism in uremia: role of parathyroid hormone. Kidney Int Suppl. 2001;78:S186–189. doi: 10.1046/j.1523-1755.2001.59780186.x. [DOI] [PubMed] [Google Scholar]
- 10.Pahl MV, Gollapudi S, Sepassi L, et al. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol Dial Transplant. 2010;25:205–212. doi: 10.1093/ndt/gfp397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto Y, Shinzato T, Amano I, et al. Relationship between susceptibility to apoptosis and Fas expression in peripheral blood T cells from uremic patients: a possible mechanism for lymphopenia in chronic renal failure. Biochem Biophys Res Commun. 1995;215:98–105. doi: 10.1006/bbrc.1995.2438. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naive and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70:371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 13.Moser B, Roth G, Brunner M, et al. Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: a comparative evaluation between non-dialysis, haemodialysis, and peritoneal dialysis. Biochem Biophys Res Commun. 2003;308:581–585. doi: 10.1016/s0006-291x(03)01389-5. [DOI] [PubMed] [Google Scholar]
- 14.Alexiewicz JM, Smogorzewski M, Fadda GZ, Massry SG. Impaired phagocytosis in dialysis patients: studies on mechanisms. Am J Nephrol. 1991;11:102–111. doi: 10.1159/000168284. [DOI] [PubMed] [Google Scholar]
- 15.Massry S, Smogorzewski M. Dysfunction of polymorphonuclear leukocytes in uremia: role of parathyroid hormone. Kidney Int Suppl. 2001;78:S195–196. doi: 10.1046/j.1523-1755.2001.59780195.x. [DOI] [PubMed] [Google Scholar]
- 16.Green NS. Yersinia infections in patients with homozygous beta-thalassemia associated with iron overload and its treatment. Pediatr Hematol Oncol. 1992;9:247–254. doi: 10.3109/08880019209016592. [DOI] [PubMed] [Google Scholar]
- 17.Hoen B, Kessler M, Hestin D, Mayeux D. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant. 1995;10:377–381. [PubMed] [Google Scholar]
- 18.Deicher R, Ziai F, Cohen G, et al. High-dose parenteral iron sucrose depresses neutrophil intracellular killing capacity. Kidney Int. 2003;64:728–736. doi: 10.1046/j.1523-1755.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 19.van Asbeck BS, Marx JJ, Struyvenberg A, Verhoef J. Functional defects in phagocytic cells from patients with iron overload. J Infect. 1984;8:232–240. doi: 10.1016/s0163-4453(84)93955-0. [DOI] [PubMed] [Google Scholar]
- 20.Waterlot Y, Cantinieaux B, Hariga-Muller C, et al. Impaired phagocytic activity of neutrophils in patients receiving haemodialysis: the critical role of iron overload. Br Med J (Clin Res Ed) 1985;291:501–504. doi: 10.1136/bmj.291.6494.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veys N, Vanholder R, Ringoir S. Correction of deficient phagocytosis during erythropoietin treatment in maintenance hemodialysis patients. Am J Kidney Dis. 1992;19:358–363. doi: 10.1016/s0272-6386(12)80454-9. [DOI] [PubMed] [Google Scholar]
- 22.Patruta SI, Edlinger R, Sunder-Plassmann G, Horl WH. Neutrophil impairment associated with iron therapy in hemodialysis patients with functional iron deficiency. J Am Soc Nephrol. 1998;9:655–663. doi: 10.1681/ASN.V94655. [DOI] [PubMed] [Google Scholar]
- 23.Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA. Changing Patterns of Anemia Management in U.S Hemodialysis Patients. Am J Medicine. doi: 10.1016/j.amjmed.2012.03.011. in press. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND. Epidemic of iron overload in dialysis population caused by excessive use of intravenous iron products: a plea for moderation. Am J Medicine. doi: 10.1016/j.amjmed.2012.02.009. In press. [DOI] [PubMed] [Google Scholar]
- 25.Rostoker G, et al. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents. Am J Medicine. doi: 10.1016/j.amjmed.2012.01.015. In press. [DOI] [PubMed] [Google Scholar]
- 26.Chonchoi M. Neutrophiol dysfunction and infection risk in end-stage renal disease. Semin Dial. 2006;19:291–6. doi: 10.1111/j.1525-139X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 27.Bullen JJ. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 28.Ward CG, Hammond JS, Bullen JJ. Effect of iron compounds on antibacterial function of human polymorphs and plasma. Infect Immun. 1986;51:723–730. doi: 10.1128/iai.51.3.723-730.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams P, Griffiths E. Bacterial transferrin receptors--structure, function and contribution to virulence. Med Microbiol Immunol. 1992;181:301–322. doi: 10.1007/BF00191543. [DOI] [PubMed] [Google Scholar]
- 30.Fishbane S. Review of issues relating to iron and infection. Am J Kidney Dis. 1999;34:S47–52. doi: 10.1053/AJKD034s00047. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal R. Proinflammatory effects of iron sucrose in chronic kidney disease. Kidney Int. 2006;69:1259–1263. doi: 10.1038/sj.ki.5000164. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 33.Anirban G, Kohli HS, Jha V, et al. The comparative safety of various intravenous iron preparations in chronic kidney disease patients. Ren Fail. 2008;30:629–638. doi: 10.1080/08860220802134631. [DOI] [PubMed] [Google Scholar]
- 34.Ganguli A, Kohli HS, Khullar M, et al. Lipid peroxidation products formation with various intravenous iron preparations in chronic kidney disease. Ren Fail. 2009;31:106–110. doi: 10.1080/08860220802599106. [DOI] [PubMed] [Google Scholar]
- 35.Maruyama Y, Nakayama M, Yoshimura K, et al. Effect of repeated intravenous iron administration in haemodialysis patients on serum 8-hydroxy-2′-deoxyguanosine levels. Nephrol Dial Transplant. 2007;22:1407–1412. doi: 10.1093/ndt/gfl789. [DOI] [PubMed] [Google Scholar]
- 36.Nakanishi T, Hasuike Y, Otaki Y, et al. Hepcidin: another culprit for complications in patients with chronic kidney disease? Nephrol Dial Transplant. 2011;26:3092–3100. doi: 10.1093/ndt/gfr410. [DOI] [PubMed] [Google Scholar]
- 37.Macdougall IC. Intravenous administration of iron in epoetin-treated haemodialysis patients--which drugs, which regimen? Nephrol Dial Transplant. 2000;15:1743–1745. doi: 10.1093/ndt/15.11.1743. [DOI] [PubMed] [Google Scholar]
- 38.Slotki I. Intravenous iron supplementation in the anaemia of renal and cardiac failure--a double-edged sword? Nephrol Dial Transplant. 2005;20(Suppl 7):vii16–23. doi: 10.1093/ndt/gfh1102. [DOI] [PubMed] [Google Scholar]
- 39.Salahudeen AK, Oliver B, Bower JD, Roberts LJ., 2nd Increase in plasma esterified F2-isoprostanes following intravenous iron infusion in patients on hemodialysis. Kidney Int. 2001;60:1525–1531. doi: 10.1046/j.1523-1755.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 40.Tovbin D, Mazor D, Vorobiov M, et al. Induction of protein oxidation by intravenous iron in hemodialysis patients: role of inflammation. Am J Kidney Dis. 2002;40:1005–1012. doi: 10.1053/ajkd.2002.36334. [DOI] [PubMed] [Google Scholar]
- 41.Guz G, Glorieux GL, De Smet R, et al. Impact of iron sucrose therapy on leucocyte surface molecules and reactive oxygen species in haemodialysis patients. Nephrol Dial Transplant. 2006;21:2834–2840. doi: 10.1093/ndt/gfl263. [DOI] [PubMed] [Google Scholar]
- 42.Pai AB, Boyd AV, McQuade CR, et al. Comparison of oxidative stress markers after intravenous administration of iron dextran, sodium ferric gluconate, and iron sucrose in patients undergoing hemodialysis. Pharmacotherapy. 2007;27:343–350. doi: 10.1592/phco.27.3.343. [DOI] [PubMed] [Google Scholar]
- 43.Kuo KL, Hung SC, Wei YH, Tarng DC. Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol. 2008;19:1817–1826. doi: 10.1681/ASN.2007101084. [DOI] [PMC free article] [PubMed] [Google Scholar]