Significance
Drinking water in response to thirst following fluid loss is a pleasant experience, whereas drinking water after thirst has been satiated is unpleasant. The pleasantness of drinking when thirsty is associated with activation in the anterior cingulate cortex and orbitofrontal region. The unpleasantness and aversion of overdrinking is associated with activations in the midcingulate cortex, insula, amygdala, and periaqueductal grey. Activations in the putamen and cerebellum, and also in the motor cortex, possibly reflect volitional effort to conduct compliant drinking in the face of mechanisms inhibiting intake. These regions may contribute to the termination of drinking. Overdrinking with hyponatraemia and cerebral edema can occur in schizophrenia, reflecting that this brain disorder can derange physiological mechanisms regulating water balance.
Keywords: fMRI, swallowing
Abstract
The instinct of thirst was a cardinal element in the successful colonization by vertebrates of the dry land of the planet, which began in the Ordovician period about 400 million y ago. It is a commonplace experience in humans that drinking water in response to thirst following fluid loss is a pleasant experience. However, continuing to drink water once thirst has been satiated becomes unpleasant and, eventually, quite aversive. Functional MRI experiments reported here show pleasantness of drinking is associated with activation in the anterior cingulate cortex (Brodmann area 32) and the orbitofrontal cortex. The unpleasantness and aversion of overdrinking is associated with activation in the midcingulate cortex, insula, amygdala, and periaqueductal gray. Drinking activations in the putamen and cerebellum also correlated with the unpleasantness of water, and the motor cortex showed increased activation during overdrinking compared with drinking during thirst. These activations in motor regions may possibly reflect volitional effort to conduct compliant drinking in the face of regulatory mechanisms inhibiting intake. The results suggestive of a specific inhibitory system in the control of drinking are unique.
The colonization of the planetary dry land by vertebrates emerging from the fresh water rivers and swamps over the Ordovician and Silurian period was dependent in major part on the development of the instincts of thirst and sodium appetite.
The genetically programmed neural organization determining the behavior embodied in these instincts is protective of the chemical integrity and volume of the circulating milieu intérieur: the extracellular fluids. With thirst, for example, in some manner presently only partly understood, the stimuli provided by sensory input from the dry mouth and desiccated pharynx, increased sodium concentration in the cerebrospinal fluid, the osmotic pressure of the carotid arterial blood, activation of the stretch receptors in the heart, and of the concentration of hormones in arterial blood is centrally integrated to contrive the intensity of the specific subjective state of thirst, and the compulsive intention to acquire water. The intensity of this primordial emotion presumptively determines the quantitative intake during drinking (1, 2).
Accurate rapid gratification over 5–10 min of body deficit occurs in species including the camel, burro, sheep, Saharan donkey, goat, cow, dog, or horse depleted of 4–8% of body weight. However, for other species, such as rat, guinea pig, hamster, and for particular consideration human, drinking is much slower. With human, other variables are operative in the gratification process, termed “the consummatory act” (3). When attesting to thirst as a result of experimental dehydration, these species may drink initially more than 50–80% of deficit (4) but then slowly complete intake to repletion. This behavior was termed “voluntary dehydration” by Adolph (5), an early pioneer in this field. Adolph studied man in the desert and was dubious of the power of thirst to predict the quantitative behavior of human drinking, although dehydration resulting in loss of 1–2% of body weight was usually corrected within 15 min (5). Other factors that modify human drinking include the strength of dry mouth and pharyngeal desiccation sensation, and also the diversity of individual cognitive experience and social conditioning. Such learning, together with other processes, such as urinary sodium excretion evoked by dehydration (6), may modify drinking behavior relative to actual water deficit.
Initial water intake in some animal species when desiccated is rapid and commensurate with the extent of fluid loss, measured by loss of body weight. Thus, putatively, the number of gulps of water taken in the process of gratification, as metered in sequence in the oropharyngeal region, is dictated by the intensity of the subjective sense of thirst. This intensity may reduce with each gulp as a result of the neural feedback conveyed to the central control organization. Multiple elements in the neural network subserving this function of integration of thirst control include circumventricular organs in the anterior wall of the third ventricle. Drinking initiates a feedback; an avalanche of neural inflow derives from mouth sensation, including alleviation of dry mouth, and this flows up the fifth cranial nerve, taste by the chorda tympani included in the seventh nerve, pharyngo-esophageal impulses metering volume swallowed by the ninth nerve, and lower esophageal and gastric sensation—including distension—by the 10th cranial nerve. This “gestalt” of inflow from drinking determined by thirst reduces the central intention to drink to the point of its obliteration, and a state of satiation ensues.
A conspicuous feature is that the drinking stops well before blood changes reflect significant absorption from the gut. Rapid intake of water consequent upon thirst, or of a salt solution when sodium-depleted, carries high survival advantage: it permits animals to go to a water or salt source, rapidly correct the deficit, and leave the place, reducing their exposure to predators that have learned to wait there.
Claude Bernard (7) observed a dehydrated horse with an esophageal fistula, where water swallowed fell out between its fore legs. The horse would drink multiple times its body deficit. The horse appeared to cease only because of exhaustion. Similar studies on sheep with esophageal fistula and other species have shown that the dehydrated sheep will drink 2.5- to 9-times its body deficit in 120 min (1, 8). Clearly, oropharyngeal neural impulses conveyed by the ninth nerve, and metering gulps swallowed, do not alone cause satiation. Satiation requires gastric distension and all elements occurring in the characteristic physiological time sequence to gratify the animal.
With sheep with esophageal fistula, tubing the known water deficit into the rumen, or into stomach in the case of other species, immediately before offering water reduces volume drunk. However, overdrinking still occurs in most instances. The upper alimentary inflow is essential to quench thirst or sodium appetite. The data from both thirst and salt appetite studies indicate that gratification involves a cascade, or “gestalt,” of sensory inflow in characteristic time sequence from the cranial nerves, as mentioned above.
Central to this gratification behavior is the question of whether there is a mechanism giving rise to a signal that causes the animal to stop drinking. It is a commonplace subjective human experience that drinking water when thirsty is generally pleasurable, with the sense of satisfying a need. However, if the subject has reached satisfaction, continuation of drinking water becomes unpleasant and eventually is strongly aversive. It is unknown whether normal cessation of intake simply results from cessation of the central stimulus as a result of neural feedback as described above, or whether there is a specific “stop” mechanism, an active protective inhibition stopping intake over and above that needed. This mechanism could avoid excessive intake, which gives rise to hyponatraemia and eventual brain edema. Clinically, such overdrinking can occur as a symptom of brain disorders, such as in schizophrenia, reflecting that this type of brain disorder disrupts the normal central physiological integration contriving apt water intake in response to replacement needs.
Our objective was to investigate how the presence or absence of thirst interacts with the act of drinking and associated brain responses. We asked participants to rate the pleasantness or unpleasantness of water and identified brain regions where drinking activation was correlated with the ratings. Pleasant drinks of water during thirst were related to activations in the pregenual anterior cingulate cortex (pACC) and orbitofrontal cortex, whereas the midcingulate cortex (MCC) showed activations related to the unpleasantness of water after thirst was satiated. Additional unpleasantness activations in subcortical and brainstem regions were also noted, possibly reflecting an inhibition of drinking. This inhibitory process could be an important mechanism to protect against the adverse consequences of excessive water intake.
Results
Exercise-Related Temperature, Weight, and Thirst.
Twenty healthy participants (11 female, 9 male; age range 19–54 y, mean 28.3 ± 10 y) reported average thirst levels of 3.5 ± 2.0 (scale range 0–10) at the outset of the experiment. Ratings of thirst increased to 6.5 ± 2.1 [t(19) = 6.8, P < 0.001] and temperature measured aurally was elevated by 0.4 ± 0.4 °C [t(19) = 3.1; P < 0.01] after participants exercised for 60 min at 60% of heart rate reserve on a stationary cycle. The exercise was associated with weight loss of 0.6 ± 0.4 kg [t(19) = 7.4; P < 0.001]. Aural temperature decreased during a subsequent 60-min cool-down period [−0.4 ± 0.3; t(19) = 2.6; P < 0.05], whereas thirst score (6.8 ± 2.1) and weight loss (0.6 ± 0.4 kg) did not show significant levels of change.
Thirst, Weight, and Subjective Ratings of Water During Drinking.
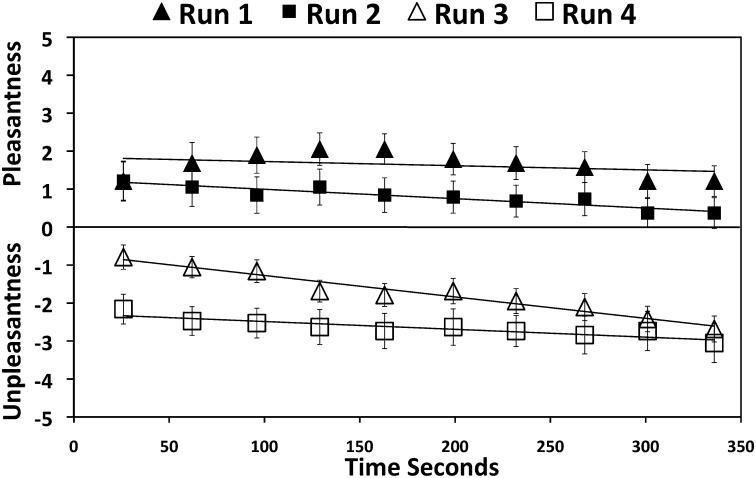
Participants reported a decrease in thirst score to 4.2 ± 1.8 [t(19) = 3.7, P < 0.01] after drinking 5-mL volumes of water on 20 occasions during the first two functional MRI (fMRI) scans, each of 6.4-min duration. The drinks of 5 mL were rated as pleasant on average (1.0 ± 1.8, scale range 0–5) during the first two scans (thirst condition) (Fig. 1). Thirst decreased to 0.3 ± 0.2 [t(19) = 8.3, P < 0.001] after participants were given ad libitum access to water and then encouraged to overdrink as much as comfortably tolerable. Participants drank an average volume of 415 ± 252 mL during ad libitum access and overdrinking. Subsequent drinks of 5 mL of water on 20 occasions were rated as unpleasant (−2.3 ± 1.5, scale range 0 to −5) during the second two scans (overdrinking condition) (Fig. 1). Successive pleasantness ratings showed decreasing levels throughout the protocol [F(9, 198) = 11.4, P < 0.001)] and weight increased by 0.7 ± 0.4 kg [t(19) = 7.3; P < 0.001] during the complete scanning period.
Fig. 1.
Participants gave ratings of the pleasantness or unpleasantness of drinking 5-mL volumes of water on 10 occasions during each of four functional brain imaging scans. Drinks were rated as pleasant during the first two scans, when participants were dehydrated and thirsty. The final two scans took place after participants drank in excess of the amount required for satiation. Increasing ratings of unpleasantness were associated with drinking water during this overdrinking condition.
Drinking-Related Activation During Thirst and Overdrinking.
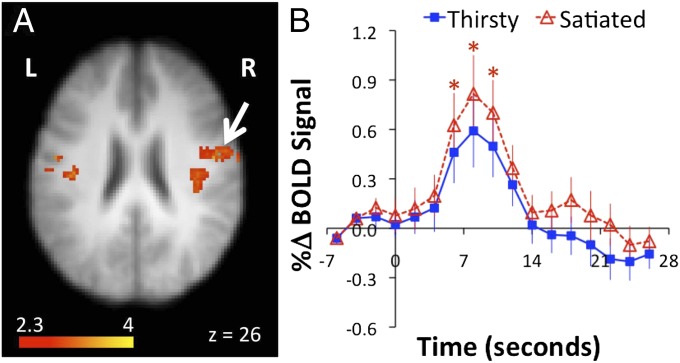
Drinking during the conditions of thirst and overdrinking activated widely distributed regions consistent with previous reports of drinking (9) (Table S1). No brain regions showed levels of drinking activation during the thirst condition that were greater than levels of drinking activation during the overdrinking condition. For the overdrinking condition compared with the thirst condition, increased bilateral drinking activation was observed in the mid and inferior primary motor cortex along with a region in the rolandic operculum (Fig. 2 and Table S2).
Fig. 2.
(A) Bilateral regions of the primary motor cortex showed increased levels of drinking-related activation during the overdrinking condition compared with the thirst condition. (B) BOLD signals measured from an activated cluster in the right motor cortex (indicated by white arrow in A) show hemodynamic responses associated with drinking during the thirst condition (closed blue squares) and during the overdrinking condition (open red triangles). Drinking events were averaged according to the timing of water placed in the mouth (time = 0 s). The signal increases associated with drinking were larger during the overdrinking condition compared with the thirst condition. *P < 0.05.
Drinking Activation Correlated with Pleasantness Ratings.
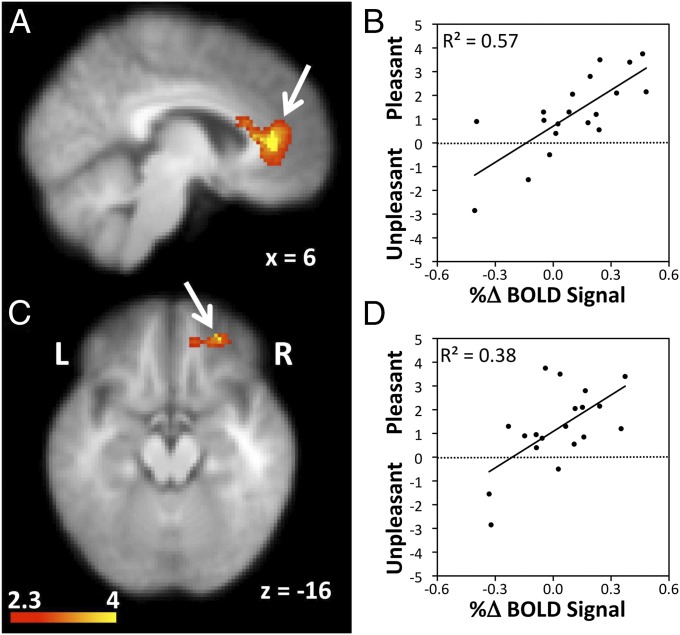
Subjective ratings after 5-mL drinks of water were correlated with drinking-related blood-oxygen level-dependent (BOLD) signal changes during the thirst condition, identifying three regions positively correlated with pleasantness ratings (Fig. 3). These regions were located in the left and right pACC and the left orbital frontal cortex (OFC) (Table S3). The majority of participants had positive signal changes during drinking and the degree of activation in these regions increased most for participants with the highest pleasantness ratings (Fig. 3).
Fig. 3.
(A) Drinking-related activation in the right pACC (indicated with arrow) was related to participants’ pleasantness ratings of water during the thirst condition. (B) Data in the scattergram was collected during two functional brain imaging scans when participants were thirsty. The average percentage signal changes in the right pACC during drinking are paired with the corresponding average ratings of pleasantness for the 5-mL drinks of water for each participant to represent the level of correlation between the two parameters. (C) Variance among participants in drinking-related activation in the right OFC (arrowed) also showed an association with pleasantness ratings of drinks of water. (D) The scattergram shows the level of correlation between pleasantness ratings and drinking-related signal changes in the right OFC.
Drinking Activation Correlated with Unpleasantness Ratings.
Subjective ratings related to 5-mL drinks of water during the overdrinking condition were correlated with drinking-related BOLD signal changes (Fig. 4 and Fig. S1). Regions showing drinking activation correlated with unpleasantness ratings occurred bilaterally in the supplementary motor area (SMA), the MCC, amygdala, putamen, posterior insula, left cerebellum, and the left midinsula (Table S4). Four regions in the brainstem showed activations that were positively correlated with unpleasantness ratings (Fig. 5). Activations in the rostral midbrain included a ventral cluster incorporating the ventral tegmental area and a dorsal cluster overlying the periaqueductal gray (PAG) (Fig. 5B). Activation incorporating the PAG was still visible in a cluster close to the dorsal midline within the caudal midbrain that extended over the dorsal nucleus of the raphe (Fig. 5C). A cluster of activation in the dorsal upper pons extended bilaterally at the level of the motor and principal sensory trigeminal nuclei (Fig. 5D). The dorsal components of a cluster in the lower pons were likely to incorporate the facial nuclei bilaterally and the pontine nuclei ventrally (Fig. 5E).
Fig. 4.
Drinking-related activations that correlated with unpleasantness ratings after overdrinking were distributed throughout the cortex and subcortical regions. (A) Variance in drinking-related activation associated with unpleasantness ratings was seen in the right SMA and MCC. (B) Unpleasantness was also associated with drinking-related activation in the left midinsula (MI), bilateral posterior insula (PI), bilateral putamen (Put), and thalamus (Th). (C) The amygdala in the left (inside circle) and right (inside square) hemispheres showed drinking-related activation that was correlated with unpleasantness ratings. (D) Drinking-related BOLD signal changes were extracted from the amygdala in the left (○) and right (■) hemispheres and plotted against participants corresponding mean unpleasantness ratings to illustrate the correlations between the two parameters.
Fig. 5.
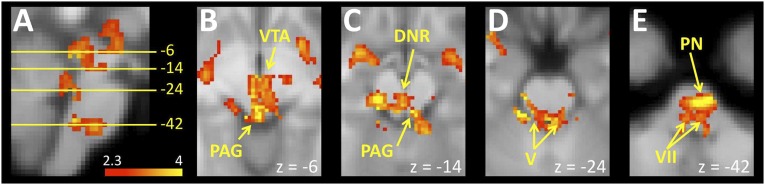
(A) A sagittal view of the brainstem shows drinking-related activations that correlated with unpleasantness ratings after overdrinking, and indicates the location of axial slices in the remaining insets in the figure. (B) An axial slice through the rostral midbrain that shows drinking activations correlated with unpleasantness ratings in a cluster incorporating the PAG and ventral tegmental area (VTA). (C) A slice through the caudal midbrain shows a cluster near the dorsal midline likely to include the PAG and dorsal nucleus of the raphe (DNR), where unpleasantness ratings were correlated with drinking-related activation. (D) Dorsal regions of the rostral pons overlying the motor and principal sensory trigeminal nuclei (V) showed drinking-related activation associated with unpleasantness ratings. (E) An axial slice through the caudal pons showed clusters of drinking-related activation associated with unpleasantness ratings in a cluster that was likely to include the facial nuclei (VII) dorsally and pontine nuclei (PN) ventrally.
Discussion
This study investigated regional brain responses associated with drinking water in relation to the subjective state of thirst in humans. Differential levels of drinking-related activity between thirsty and overdrinking conditions, together with correlations between drinking-related activity and subjective responses to drinking, have provided insights into the mechanisms underlying responses to water deficiency. The results of this study suggest that activation in pACC and OFC is related to the experience of pleasantness that accompanies drinking water when thirsty. When overdrinking, we identified increased activation in the motor cortices presumably required to drink additional water. Of the possible explanations for the origin of this increased activation, there may be a common factor. We propose that this cause is an inhibitory mechanism that prevents overdrinking from taking place postsatiation. Additionally, a distributed network of regions, including the insula, MCC, amygdala, midbrain, cerebellum, and PAG showed drinking-related activation related to the experience of unpleasantness that accompanies drinking water when already fully satiated.
Drinking-Related Regional Brain Activation During Thirst.
During the thirst condition, participants with the highest pleasantness ratings showed the greatest activation in the OFC and pACC. The activation of these regions in relation to pleasantness indicates they could be involved in the generation of this subjective state.
Activity in the OFC has been associated with subjective states of pleasantness produced in response to sensory stimuli presented in the auditory (10), somatosensory (11), olfactory (12), and gustatory (13) domains. OFC activation has also been observed in relation to abstract concepts like monetary reward (14). The location of the OFC activation in the present study is similar to a region activated bilaterally in a previous study that investigated the effect of thirst on the taste of water, including its subjective appraisal (15). The present findings may thus represent an initial subjective appraisal regarding the pleasantness of water in the mouth, consistent with the results of this earlier report.
Earlier studies have also linked pACC activity to thirst. From the very first neuroimaging study of thirst (16), this region has consistently shown activation in response to thirst using different imaging techniques (i.e., PET, arterial spin labeling) and varied experimental manipulations of physiology (17–19). In addition to being involved in the neural representation of thirst, the pACC is also part of the cortical network involved in swallowing (20, 21), and in monkey it has been demonstrated that the cingulate cortex has cortical projections to pons (22), an area that contains nuclei implicated in the initiation of swallowing (23, 24). Furthermore, in a study that analyzed the response of the cortical swallowing network to stimulation of the esophagus with acid, a region of the pACC was activated (25) that is very similar to the activated region observed in the present study.
The pACC is also prominently associated with emotional responses, with several functional imaging studies of emotion showing pACC activation in relation to pleasant subjective states (26), including responses to pleasant touch (11) and activation levels related to pleasantness ratings of odors (12). In contradistinction to the bulk of studies, there has been a report by Kulkarni et al. (27) of activation levels in the pACC correlating with the unpleasantness of pain. However, alternative studies have identifed the MCC as the likely locus of pain unpleasantness (28, 29). The pACC is replete with high levels of opioid receptors and has been implicated in modulation of pain (30–32). Conditions of increased pain unpleasantness could recruit pain-inhibition responses, which would explain the association between activation in the pACC and pain unpleasantness ratings in the study by Kulkarni et al. (27). On balance, the pACC activation observed in the present study is likely to be involved in producing a pleasant subjective state in response to drinking water when thirsty.
Drinking-Related Regional Brain Activation During Overdrinking.
Regions of the primary motor cortex associated with swallowing showed significantly increased activation when drinking was initiated in the gratified state compared with the thirsty state. There are several potential explanations for the origin of this increased activation. For example, the activation may reflect the influence of an attention mechanism that transfers an otherwise automatic response into a consciously controlled response that now involves the motor cortex. Alternatively, it may simply reflect a situation where swallowing becomes more effortful once the swallowing becomes a subjectively unpleasant experience. A problem with these explanations, however, is that they do not provide any insights into why the attentional mechanism was recruited or what caused the unpleasant experience. One answer is that the explanations reflect a response to an inhibitory mechanism that prevents swallowing water after rehydration and satiation of thirst. Thus, the observed increase in motor activity, whether it is via attention or another mechanism, is needed to overcome the inhibition of swallowing provided by this inhibitory mechanism. Three lines of evidence favor this explanation. First, swallowing is largely an automatic reflex process that, once initiated, is unlikely to require additional motor cortex output to be completed (33). This theory suggests that the motor cortex makes a contribution only to the initiation of swallowing. Second, it has been observed that distention of the stomach can lead to inhibition of swallowing events in the decerebrate rat (34). Thus, if a similar response to gastric distension were present in humans, increased input from the motor cortex would be required in order for the swallowing act to be initiated. Third, in the present study regions in the medulla that correspond to nuclei responsible for the automated control of swallowing (33) did show drinking-related activations, but these were neither state-dependent (i.e., not related to thirst nor overdrinking) nor were they related to ratings of pleasantness or unpleasantness. As the drinking protocol was the same in both the thirsty and overdrinking conditions, this result is consistent with these regions being responsible for an automated process that is unaffected by subjective state. Pontine areas corresponding to nuclei involved in the initiation of swallowing (23, 24) showed increased drinking-related activation that correlated with unpleasantness. A parsimonious explanation for this outcome is that the increased activation of these regions occurs in the face of inhibitory inputs that are responsible, under normal circumstances, for preventing the initiation of swallowing.
The putative inhibition of swallowing water in excess of deficit could have implications for motor control in regions other than the motor cortex. It is salient to note that increasing unpleasantness was associated with levels of swallowing activation in the supplementary motor area, putamen, and cerebellum. These relationships could represent relatively greater levels of output from the regions in association with the execution of an unfamiliar action, namely the willed instigation of a normally automatic motor pattern that is momentarily inhibited because of the prevailing regulatory state.
One potential source of the inhibitory inputs is interoceptive signals originating from the cranial nerves implicated in the drinking and gratification process by early animal studies, as reviewed by Fitzsimmons (2) and Wolf (4) and also described in the introductory remarks. The influence of interoceptive input on pontine regions responsible for initiating swallowing therefore represents a plausible account of how the termination of drinking might occur.
In addition to the interoceptive input originating from the cranial nerves, the amygdala and the PAG are possibly involved in the inhibition of swallowing. Although these regions were not the only areas activated in relation to unpleasantness ratings, the functional and neuronanatomical characteristics of both make it feasible that they contribute to the inhibitory process. The functional role of the amygdala in aversive states is well documented (35), and includes processes associated with taste aversion (36). Recent viral tract-tracing studies have highlighted direct outputs from the amygdala to the premotor nuclei controlling muscles associated with the initiation of swallowing (37, 38). Efferent connections from the amygdala to the PAG are also well established (39), and animal experiments have shown that stimulation of the PAG is associated with marked inhibition of swallowing (40). It is therefore likely that, in humans, unpleasantness-related drinking activation in the amygdala and the PAG is also associated with the inhibition of swallowing.
It is notable that the regions we have ascribed with a role in drinking inhibition are distinct from a network incorporating the inferior frontal gyrus, ventromedial prefrontal cortex, and SMA, which has been implicated in motor inhibition more generally (41). However, there is an important distinction between the experiment herein and the paradigms usually used to assess response inhibition. Procedures such as go/no-go tasks, which are typically used to assess inhibitory control, identify processes involved in the conscious suppression of an action in the face of a prepotent impulse. In our study a conscious effort is needed to initiate the action of overdrinking, rather than it being a case of suppressing action.
Accompanying the activation of the amygdala and PAG during unpleasantness-related drinking was the activation of two other regions that are likely to represent the experience of unpleasantness reflected in participants’ ratings. The MCC has frequently been implicated in interoceptive sensations and affective states by functional imaging experiments in humans (26), including the unpleasantness of pain (28, 29), the intensity of thirst (19), thermal discomfort (42), dyspnea (43), air hunger (44), and ratings of the urge-to-cough (45). Similarly, the midinsula has also been consistently activated in studies of interoception (46), with the insula itself having been proposed as an integrative site for emotional responses (47).
Under normal conditions of satiation, the inhibition of swallowing and the accompanying experience of unpleasantness would be sufficient to prevent drinking from continuing. During the present study, however, the experimental protocol required compliant participants to continue drinking despite being satiated, an act requiring the pontine nuclei to continually initiate the automated act of swallowing. To make this possible, additional input would be needed to overcome the inhibitory influences that normally prevent swallowing from occurring. The increased activation of the pontine nuclei may therefore not only reflect the addition of inhibitory inputs but also input originating from the cortex, reflected in the observed increase in motor cortex activation. This regulatory input may reflect the need for cortical control to ensure swallowing continues.
A potential problem for the explanation presented above is that components of the neural network we have proposed in connection with overdrinking, such as the amygdala or PAG, may actually reflect regions that are activated as part of a generalized arousal response and specific attention following an unpleasant stimulus. Although the activations observed in the correlation analysis are therefore likely to reflect a network related to overdrinking, disambiguating the specific functions of each activated region in terms of inhibition, general arousal, or other processes will require further research.
Implications.
Clinically, the issue of overdrinking is important because it occurs as a symptom of schizophrenia (48), suggesting that one of the many consequences of this brain disorder is disruption to the normal central integration contriving apt water intake. In addition to the other devastating impacts of the disorder, disrupted water intake can have a significant negative effect on life expectancy (49). Excess secretion of antidiuretic hormone may also contribute to water intoxication in people with schizophrenia (50). Excess intake of water can also be engendered by incorrect ideas of appropriate hydration in marathon runners, as increased body weight associated with hyponatraemia has been recorded in runners in the Boston Marathon (51).
Conclusion
The results of the study indicate that drinking when thirsty is accompanied by a subjective state of pleasantness associated with activations in the pACC and OFC. Following the satiation of thirst, subsequent compliant overdrinking is accompanied by a subjective experience of unpleasantness and aversion, which is inhibiting of drinking behavior and is associated with activations in the MCC, the left midinsula, amygdala, and PAG. Cortical control, reflected in increased activity in the motor cortex, may be required for continued compliant overdrinking in the face of physiological regulatory mechanisms of inhibition. The activations in the cerebellum, putamen, and SMA also observed may have a similar basis. Precise delineation of the components of activated areas specifically subserving inhibition of overdrinking water from those subserving attention and arousal is a matter for further study. The findings here are unique and may have some clinical importance.
Experimental Procedures
The experimental protocol was approved by the Melbourne Health Human Research Ethics Committee (approval #2010.106) and informed consent was obtained from participants before they commenced the study. The study consisted of a single session made up of three primary components: exercise (60 min), cool down (60 min), and MRI scanning (60 min) (Fig. S2). Ratings of thirst were made using a 0–10 rating scale according to a previously reported procedure (18). The level of cycling exercise was standardized by calculating the heart rate reserve (HRR) for each participant: HRR = (220 – age in years) – resting heart rate (RHR), and then ensuring that participants maintained their heart rate at the sum of the RHR plus 60% of the HRR for the duration of the exercise period, using feedback on their heart rate provided by a telemetric device.
Scanning involved two physiological conditions, the first of which involved scanning during the experience of thirst. Participants were subsequently removed from the scanner and asked to drink to satiation. They were then instructed to drink further water to the comfortable limits of their tolerance. The subjects returned to the scanner and two further scans were performed. This process represented the overdrinking condition. During all four scanning runs, 5 mL of water was delivered to the participant’s mouth on average every 30 s. Water was administered by one of the investigators using a syringe connected to a plastic tube. Drinking was cued by instructions that appeared on a screen at the foot of the scanner bed (Fig. S2). Ten seconds after water was delivered to the mouth, a participant was cued to rate the pleasantness of the water on a 0–10 point scale, with ratings of 0–4 representing decreasing levels of unpleasantness, 5 representing a neutral rating (neither pleasant nor unpleasant), and 6–10 representing increasing levels of pleasantness. Participants imbibed 50 mL of water during each of the four functional scans for a total of 200 mL during cued drinking in the scanner.
Image Acquisition.
Scanning was performed at the Murdoch Children’s Research Institute (Melbourne, Australia) using a Siemens Trio 3T scanner (Siemens) with a 32-channel head coil. Structural T1-weighted images were acquired in the sagittal plane [192 slices; 0.90 mm thickness; 0.84 × 0.84 mm2 in-plane resolution; echo time (TE) 2.6 ms; repetition time (TR) 1,900 ms; flip angle 9°]. Echo-planar images (EPI) were acquired in the transaxial plane (33 slices; 4.5 mm thickness; 3.3 × 3.3 mm2 in-plane resolution; TE 35 ms; TR 2,000 ms; flip angle 90°) during scans of 6.4-min duration that incorporated 192 sequential images.
Analysis.
Statistical analyses of the physiological parameters and thirst ratings were performed using SPSS 15.0 for Windows. A repeated-measures ANOVA was used to test for differences in pleasantness/unpleasantness ratings related to drink order (1–10), scanning run (first or second), thirst condition (thirsty, satiated), and any interaction between the principal factors. Preprocessing and analysis of functional images was performed using standard procedures with FEAT, v5.98 (52). Regressors for drinking and rating events were included in a model that included motion correction parameters as confound regressors, as well as confounds to take account of physiological noise because swallowing is associated with respiratory maneuvers (53, 54). The mean of each participant’s ratings during thirst scans were included as regressors to explain between-participant variance in drinking activation during the thirst condition. Drinking activation during the overdrinking state was similarly correlated with participants’ subjective experiences by using the ratings from overdrinking scans. Significant activations were determined using a single-voxel inclusion threshold of z > 2.3 (P < 0.01) and a cluster-level threshold of Pcorr < 0.05, corrected for multiple comparisons (55).
Supplementary Material
Acknowledgments
The authors thank Michael Kean of the Children’s MRI Centre (Melbourne, Australia) for his assistance during the execution of the study. This work was supported by the G. Harold and Leila Y. Mathers Charitable Foundation, the Search Foundation, S. Baillieu Myer, Diana Gibson, Robert Albert, Dr Mark Nelson, Andrew Abercrombie, Nielma Gantner, and the National Health and Medical Research Council of Australia.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403382111/-/DCSupplemental.
References
- 1.Denton D. The Hunger for Salt. An Anthropological, Physiological and Medical Analysis. Heidelberg: Springer; 1982. [Google Scholar]
- 2.Fitzsimons JT. The Physiology of Thirst. Cambridge, UK: Cambridge Univ Press; 1972. [Google Scholar]
- 3.Tinbergen N. The Study of Instinct. Oxford, UK: Clarendon; 1958. [Google Scholar]
- 4.Wolf AV. Thirst. Physiology of the Urge to Drink and Problems of Water Lack. Springfield, IL: Charles C. Thomas; 1958. [Google Scholar]
- 5.Adolph EF. Physiology of Man in the Desert. New York: Interscience; 1947. [Google Scholar]
- 6.McKinley MJ, Denton DA, Nelson JF, Weisinger RS. Dehydration induces sodium depletion in rats, rabbits, and sheep. Am J Physiol. 1983;245(2):R287–R292. doi: 10.1152/ajpregu.1983.245.2.R287. [DOI] [PubMed] [Google Scholar]
- 7.Bernard C. (1856) Leçons de physiologie expérimentale appliquée à la médecine, faites au collége de France: Cours du semestre d'été 1855 (J.-B. Baillière et fils, Paris), pp 49–52. [Google Scholar]
- 8.Bott E, Denton DA, Weller S. Water drinking in sheep with oesophageal fistulae. J Physiol. 1965;176:323–336. doi: 10.1113/jphysiol.1965.sp007553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30(8):2426–2439. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2(4):382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET, et al. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13(3):308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 12.Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18(3):695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- 13.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 14.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 15.de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90(3):1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- 16.Denton D, et al. Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc Natl Acad Sci USA. 1999;96(9):5304–5309. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell MJ, et al. Cortical activation and lamina terminalis functional connectivity during thirst and drinking in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R623–R631. doi: 10.1152/ajpregu.00817.2010. [DOI] [PubMed] [Google Scholar]
- 18.Farrell MJ, et al. Unique, common, and interacting cortical correlates of thirst and pain. Proc Natl Acad Sci USA. 2006;103(7):2416–2421. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell MJ, et al. Effect of aging on regional cerebral blood flow responses associated with osmotic thirst and its satiation by water drinking: A PET study. Proc Natl Acad Sci USA. 2008;105(1):382–387. doi: 10.1073/pnas.0710572105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamdy S, et al. Cortical activation during human volitional swallowing: An event-related fMRI study. Am J Physiol. 1999;277(1 Pt 1):G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 21.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85(2):938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 22.Glickstein M, May JG, 3rd, Mercier BE. Corticopontine projection in the macaque: The distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235(3):343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- 23.Athanassiadis T, Olsson KA, Kolta A, Westberg KG. Identification of c-Fos immunoreactive brainstem neurons activated during fictive mastication in the rabbit. Exp Brain Res. 2005;165(4):478–489. doi: 10.1007/s00221-005-2319-5. [DOI] [PubMed] [Google Scholar]
- 24.Athanassiadis T, Westberg KG, Olsson KA, Kolta A. Physiological characterization, localization and synaptic inputs of bursting and nonbursting neurons in the trigeminal principal sensory nucleus of the rat. Eur J Neurosci. 2005;22(12):3099–3110. doi: 10.1111/j.1460-9568.2005.04479.x. [DOI] [PubMed] [Google Scholar]
- 25.Kern M, Chai K, Lawal A, Shaker R. Effect of esophageal acid exposure on the cortical swallowing network in healthy human subjects. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G152–G158. doi: 10.1152/ajpgi.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulkarni B, et al. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21(11):3133–3142. doi: 10.1111/j.1460-9568.2005.04098.x. [DOI] [PubMed] [Google Scholar]
- 28.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 29.Tölle TR, et al. Region-specific encoding of sensory and affective components of pain in the human brain: A positron emission tomography correlation analysis. Ann Neurol. 1999;45(1):40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wager TD, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 32.Bantick SJ, et al. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(Pt 2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 33.Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24(3):333–348. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- 34.Kurozumi C, Yamagata R, Himi N, Koga T. Emetic stimulation inhibits the swallowing reflex in decerebrate rats. Auton Neurosci. 2008;140(1–2):24–29. doi: 10.1016/j.autneu.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 35.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chem Senses. 2007;32(1):105–109. doi: 10.1093/chemse/bjj045. [DOI] [PubMed] [Google Scholar]
- 37.Van Daele DJ, Cassell MD. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience. 2009;162(2):501–524. doi: 10.1016/j.neuroscience.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Daele DJ, Fazan VP, Agassandian K, Cassell MD. Amygdala connections with jaw, tongue and laryngo-pharyngeal premotor neurons. Neuroscience. 2011;177:93–113. doi: 10.1016/j.neuroscience.2010.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantyh PW. Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol. 1982;206(2):146–158. doi: 10.1002/cne.902060205. [DOI] [PubMed] [Google Scholar]
- 40.Sessle BJ, Ball GJ, Lucier GE. Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Res. 1981;216(1):145–161. doi: 10.1016/0006-8993(81)91283-x. [DOI] [PubMed] [Google Scholar]
- 41.Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37(1):11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Farrell MJ, et al. Brain activation associated with ratings of the hedonic component of thermal sensation during whole-body warming and cooling. J Therm Biol. 2011;36(1):57–63. [Google Scholar]
- 43.von Leupoldt A, et al. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48(1):200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Liotti M, et al. Brain responses associated with consciousness of breathlessness (air hunger) Proc Natl Acad Sci USA. 2001;98(4):2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farrell MJ, Cole LJ, Chiapoco D, Egan GF, Mazzone SB. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage. 2012;61(4):1324–1335. doi: 10.1016/j.neuroimage.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 48.de Leon J, et al. Polydipsia and water intoxication in a long-term psychiatric hospital. Biol Psychiatry. 1996;40(1):28–34. doi: 10.1016/0006-3223(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 49.Hawken ER, et al. Mortality over a 20-year period in patients with primary polydipsia associated with schizophrenia: A retrospective study. Schizophr Res. 2009;107(2–3):128–133. doi: 10.1016/j.schres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 50.Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Relat Psychoses. 2010;4(2):115–123. doi: 10.3371/CSRP.4.2.3. [DOI] [PubMed] [Google Scholar]
- 51.Almond CS, et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352(15):1550–1556. doi: 10.1056/NEJMoa043901. [DOI] [PubMed] [Google Scholar]
- 52.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47(3):1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31(8):2948–2958. doi: 10.1523/JNEUROSCI.4597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.