Significance
This paper describes the successful therapeutic application of properdin, a positive regulator of complement activation. Recombinant expression of properdin generated a highly polymerized artificial form of properdin, Pn, with significantly higher functional activity than the lower-grade properdin polymers in serum. Adding low pharmacologic quantities of Pn to serum markedly enhanced complement deposition on Neisseria meningitidis and Streptococcus pneumoniae and dramatically boosted serum lysis of meningococci. In mouse models of infection for these two major human pathogens, a single low-dose application of Pn significantly reduced bacteremia and markedly increased survival rates. Interestingly, therapeutic induction of massive complement mediated lysis of meningococci did not induce septic shock symptoms through the release of bacterial toxins.
Abstract
Modern medicine has established three central antimicrobial therapeutic concepts: vaccination, antibiotics, and, recently, the use of active immunotherapy to enhance the immune response toward specific pathogens. The efficacy of vaccination and antibiotics is limited by the emergence of new pathogen strains and the increased incidence of antibiotic resistance. To date, immunotherapy development has focused mainly on cytokines. Here we report the successful therapeutic application of a complement component, a recombinant form of properdin (Pn), with significantly higher activity than native properdin, which promotes complement activation via the alternative pathway, affording protection against N. menigitidis and S. pneumoniae. In a mouse model of infection, we challenged C57BL/6 WT mice with N. menigitidis B-MC58 6 h after i.p. administration of Pn (100 µg/mouse) or buffer alone. Twelve hours later, all control mice showed clear symptoms of infectious disease while the Pn treated group looked healthy. After 16 hours, all control mice developed sepsis and had to be culled, while only 10% of Pn treated mice presented with sepsis and recoverable levels of live Meningococci. In a parallel experiment, mice were challenged intranasally with a lethal dose of S. pneumoniae D39. Mice that received a single i.p. dose of Pn at the time of infection showed no signs of bacteremia at 12 h postinfection and had prolonged survival times compared with the saline-treated control group (P < 0.0001). Our findings show a significant therapeutic benefit of Pn administration and suggest that its antimicrobial activity could open new avenues for fighting infections caused by multidrug-resistant neisserial or streptococcal strains.
Pneumococcal and meningococcal infectious diseases remain a serious threat to public health. Streptococcus pneumoniae is the leading cause of community-acquired pneumonia and a major cause of otitis media, septicemia, and meningitis (1, 2). S. pneumoniae is responsible for ∼1.2 million deaths per year worldwide, with young children and immunocompromised patients at particular risk (3). Neisseria meningitidis causes epidemic bacterial meningitis and septicemia, with high mortality in children and young adults (4). The impact of meningococcal disease on human health is defined by both the risk and the severity of invasive meningococcal infections, with unacceptably high mortality rates, ranging from 10% in patients under optimal clinical therapy with the latest generation of antibiotics to up to 40% in patients with untreated septicemia. Almost one-third of those who survive invasive infections are left with long-term disabilities and long-term morbidity. Globally, the World Health Organization estimates that ∼1.2 million cases of invasive meningococcal infections occur annually, leading to more than 135,000 fatalities (5).
Vaccination programs have reduced the rates of infection in developed countries, but neonates and elderly adults remain especially vulnerable (6, 7). The efficacy of vaccination is further limited by the emergence of new strains of S. pneumoniae and N. meningitidis.
The complement system plays a major role in the host resistance to both pathogens (8–13). Complement is activated via three routes: the classical pathway, the lectin pathway, and the alternative pathway. Activation of the classical and lectin pathways is mediated by specific recognition molecules. Binding of C1q to the bacterial surface or the Fc region of antibody initiates the classical pathway. The lectin pathway is initiated by carbohydrate recognition molecules, including mannan-binding lectin, ficolins, and collectin 11, which bind directly to bacterial polysaccharides. Activation of the classical or lectin pathway leads to the formation of a C3 convertase (C4b2a), which splits C3 into the biologically active fragments, C3b and C3a. C3b can bind covalently to an activating surface, and hundreds of molecules of C3b can be deposited in close proximity to the C3 convertase complex. Accumulation of C3b close to C4b2a forms the classical pathway C5 convertase C4b2a(3b)n, in which C4b and C3b form a binding site for C5, orienting it for cleavage by C2a (14, 15).
The mechanisms initiating the alternative pathway are less well understood. It is widely accepted that the alternative pathway maintains a continuous state of low-rate activation, which is held in check by potent negative regulators of activation on nonactivating surfaces, such as the surface of host cells. Turnover of the alternative pathway is initiated either by the provision of C3b via the classical pathway, the lectin pathway, or complement-independent proteolysis of C3 or by the spontaneous hydrolysis of C3 to form C3(H2O). C3b or C3(H2O) bind factor B to form either the C3bB or C3(H2O)B zymogen complex. In this complex, factor B is cleaved by factor D, releasing a Ba fragment. The activated C3bBb or C3(H2O)Bb fragments are themselves C3 convertases, which in turn cleave more C3 into C3a and C3b. Unchecked, the accumulation of C3b rapidly leads to the formation of more alternative pathway convertase complexes, resulting in a physiologically critical positive feedback mechanism—the amplification loop of complement activation (16). The alternative pathway thus amplifies complement activation initiated by any of the three pathways, making it an attractive target for therapeutic intervention designed to modulate complement-mediated immunity and/or inflammatory processes (17).
Deposition of C3b and iC3b on the bacterial surface is a key step in the immune response against S. pneumoniae, because complement-mediated opsonisation is essential for clearance of S. pneumoniae through phagocytosis (8). Lysis of bacteria, owing to formation of the membrane attack complex complex, is the critically important biological activity of complement in the defense against N. meningitidis (10). Inherited or acquired deficiencies of the alternative pathway are associated with a high risk of recurrent bacterial infection. Factor B deficiencies significantly increase the risk of S. pneumoniae and Pseudomonas aeruginosa infection (9, 18). In a mouse model of properdin deficiency, the severity of polymicrobial peritonitis was significantly greater in deficient mice compared with their WT littermates (19). Properdin deficiency in humans has been associated with a high risk of meningococcal infections, especially with unusual infective serotypes, such as W-135 and Y (10, 20, 21). In addition, opsonophagocytosis of S. pneumoniae was found to be severely compromised in properdin-deficient sera, and reconstitution of properdin-deficient sera with purified properdin restored the opsonic activity and killing of S. pneumoniae by polymorphonuclear leukocytes (22, 23).
Properdin is the only known positive physiological regulator of complement activation. It stabilizes and extends the half-life of the surface-bound C3 convertase C3bBb, and inhibits its degradation by factor I (24–26). In their pioneering 1954 work, Pillemer et al. (26) first described properdin as a serum protein that mediates complement activation and antimicrobial activity in absence of antibodies.
Properdin is present in serum at a concentration of ∼5–15 μg/mL (27). Unlike most other complement components, properdin is not synthesized in the liver but rather is expressed by other cells, including monocytes, T cells, mast cells, and granulocytes (19, 28–30). Properdin monomers can assemble into dimers (P2), trimers (P3), and tetramers (P4), formed by head-to-tail association of monomers (each ∼53 kDa) (31, 32). Properdin aggregates, so-called “activated” properdin (Pn), are considered artificial higher-order oligomers formed during the purification of properdin from plasma or during subsequent freeze–thaw cycles (33). The functional activity of properdin increases with the size of the polymers formed (34). By increasing the half-life of the alternative pathway C3 convertase, properdin antagonizes the functional activity of complement factor H, an abundantly expressed plasma component, which promotes inactivation of the alternative pathway C3 convertase and of all C5 convertases of complement by accelerating the decay of these enzyme complexes through binding to complex-bound C3b and by serving as a cofactor in the factor I-mediated conversion of C3b to its inactive form, termed iC3b (35). Interestingly, the two pathogens used in this study were previously shown to express distinct microbial surface components that sequester factor H from host plasma, leading to resistance to the complement-mediated immune clearance of these pathogens (36, 37).
In the present study, we addressed the role of the alternative pathway and the effect of administration of recombinant properdin as a tool for boosting alternative pathway activity to augment the immune response against S. pneumoniae or N. meningitidis.
Results
Recombinant Pn Enhances Alternative Pathway Activity.
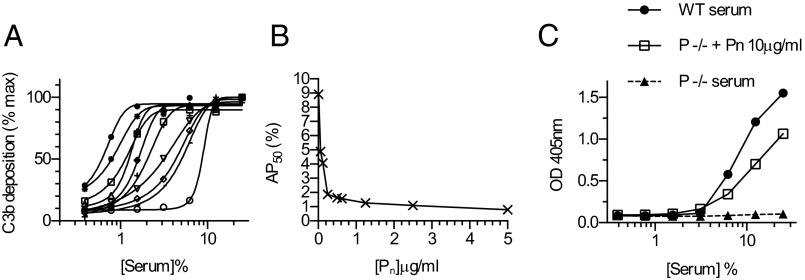
Properdin is normally found in the circulation as dimers, trimers, or tetramers of the basic 53-kDa glycopeptide. We found that recombinant properdin expressed in Chinese hamster ovary (CHO)-K1 cells contained mainly higher-order oligomers of a total molecular weight of ≥300 kDa (SI Appendix, Figs. S1 and S2). Highly polymerized recombinant Pn proved to be extremely effective at augmenting alternative pathway activation in vitro. As shown in Fig. 1 A and B, the addition of as little as 78 ng/mL of recombinant human Pn to normal human serum (NHS) was sufficient to halve the concentration of serum required for 50% maximal alternative pathway activation (AP50) from 9% to 4.5%. Given that the concentration of native properdin in 4.5% serum is ∼0.7 μg/mL, these results suggest that the Pn material is an order of magnitude more active than native properdin.
Fig. 1.
The addition of recombinant Pn allows the alternative pathway to work at serum concentrations beyond the dilution limit of NHS without added extrinsic properdin, and restores alternative pathway functional activity in properdin-deficient mouse serum. Alternative pathway functional activity was assayed by measuring C3b deposition on zymosan-coated microtiter plates using veronal buffer containing Mg2+ and EGTA. (A) Serial dilutions of human serum (0.39–20%) preincubated with recombinant human Pn at concentrations ranging from 0 to 10 μg/mL. Sigmoid curves were fitted to the data, and the AP50 was calculated for each Pn concentration. (B) AP50 values obtained from A, plotted as a function of Pn concentration. (C) Addition of excess recombinant murine Pn to serum from properdin-deficient (P−/−) mice restored C3b deposition to approximately the same level as seen in WT murine serum. Data are reported as means of duplicates and are representative of two independent experiments.
Recombinant mouse Pn (final concentration, 10 μg/mL) reconstituted alternative pathway C3b/iC3b deposition in properdin-deficient mouse serum (Fig. 1C).
We next measured C3b deposition on the surfaces of S. pneumoniae and N. menigitidis by FACS analysis, under conditions that permitted alternative pathway activation but excluded activation of the lectin and classical pathways, using barbital- buffered saline (BBS) with Mg2+ and EGTA. Little or no C3b deposition was observed using either 5% (vol/vol) human serum or 15% (vol/vol) murine serum (Fig. 2). The addition of 5 μg/mL human Pn (Fig. 2 A and C) or 10 μg/mL murine Pn (Fig. 2 B and D) to the sera led to major C3b or iC3b deposition on both species of bacteria. Taken together, these results indicate that the concentration of properdin in NHS and murine serum is a limiting factor in alternative pathway activation.
Fig. 2.
Properdin enhances C3 opsonisation of S. pneumoniae and N. meningitidis. Here 1 × 105 S. pneumoniae D39 or N. meningitis B-MC58 cells were opsonized with 5% NHS or 15% WT mouse serum, with and without the addition of 5 μg/mL recombinant Pn in veronal buffer containing Mg2+ and EGTA. C3b deposition was assayed by FACS analysis. Green represents bacteria opsonized with serum alone; red, bacteria opsonised with serum plus recombinant Pn; purple, unopsonised bacteria. (A) S. pneumoniae D39 with NHS with and without human Pn. (B) S. pneumoniae D39 with WT mouse serum with and without murine Pn. (C) N. meningitis B-MC58 with NHS with and without human Pn. (D) N. meningitis B-MC58 with WT mouse serum with and without murine Pn.
Recombinant Pn Enhances the Killing of N. meningitidis.
The contribution of the alternative pathway to the lysis of N. menigitidis is shown in Fig. 3. Enhanced killing of N. meningitidis B-MC58 was achieved when WT mouse serum or NHS was supplemented with 5 μg/mL mouse or human Pn.
Fig. 3.
Bactericidal activity of serum against N. meningitidis serogroup B. (A) 1 × 105 N. meningitidis B-MC58 cells were incubated with 20% NHS with and without the addition of 5 μg/mL recombinant Pn. Samples were obtained at the time points indicated, and viable counts were determined. Heat-inactivated NHS (HI) served as a negative control. (B) Results obtained using WT murine serum in an otherwise identical experiment. *P < 0.05; **P < 0.01, ANOVA.
To evaluate the therapeutic benefit of recombinant properdin in a murine model of N. meningitidis infection, age-matched WT female C57BL/6 mice were tested in three groups. Mice in the first group were given recombinant Pn i.p. at 6 h before infection, the second group was treated with recombinant Pn at 3 h after infection, and the third group was injected with saline only before infection. Mice were infected i.p. with a high dose (8 × 106 cfu/mouse) of N. meningitidis serogroup B, strain B-MC58 and monitored for 1 wk. Blood samples were obtained after 12 h to measure bacterial load. Mice were scored for signs of disease throughout the experiment.
At 36 h after infection, 90% of the mice pretreated with Pn survived, whereas none of the control mice survived (Fig. 4A). (The control mice had reached the severity endpoint of disease progression and were euthanized.) Surviving treated mice recovered completely at 48 h, with no signs of disease. Although mice treated with Pn at 3 h postinfection did not survive the infection, they had significantly longer survival times (P = 0.0007) compared with the control nontreated group (Fig. 4B). At 12 h postinfection, mice treated with Pn had significantly less bacteremia than nontreated controls (Fig. 4 C and D). During the early stage of infection at 6 h, 12 h, and 18 h, mice treated with Pn either before or after infection had better clinical scores compared with the nontreated control group (Fig. 4 E and F).
Fig. 4.
Pn treatment reduces the severity of N. meningitidis infection. Mice were infected i.p. with 8 × 106 cfu of N. meningitidis B-MC58, as described in Materials and Methods. Treated mice received 100 μg/animal of recombinant murine Pn at 6 h before (A, C, and E) or 3 h after (B, D, and F) infection. Control animals were injected with PBS. (A and B) Properdin treatment significantly increased the survival of mice in both treatment groups (Mantel–Cox log-rank test; n = 10/group). (C and D) Properdin treatment significantly reduced the bacterial load in tail blood samples collected at 12 h after infection. Data are mean ± SEM. **P < 0.01; ***P < 0.001, Student t test; n = 10/group. (E and F) Clinical scores for the infected mice. Data are mean ± SEM. *P < 0.05, Student t test.
Recombinant Pn Protects Mice from S. pneumoniae Infection.
To assess the extent to which recombinant properdin helps in the protection against S. pneumoniae infection, two groups of 10-wk-old C57BL/6 mice were infected with S. pneumoniae D39 via the intranasal route. One group of mice received 100 μg/mouse of recombinant Pn by i.p. injection at the time of infection, and the other group received saline only. Mice were monitored for the signs of disease development.
The treated mice had significantly longer survival times compared with the nontreated control group (P < 0.0001; Fig. 5A). At 36 h postinfection, 70% of the control mice had reached the severity endpoint of disease progression and were euthanized, whereas all mice in the Pn-treated group exhibited very few or no signs of disease at that time point. Interestingly, mice treated with Pn at the time of infection showed no bacteremia at 6 h and 12 h postinfection, whereas control mice clearly showed significantly higher bacterial loads at these time points. The treated mice developed bacteremia by 12 h postinfection (Fig. 5B). We also assessed the protective effect of Pn treatment when applied 6 h before intranasal infection with S. pneumoniae. Significantly reduced bacteremia was seen at 12 h and 24 h postinfection (SI Appendix, Fig. S3).
Fig. 5.
Properdin treatment reduces the severity of S. pneumoniae infection. Mice were infected intranasally with 2.5 × 106 cfu of S. pneumoniae D39. Animals in the treated group were injected i.p. with 100 μg of recombinant murine Pn at the time of infection. (A) The Pn-treated mice had significantly improved survival from S. pneumoniae infections (Mantel–Cox log-rank test; n = 10/group). (B) Viable S. pneumoniae counted in peripheral blood at the indicated time points after infection. Data are mean ± SEM; n = 10. *P < 0.05; ***P < 0.001, Student t test at each time point.
Discussion
A recent in vitro study showed that purified native properdin containing higher-order oligomers, an artifact arising during purification and/or storage, binds to the surface of both N. meningitidis and N. gonorrheae and increases the level of C3 deposition when the bacteria are exposed to serum (38). Other studies have shown a similar effect of higher-order properdin polymers on alternative pathway activity in vitro (39). Encouraged by these findings, we investigated the use of recombinant Pn as a bactericidal agent under in vitro and in vivo conditions.
Kemper et al. (40) postulated that properdin can even act as a pattern recognition molecule, binding to pathogen surfaces and to apoptotic and necrotic cells, where it initiates the formation of alternative pathway convertases. The molecular events that would allow properdin to specifically discriminate between activating and nonactivating surfaces remain unclear, however. More recent work has shown that properdin binding to zymosan and Escherichia coli does not occur in the absence of C3 deposition, indicating that the binding of properdin to pathogen surfaces is not the primary event initiating alternative pathway activation (41). Our results show that unfractionated recombinant Pn is a highly oligomerized material with significantly higher functional activity than the same amount of native properdin purified from either human or mouse serum. We found that Pn binds efficiently to the surface of N. meningitidis as well as to the surface of S. pneumoniae, and recruits the deposition of C3b and iC3b from serum through activation of the alternative pathway, whereas similar quantities of native purified serum properdin (dimers, trimers and tetramers) failed to bind to the surface of these pathogens in parallel experiments (SI Appendix, Figs. S1 and S2).
Alternative pathway functional activity is usually lost when serum is diluted to concentrations of <8%. Here we report that supplementation of highly diluted NHS or WT murine serum with Pn resulted in measurable alternative pathway activation at serum concentrations <1% and <3%, respectively, indicating that properdin concentration is a key limiting factor in the loss of alternative pathway functional activity in diluted serum (Fig. 1). Pn supplementation led to a corresponding increase in opsonization of both S. pneumoniae and N. meningitidis with C3b (Fig. 2) and serum bactericidal activity against N. meningitidis (Fig. 3).
Low-dose application of recombinant murine Pn at 6 h before infection with N. meningitidis proved to be highly effective, with an increase in survival rate from 0 to 90% and a correspondingly large decrease (5 log) in bacteremia. Application of Pn at 3 h after infection was less effective, but nonetheless led to significantly prolonged survival times and a 2-log drop in peripheral blood bacterial load (Fig. 4). Similarly, low-dose application of recombinant murine Pn in mice infected with a lethal dose of S. pneumoniae significantly decreased the bacterial load in blood during the first 12 h postinfection. Pn treatment significantly prolonged survival in these mice compared with the untreated control mice (Fig. 5).
A striking feature of our results is that Pn-mediated augmentation of alternative pathway activity supports two rather diverse complement-mediated clearance mechanisms. Whereas N. meningitidis is susceptible mainly to terminal pathway-mediated lysis (11). S. pneumoniae is resistant to complement lysis but is usually cleared by phagocytosis after complement-mediated opsonization (8).
Studying the effects of Pn administration on the clearance of other pathogens is an aspect of ongoing work in our laboratory. In general, the therapeutic application of Pn may be a useful adjunct to antibiotic therapy in the treatment of infections caused by N. meningitidis, S. pneumoniae, and possibly other pathogens, particularly when treating multidrug-resistant strains. In our experimental model of N. meningitidis infection, Pn administration was likely to increase bacterial lysis and thereby also the release of bacterial endotoxins; however, under no circumstances did Pn administration cause signs of an overwhelming endotoxin shock, and the Pn-treated animals exhibited a significant degree of protection during the course of infection compared with their sham-treated littermates. This was seen regardless whether Pn was given at 6 h before infection or at 3 h or 6 h after infection. Thus, we hypothesize that complement activation also may occur on the surface of the released bacterial endotoxins, neutralizing the detrimental effects of endotoxin by C3 deposition and clearance although complement-receptor bearing host cells (42). In patients with inherited properdin deficiencies, who are known to be at high risk for frequent and severe infections with N. meningitidis and S. pneumoniae (12, 23, 43, 44), the prophylactic administration of Pn might alleviate the predisposition to recurrent and severe infections.
Materials and Methods
Ethics Statement.
All animal experiments were authorized by the United Kingdom Home Office (Animals Scientific Procedures Act 1986; Home Office project license 80/2111) and approved by the University of Leicester’s Animal Welfare Committee. Every effort was made to minimize suffering, and mice were humanely euthanized as soon as they became lethargic during infection experiments.
Materials.
Unless stated otherwise, all reagents were obtained from Sigma-Aldrich.
Bacteria.
S. pneumoniae serotype 2, strain D39 was obtained from the National Collection of Type Cultures (NCTC 7466). N. meningitidis serotype B strain MC58 was kindly provided by Dr. C. Bayliss, University of Leicester, Leicester, UK. Bacteria were cultured and passaged through mice as described previously (45), then recovered and stored at −80 °C. As required, suspensions were thawed at room temperature, and bacteria were harvested by centrifugation before resuspension in sterile PBS.
Mice and Sera.
C57/BL6 female mice were purchased from Charles River Laboratories. Blood was obtained by cardiac puncture. Blood samples were left to clot on ice for 2 h, and serum was separated and stored at −80 °C. Human blood was obtained from healthy volunteers who had provided written, informed consent, as required by the local Ethics Committee. Serum was isolated and stored as described above. Properdin-deficient mouse serum was kindly provided by Dr. C. Stover, University of Leicester, Leicester, UK (19).
Recombinant Properdin.
The coding sequence of full-length murine and human properdin was amplified from mouse or human liver cDNA using the following primer pairs: mP_F, 5′-AAG CTT ATG CCT GCT GAA ATG CAA GCC C-3′ and mP_R, 5′-CTC GAG AGT AGG GTT TCT TCT CTT CTG GGT CTT T-3′, and hP_F, 5′-AAG CTT ATG ATC ACA GAG GGA GCG CAG-3′ and hP_R, 5′-CTC GAG AGT AGA GTT CCT CTT CCT CAG GGT CTT TGC A-3′.
These primers introduced restriction sites for HindIII and XhoI for subsequent cloning into pSecTag2/HygroB (Invitrogen) in frame with and upstream of nucleotides encoding a 6-histidine tag and stop codon. Reverse primers replaced the stop codon (TAA) of the murine properdin cDNA. CHO cells were transfected using GeneJuice reagent (Novagen) according to the manufacturer’s protocol. Stable transfected cell lines were generated by selection on Hygromycin B (300 μg/mL; Invitrogen).
CHO-K1 cells expressing human and murine properdin were adapted to grow in CHO serum-free II medium (Invitrogen) containing 10 U/mL penicillin, 10 μg/mL streptomycin (Gibco), and 300 μg/mL Hygromycin B (Invitrogen). After 72 h of incubation, medium was harvested, and cell debris was removed before purification by centrifugation. Recombinant properdin was purified by loading 500 mL of cell supernatant diluted with an equal volume of 2× loading buffer (0.1 M phosphate buffer, pH 7.4, containing 100 mM NaCl and 5 mM imidazole) onto a HisGravi Trap column (GE Healthcare) and allowing it to flow through the column by gravity. The column was then washed with 20 mL of wash buffer (0.1 M phosphate buffer, pH 7.4, containing 100 mM NaCl and 25 mM imidazole). Recombinant properdin was eluted in 1-mL fractions using elution buffer (0.1 M phosphate buffer, pH 7.4, containing 100 mM NaCl and 500 mM imidazole). Protein-containing fractions were identified by SDS/PAGE, followed by Western blot analysis.
Complement C3b Deposition Assay.
To measure C3b deposition, Nunc MaxiSorb microtiter plates were coated with 1 μg/well of mannan in coating buffer (15 mM Na2CO3 and 35 mM NaHCO3, pH 9.6). After overnight incubation, wells were blocked with 0.1% human serum albumin in Tris-buffered saline (TBS; 10 mM Tris⋅HCl and 140 mM NaCl, pH 7.4) and then washed with TBS containing 0.05% Tween 20. Human or mouse serum samples diluted in barbital-buffered saline (BBS)/EGTA/Mg+2 buffer were added to the plate, followed by a 1.5-h incubation at 37 °C. The plate was rewashed, and bound C3b was detected using rabbit anti-human C3c (Dako) that cross-reacts with mouse C3c, followed by alkaline phosphatase-conjugated goat anti-rabbit IgG and the colorimetric substrate pNPP. To study the effect of recombinant properdin on alternative pathway activity, different concentrations of recombinant proteins were added to diluted human or murine sera before incubation on the mannan-coated plate.
Serum Bactericidal Assay.
Killing of N. meningitidis B-MC58 by serum was estimated by measuring the decrease in viable count over time, as described previously with modifications (46). A frozen stock of N. meningitidis B-MC58 was resuspended in HBSS containing 1.2 mM Ca2+ and 1.2 mM Mg2+ (pH 7.4; Invitrogen) to a concentration of 106 cells/mL. N. meningitidis (105 cells) was then opsonized with mouse or human serum, with or without added recombinant mouse or human Pn, for 2 h at 37 °C in a final volume of 250 μL on a rotatory mixer. Samples were obtained at 0, 30, 60, and 120 min. To measure viable bacteria, samples were serially diluted in HBSS and plated onto brain heart infusion (BHI) agar supplemented with Levinthal’s broth (10%, vol/vol), followed by overnight incubation at 37 °C in 5% (vol/vol) CO2 (47).
FACS Analysis.
S. pneumoniae D39 and N. meningitidis B-MC58 samples were washed twice with TBS buffer and resuspended in BBS/EGTA/Mg+2 buffer to a final concentration of 106 cfu/mL. Bacterial suspension (100 µL) was opsonised with 5% (vol/vol) NHS or 15% (vol/vol) WT mouse serum for 1 h at 37 °C with or without recombinant Pn. Nonopsonized bacteria served as a negative control. After opsonization, the bacterial samples were washed twice with TBS buffer, and bound C3b was detected using FITC-conjugated rabbit anti-human C3c (Dako). Fluorescence intensity was measured with a FACSCalibur cell analyzer (BD Biosciences).
Infection Experiments.
These experiments were performed using 10-wk-old female C57BL/6 WT mice (Charles River Laboratory). For the S. pneumoniae infection model, mice were lightly anesthetized with 2.5% (vol/vol) fluothane (AstraZeneca) over oxygen (1.5–2 L/min), after which 50 μL of PBS containing 2.5 × 106 cfu of S. pneumoniae D39 was administered into the mouse nostrils. The mice were treated with recombinant murine Pn 100 μg/mouse i.p. at 0 h or at 0 h and 12 h postinfection. Control mice received saline only.
For the N. meningitidis infection experiment, mice were injected i.p. with iron dextran (400 mg/kg; Sigma-Aldrich) at 12 h before infection. The next day, 100 µL of passaged N. meningitidis B-MC58 suspension (8 × 106 cfu/mL) was coadministered with iron dextran (400 mg/kg). Recombinant murine Pn (100 μg/mouse) was injected i.p. at either 0 h or 3 h postinfection. Mice treated with PBS instead of recombinant Pn served as a control. The inoculum dose was confirmed by viable count after plating on blood agar in cases of S. pneumoniae D39 or on on BHI agar with 5% (vol/vol) Levanthal's supplement for cases of N. meningitidis. Mice were monitored for progression of clinical signs and euthanized when they became severely lethargic (48). Blood samples were obtained at predetermined time points, and viable counts were calculated after serial dilution in PBS and plating out in the corresponding plates as described above.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council UK (Grant G0801952) and The Medicines Company. W.J.S. is a Royal Society/Wolfson Foundation Research Merit Award holder; A.H. and K.S.H. were supported by a PhD studentship from Hazara University, Pakistan; B.M.S. was supported by a PhD studentship of the Kurdistan Regional Government, Iraq; and S.A. was supported by a studentship of Najran University, Saudi Arabia.
Footnotes
Conflict of interest statement: D.G. is an employee and shareholder of The Medicines Company. The University of Leicester and The Medicines Company hold patents covering the use of Pn. The intellectual property behind the Pn technology is protected by the international patent application PCT/US2012/053315.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401011111/-/DCSupplemental.
References
- 1.Kyaw MH, et al. Invasive pneumococcal disease in Scotland, 1999-2001: Use of record linkage to explore associations between patients and disease in relation to future vaccination policy. Clin Infect Dis. 2003;37(10):1283–1291. doi: 10.1086/379016. [DOI] [PubMed] [Google Scholar]
- 2.Miller E, et al. Epidemiology of invasive and other pneumococcal disease in children in England and Wales, 1996-1998. Acta Paediatr Suppl. 2000;89(435):11–16. doi: 10.1111/j.1651-2227.2000.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 3.Neill DR, et al. T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice. PLoS Pathog. 2012;8(4):e1002660. doi: 10.1371/journal.ppat.1002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibarz-Pavón AB, et al. Epidemiology, molecular characterization and antibiotic resistance of Neisseria meningitidis from patients ≤15 years in Manhiça, rural Mozambique. PLoS ONE. 2011;6(6):e19717. doi: 10.1371/journal.pone.0019717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Weekly Epidemiological Record No. 37. 2001 Available at http://www.who.int/docstore/wer/pdf/2001/wer7637.pdf. Accessed October 2010. [Google Scholar]
- 6.Boulton J. Meninigitis immunisation: Challenges, successes and new developments. Br J Nurs. 2013;22(1):20–25. doi: 10.12968/bjon.2013.22.1.20. [DOI] [PubMed] [Google Scholar]
- 7.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364(9431):365–367. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 8.Ali YM, et al. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 2012;8(7):e1002793. doi: 10.1371/journal.ppat.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JS, et al. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci USA. 2002;99(26):16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprong T, et al. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood. 2006;107(12):4865–4870. doi: 10.1182/blood-2005-07-2820. [DOI] [PubMed] [Google Scholar]
- 11.Kugelberg E, Gollan B, Tang CM. Mechanisms in Neisseria meningitidis for resistance against complement-mediated killing. Vaccine. 2008;26(Suppl 8):I34–I39. doi: 10.1016/j.vaccine.2008.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Späth PJ, et al. Properdin deficiency in a large Swiss family: Identification of a stop codon in the properdin gene, and association of meningococcal disease with lack of the IgG2 allotype marker G2m(n) Clin Exp Immunol. 1999;118(2):278–284. doi: 10.1046/j.1365-2249.1999.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linton SM, Morgan BP. Properdin deficiency and meningococcal disease—identifying those most at risk. Clin Exp Immunol. 1999;118(2):189–191. doi: 10.1046/j.1365-2249.1999.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arlaud GJ, et al. Structural biology of the C1 complex of complement unveils the mechanisms of its activation and proteolytic activity. Mol Immunol. 2002;39(7-8):383–394. doi: 10.1016/s0161-5890(02)00143-8. [DOI] [PubMed] [Google Scholar]
- 15.Carroll MV, Sim RB. Complement in health and disease. Adv Drug Deliv Rev. 2011;63(12):965–975. doi: 10.1016/j.addr.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Lachmann PJ. The amplification loop of the complement pathways. Adv Immunol. 2009;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]
- 17.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176(3):1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 18.Mueller-Ortiz SL, Drouin SM, Wetsel RA. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect Immun. 2004;72(5):2899–2906. doi: 10.1128/IAI.72.5.2899-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stover CM, et al. Properdin plays a protective role in polymicrobial septic peritonitis. J Immunol. 2008;180(5):3313–3318. doi: 10.4049/jimmunol.180.5.3313. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4(3):359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Söderström C, Braconier JH, Danielsson D, Sjöholm AG. Bactericidal activity for Neisseria meningitidis in properdin-deficient sera. J Infect Dis. 1987;156(1):107–112. doi: 10.1093/infdis/156.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Braconier JH, Odeberg H, Sjöholm AG. Granulocyte phagocytosis of Streptococcus pneumoniae in properdin-deficient serum. Infect Immun. 1983;40(1):219–224. doi: 10.1128/iai.40.1.219-224.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schejbel L, Rosenfeldt V, Marquart H, Valerius NH, Garred P. Properdin deficiency associated with recurrent otitis media and pneumonia, and identification of male carrier with Klinefelter syndrome. Clin Immunol. 2009;131(3):456–462. doi: 10.1016/j.clim.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Farries TC, Lachmann PJ, Harrison RA. Analysis of the interaction between properdin and factor B, components of the alternative-pathway C3 convertase of complement. Biochem J. 1988;253(3):667–675. doi: 10.1042/bj2530667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon DT, Austen KF. Properdin: Binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillemer L, et al. The properdin system and immunity, I: Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 27.Schwaeble WJ, Reid KB. Does properdin crosslink the cellular and the humoral immune response? Immunol Today. 1999;20(1):17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 28.Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med. 1980;151(3):501–516. doi: 10.1084/jem.151.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirthmueller U, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158(9):4444–4451. [PubMed] [Google Scholar]
- 30.Schwaeble W, et al. Properdin, a positive regulator of complement activation, is expressed in human T cell lines and peripheral blood T cells. J Immunol. 1993;151(5):2521–2528. [PubMed] [Google Scholar]
- 31.Smith CA, Pangburn MK, Vogel CW, Müller-Eberhard HJ. Molecular architecture of human properdin, a positive regulator of the alternative pathway of complement. J Biol Chem. 1984;259(7):4582–4588. [PubMed] [Google Scholar]
- 32.Pangburn MK. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J Immunol. 1989;142(1):202–207. [PubMed] [Google Scholar]
- 33.Ferreira VP, Cortes C, Pangburn MK. Native polymeric forms of properdin selectively bind to targets and promote activation of the alternative pathway of complement. Immunobiology. 2010;215(11):932–940. doi: 10.1016/j.imbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JM, Wiedemann H, Timpl R, Reid KB. Characterization of mutant forms of recombinant human properdin lacking single thrombospondin type I repeats: Identification of modules important for function. J Immunol. 1995;155(12):5777–5785. [PubMed] [Google Scholar]
- 35.Schwaeble W, et al. Human complement factor H: Expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 1987;17(10):1485–1489. doi: 10.1002/eji.1830171015. [DOI] [PubMed] [Google Scholar]
- 36.Malito E, et al. Defining a protective epitope on factor H binding protein, a key meningococcal virulence factor and vaccine antigen. Proc Natl Acad Sci USA. 2013;110(9):3304–3309. doi: 10.1073/pnas.1222845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meri T, et al. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 2013;9(4):e1003308. doi: 10.1371/journal.ppat.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal S, et al. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J Immunol. 2010;185(1):507–516. doi: 10.4049/jimmunol.0903598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farries TC, Finch JT, Lachmann PJ, Harrison RA. Resolution and analysis of “native” and “activated” properdin. Biochem J. 1987;243(2):507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci USA. 2008;105(26):9023–9028. doi: 10.1073/pnas.0801015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harboe M, et al. The role of properdin in zymosan- and Escherichia coli-induced complement activation. J Immunol. 2012;189(5):2606–2613. doi: 10.4049/jimmunol.1200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali YM, et al. Human L-ficolin, a recognition molecule of the lectin activation pathway of complement, activates complement by binding to pneumolysin, the major toxin of Streptococcus pneumoniae. PLoS ONE. 2013;8(12):e82583. doi: 10.1371/journal.pone.0082583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Densen P, Weiler JM, Griffiss JM, Hoffmann LG. Familial properdin deficiency and fatal meningococcemia: Correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316(15):922–926. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen HE, Koch C, Magnussen P, Lind I. Complement deficiencies in selected groups of patients with meningococcal disease. Scand J Infect Dis. 1989;21(4):389–396. doi: 10.3109/00365548909167442. [DOI] [PubMed] [Google Scholar]
- 45.Kadioglu A, et al. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68(2):492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estabrook MM, Griffiss JM, Jarvis GA. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65(11):4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayliss CD, et al. Neisseria meningitidis escape from the bactericidal activity of a monoclonal antibody is mediated by phase variation of lgtG and enhanced by a mutator phenotype. Infect Immun. 2008;76(11):5038–5048. doi: 10.1128/IAI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wölbeling F, et al. Lung function and inflammation during murine Pseudomonas aeruginosa airway infection. Immunobiology. 2011;216(8):901–908. doi: 10.1016/j.imbio.2011.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.