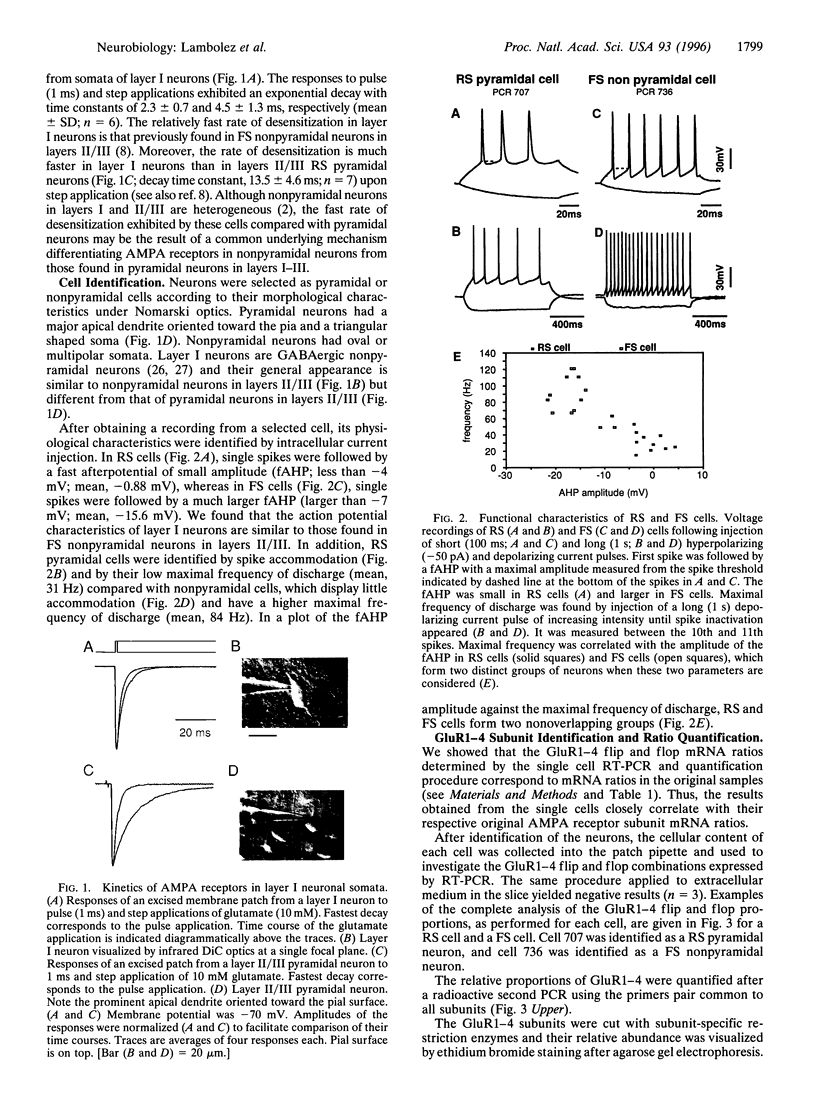
Abstract
In the cortex fast excitatory synaptic currents onto excitatory pyramidal neurons and inhibitory nonpyramidal neurons are mediated by alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors exhibiting cell-type-specific differences in their kinetic properties. AMPA receptors consist of four subunits (GluR1-4), each existing as two splice variants, flip and flop, which critically affect the desensitization properties of receptors expressed in heterologous systems. Using single cell reverse transcription PCR to analyze the mRNA of AMPA receptor subunits expressed in layers I-III neocortical neurons, we find that 90% of the GluR1-4 in nonpyramidal neurons are flop variants, whereas 92% of the GluR1-4 in pyramidal neurons are flip variants. We also find that nonpyramidal neurons predominantly express GluR1 mRNA (GluR1/GluR1-4 = 59%), whereas pyramidal neurons contain mainly GluR2 mRNA (GluR2/GluR1-4 = 59%). However, the neuron-type-specific splicing is exhibited by all four AMPA receptor subunits. We suggest that the predominance of the flop variants contributes to the faster and more extensive desensitization in nonpyramidal neurons, compared to pyramidal cells where flip variants are dominant. Alternative splicing of AMPA receptors may play an important role in regulating synaptic function in a cell-type-specific manner, without changing permeation properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour B., Keller B. U., Llano I., Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994 Jun;12(6):1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Baughman R. W., Gilbert C. D. Aspartate and glutamate as possible neurotransmitters in the visual cortex. J Neurosci. 1981 Apr;1(4):427–439. doi: 10.1523/JNEUROSCI.01-04-00427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet P., Audinat E., Lambolez B., Crépel F., Rossier J., Iino M., Tsuzuki K., Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994 Feb;12(2):383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Jonas P., Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J Physiol. 1992 Dec;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Young W. S., Legendre P., Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992 Nov 27;258(5087):1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Gabbott P. L., Somogyi P. Quantitative distribution of GABA-immunoreactive neurons in the visual cortex (area 17) of the cat. Exp Brain Res. 1986;61(2):323–331. doi: 10.1007/BF00239522. [DOI] [PubMed] [Google Scholar]
- Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995 Jul;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Activation and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron. 1992 Nov;9(5):991–999. doi: 10.1016/0896-6273(92)90250-h. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Different glutamate receptor channels mediate fast excitatory synaptic currents in inhibitory and excitatory cortical neurons. Neuron. 1993 Dec;11(6):1083–1091. doi: 10.1016/0896-6273(93)90221-c. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Racca C., Sakmann B., Seeburg P. H., Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994 Jun;12(6):1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Jonas P., Spruston N. Mechanisms shaping glutamate-mediated excitatory postsynaptic currents in the CNS. Curr Opin Neurobiol. 1994 Jun;4(3):366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995 Apr;15(4):2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann D. M. Quantal variability of excitatory transmission in the hippocampus: implications for the opening probability of fast glutamate-gated channels. Proc Biol Sci. 1993 Jul 22;253(1336):107–116. doi: 10.1098/rspb.1993.0088. [DOI] [PubMed] [Google Scholar]
- Lambolez B., Audinat E., Bochet P., Crépel F., Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992 Aug;9(2):247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- Lambolez B., Curutchet P., Stinnakre J., Bregestovski P., Rossier J., Prado de Carvalho L. Electrophysiological and pharmacological properties of GluR1, a subunit of a glutamate receptor-channel expressed in Xenopus oocytes. Neurosci Lett. 1991 Feb 11;123(1):69–72. doi: 10.1016/0304-3940(91)90160-u. [DOI] [PubMed] [Google Scholar]
- Livsey C. T., Costa E., Vicini S. Glutamate-activated currents in outside-out patches from spiny versus aspiny hilar neurons of rat hippocampal slices. J Neurosci. 1993 Dec;13(12):5324–5333. doi: 10.1523/JNEUROSCI.13-12-05324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H., Mosbacher J., Melcher T., Höger T., Geiger J. R., Kuner T., Monyer H., Higuchi M., Bach A., Seeburg P. H. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994 Dec 9;266(5191):1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Blackstone C. D., Levey A. I., Huganir R. L., Price D. L. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993 Mar;53(2):327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Mosbacher J., Schoepfer R., Monyer H., Burnashev N., Seeburg P. H., Ruppersberg J. P. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994 Nov 11;266(5187):1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Iino M., Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol. 1991 Jul;66(1):2–11. doi: 10.1152/jn.1991.66.1.2. [DOI] [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Mayer M. L. Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol. 1994 Jul;46(1):129–138. [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Raman I. M., Zhang S., Trussell L. O. Pathway-specific variants of AMPA receptors and their contribution to neuronal signaling. J Neurosci. 1994 Aug;14(8):4998–5010. doi: 10.1523/JNEUROSCI.14-08-04998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993 May;54(2):347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. Fluctuations in pyramid-pyramid excitatory postsynaptic potentials modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience. 1993 May;54(2):329–346. doi: 10.1016/0306-4522(93)90256-f. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Zhang S., Raman I. M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993 Jun;10(6):1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Vickers J. C., Huntley G. W., Edwards A. M., Moran T., Rogers S. W., Heinemann S. F., Morrison J. H. Quantitative localization of AMPA/kainate and kainate glutamate receptor subunit immunoreactivity in neurochemically identified subpopulations of neurons in the prefrontal cortex of the macaque monkey. J Neurosci. 1993 Jul;13(7):2982–2992. doi: 10.1523/JNEUROSCI.13-07-02982.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993 Jun;3(3):291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]





