Significance
Embryonal rhabdomyosarcoma (ERMS) is a cancer of skeletal muscle and is one of the most common pediatric cancers of soft tissue. There is no effective treatment for patients with relapsed ERMS, with less than 50% surviving the disease. The self-renewing and molecularly defined tumor propagating cells (TPCs) drive continued tumor growth and relapse. Yet to date, drugs targeting ERMS self-renewal and differentiation of TPCs have not been identified. Our study describes a large-scale chemical screen to identify targetable pathways essential for modulating self-renewal and differentiation of ERMS and demonstrates the feasibility of inducing differentiation of TPCs in ERMS by small molecules.
Abstract
Embryonal rhabdomyosarcoma (ERMS) is a common pediatric malignancy of muscle, with relapse being the major clinical challenge. Self-renewing tumor-propagating cells (TPCs) drive cancer relapse and are confined to a molecularly definable subset of ERMS cells. To identify drugs that suppress ERMS self-renewal and induce differentiation of TPCs, a large-scale chemical screen was completed. Glycogen synthase kinase 3 (GSK3) inhibitors were identified as potent suppressors of ERMS growth through inhibiting proliferation and inducing terminal differentiation of TPCs into myosin-expressing cells. In support of GSK3 inhibitors functioning through activation of the canonical WNT/β-catenin pathway, recombinant WNT3A and stabilized β-catenin also enhanced terminal differentiation of human ERMS cells. Treatment of ERMS-bearing zebrafish with GSK3 inhibitors activated the WNT/β-catenin pathway, resulting in suppressed ERMS growth, depleted TPCs, and diminished self-renewal capacity in vivo. Activation of the canonical WNT/β-catenin pathway also significantly reduced self-renewal of human ERMS, indicating a conserved function for this pathway in modulating ERMS self-renewal. In total, we have identified an unconventional tumor suppressive role for the canonical WNT/β-catenin pathway in regulating self-renewal of ERMS and revealed therapeutic strategies to target differentiation of TPCs in ERMS.
Tumor-propagating cells (TPCs) have the capacity for self-renewal, sustain tumor growth, and initiate relapse disease. They also differentiate to give rise to all cell types contained within the tumor. Molecularly defined TPCs have been identified in a variety of cancers, such as acute myeloid leukemia, breast, colon, brain, and prostate cancers (1–5), confirming that sustained tumor growth is driven by TPCs in a large fraction of cancers. Drugs that deplete TPCs by inhibiting self-renewal and inducing differentiation have become a promising therapy for a subset of human cancers. For example, acute promyelocytic leukemia (APL) was nearly universally lethal before the introduction of all-trans-retinoic acid-induced differentiation therapy, which now has cure rates approximating 80% (6). Differentiation therapy in solid tumors has garnered renewed interest over the past decade (7–10), yet description of these approaches in sarcomas is only now being appreciated. In two studies, peroxisome proliferator-activated receptor γ agonists were able to induce differentiation in a subset of patients with liposarcoma and myxoid liposarcoma, respectively (11, 12), suggesting that differentiation therapy will be possible in sarcoma. However, a role for these drugs in specifically suppressing self-renewal and inducing differentiation within the TPC subpopulation has not been reported.
Embryonal rhabdomyosarcoma (ERMS) is a common soft tissue malignancy of childhood, with tumor cells being arrested in early stages of muscle differentiation. The prognosis for relapse—driven by retention of self-renewing TPCs after treatment—remains dismal, with 50% of patients succumbing to their disease. Using a zebrafish transgenic model of ERMS where an activated form of Kirsten rat sarcoma viral oncogene (K-RAS) is expressed in early muscle cells, we have identified a molecularly defined population of cells that drive continued tumor growth (13, 14). These TPCs express muscle stem cell markers, including cMet, m-cadherin, and myogenic factor 5 (myf5), and exhibit a conserved gene expression profile found in human and mouse activated satellite cells. RAS is the dominant oncogenic pathway in human ERMS (14, 15), making our transgenic model useful for understanding important aspects of human disease. Importantly, the zebrafish ERMS model also shows morphologic features resembling the human counterpart, expresses clinical diagnostic markers, and shares common molecular pathways with human and mouse disease as assessed by expression profiling, array CGH studies, and cross-species functional analysis (14, 16). Building on these findings and the use of the zebrafish ERMS model for in vivo drug discovery (17), novel therapies that modulate TPC function and/or induce differentiation will likely provide new treatment options for relapsed or metastatic ERMS.
Here we report the completion of a large-scale chemical genetic screen to identify drugs that induce differentiation of human ERMS and suppress tumor growth in vivo by modulating the frequency of the TPCs. Glycogen synthase kinase 3 (GSK3) inhibitors emerged as a promising class of lead compounds. These inhibitors activate the canonical WNT pathway by stabilizing β-catenin, resulting in reduced tumor growth, differentiation of TPCs, and significant reduction in self-renewal capacity in vivo. Because canonical WNT/β-catenin signaling is essential for transition from muscle stem cell proliferation to myogenic differentiation during regeneration (18), our findings suggest that the same developmental pathways that regulate muscle stem cell self-renewal also contribute to tumorigenesis in ERMS. In total, our work has identified the unconventional role of the canonical WNT/β-catenin pathway in regulating differentiation and self-renewal of ERMS. Moreover, our work has demonstrated the feasibility of using small-molecule inhibitors to induce differentiation of TPCs in ERMS, representing a potential therapeutic strategy for the treatment of primary and relapsed ERMS.
Results
A Large-Scale Chemical Screen Identifies Compounds That Induce Terminal Differentiation of Human ERMS Cells.
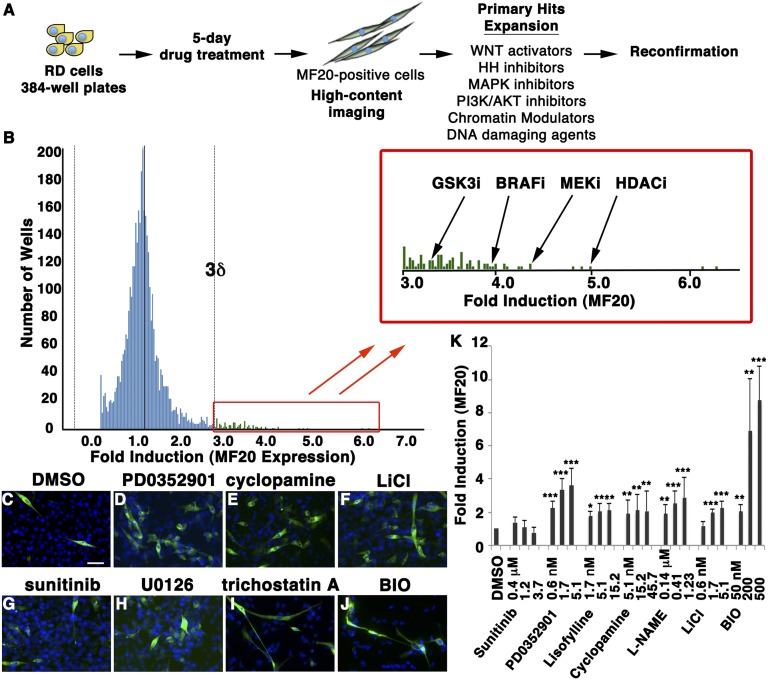
A high-content imaging assay was developed to assess drug effects on the differentiation of human ERMS RD cells into non–self-renewing, terminally differentiated, myosin-expressing cells (Fig. 1A). Using this assay, a large-scale chemical screen was completed using ∼40,000 compounds from the Genomics Institute of Novartis Foundation and Massachusetts General Hospital compound collection, including 4,574 biologically active drugs and 47% of US Food and Drug Administration-approved compounds (Fig. 1B). After 5 d of drug treatment, cells were assessed for myosin heavy chain (MF20) expression using a high-content imaging platform. Hits were defined as exhibiting ≥2.5-fold increase in the number of MF20-positive cells compared with the mean for all chemicals assessed (3 SDs above background). With this threshold, 91 hits were identified (∼0.2% hit rate) with a Z’ score of 0.52. Hit compounds predominantly represent six classes of drugs, including inhibitors of GSK3, Raf/MEK protein kinase, PI3-kinase/AKT protein kinase, Hedgehog pathway, and histone deacetylases (HDACs), as well as DNA-damaging agents (representative images shown in Fig. 1 C–J). Twelve compounds representative of these six classes of drugs induced MF20 expression in reconfirmation assays (Fig. 1K).
Fig. 1.
A large-scale, high-content imaging screen identifies compounds that induce differentiation of human ERMS cells. (A) Schematic of the experimental design. (B) Results for the differentiation screen. Lead compounds that increase the frequency of MF20-expressing RD cells are shown in the magnified box (>3δ). (C–J) Representative images of the MF20 (green) and DAPI (blue) staining in drug-treated cells. Control (DMSO, C) and a negative control compound (sunitinib, G) are shown. Blue, DAPI staining. (K) Quantification of MF20 induction in treated RD cells. Error bars denote ±1 SD. *P < 0.005; **P < 0.001; ***P < 0.0005.
A Secondary Transplant Screen Identifies Drugs That Inhibit ERMS Growth in Vivo.
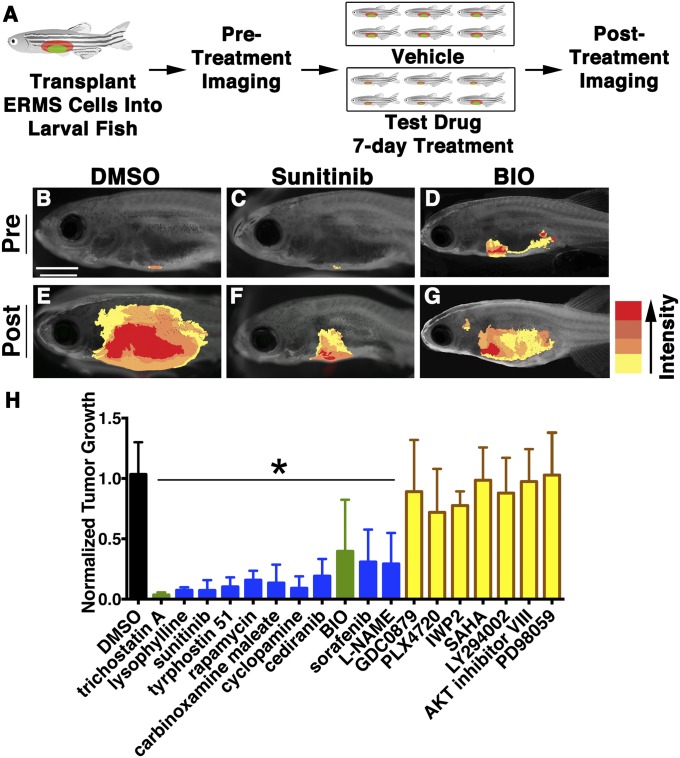
The confirmed hits identified in the human differentiation screen (12 compounds) were combined with the top hits from a zebrafish embryonic screen (83 compounds) that identified compounds with anti-RAS activity (17). This panel of 95 drugs was assessed for their effect on growth in transplanted zebrafish RAS-induced ERMS (schematic in Fig. 2A; ∼2,000 fish transplanted for the screen). Compounds that inhibited tumor growth by 50% in ≥50% of surviving animals were considered hits (Fig. 2 B–H, Fig. S1A, and Table S1). Standard t test analysis verified that the reduction in tumor volume was significant between the drug- and control-treated groups (P < 0.05). Thirteen compounds reduced ERMS tumor growth in vivo, of which 11 (9 anti-RAS compounds and 2 from the human differentiation screen) suppressed tumor growth in a larger cohort of transplant animals derived from independently arising ERMS (Fig. S1 B–E, P < 0.05), confirming the high stringency of our screen in identifying reproducible candidate lead compounds that suppress ERMS growth.
Fig. 2.
A secondary screen identifies lead compounds that suppress ERMS tumor growth in live zebrafish. (A) Schematic of the secondary screen completed in zebrafish transplanted with fluorescent-labeled ERMS. (B–G) Pretreatment images for DMSO (B), sunitinib (C), and BIO (D), with corresponding posttreatment images (E, F, and G, respectively). Tumor volume is indicated by the heat map (Right). (Scale bar, 2 mm.) (H) Summary of tumor volume changes in animals treated with compounds that inhibit RAS-signaling in embryonic zebrafish (blue), representative compounds of major classes of hits identified in the human differentiation screen (yellow), or common hits from both screens (green). Error bars equal SD. *Statistical significance by Student t test, with P < 0.05.
Each hit was assessed for effects on various aspects of tumorigenesis, including proliferation, apoptosis, and neovascularization (Fig. S2). Trichostatin A (an HDAC inhibitor) and 6-bromoindirubin-3′-oxime (BIO, a GSK3 inhibitor), were the only hits that induced RD cell differentiation and reduced tumor growth in vivo (Fig. 2H), each exerting additional effects on apoptosis and proliferation, respectively (Fig. S2B). Although the effect of HDAC inhibitors on tumor suppression has been shown on a panel of human RMS cell lines (19, 20), the impact of GSK3 inhibitors in ERMS tumor growth, self-renewal, and differentiation remains to be fully described. Thus, we focused on further functional characterization of BIO and GSK3 inhibitors in regulating ERMS growth.
GSK3 Inhibitors Activate the WNT/β-Catenin Pathway to Stimulate Differentiation of Human ERMS Cells.
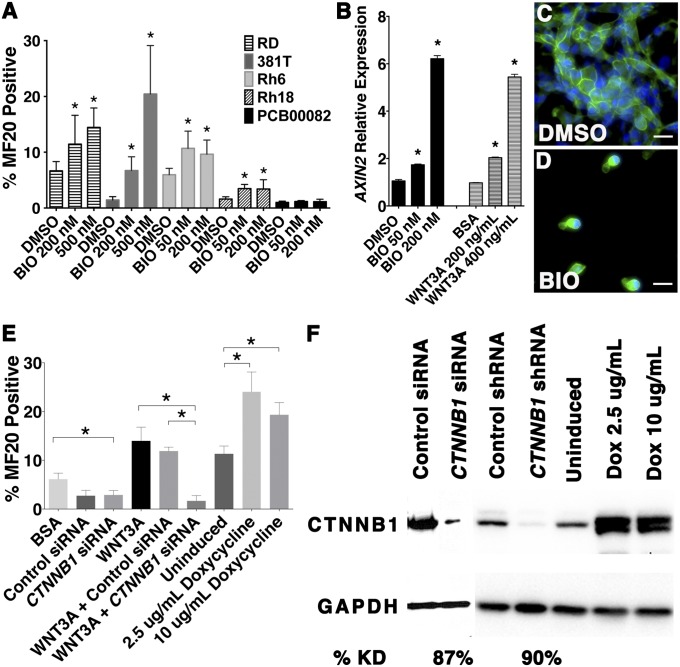
To assess the broad utility of GSK3 inhibitors to induce differentiation of RMS cells, BIO was assessed for differentiation effects in a panel of human ERMS and alveolar rhabdomyosarcoma (ARMS) cell lines. Each cell line was treated with DMSO control or BIO for 72 h under differentiating culture conditions, and the frequency of MF20-stained cells was assessed. BIO treatment significantly increased the number of MF20-positive cells in four of five ERMS cell lines tested (RD, 381T, Rh6, and Rh18; Fig. 3A). Two additional GSK3 inhibitors, CHIR 98014 and CHIR 99021, were able to induce differentiation in RD and 381T cells (P < 0.05; Fig. S3A), validating that induced differentiation can be generalized to GSK3 inhibitors as a class of compounds. By contrast, BIO did not induce differentiation of human ARMS cells (Rh3, Rh5, Rh7, and Rh30; Fig. S3B), suggesting that the differentiation effects of GSK3 inhibitors are likely specific to ERMS.
Fig. 3.
Activation of canonical WNT/β-catenin pathway induces differentiation of human ERMS cells. (A) Graphic summary of MF20-positive cells in human ERMS treated with DMSO or BIO. *Statistically significant differences between experimental and control treated cells (P < 0.05). (B) AXIN2 expression assessed by real-time RT-PCR. (C and D) β-Catenin staining (green) in the presence of DMSO (C) or BIO (D). Blue, DAPI. (Scale bars, 50 μM.) (E) Quantification of MF20-positive cells in human ERMS treated with recombinant WNT3a, CTNNB1 siRNA, and doxycycline-inducible CTNNB1S33Y. *Significant difference for bracketed comparisons (P < 0.05, t test). (F) Western blot analysis.
BIO, CHIR 98014, and CHIR 99021 activate the canonical WNT pathway through decreased phosphorylation and subsequent stabilization of β-catenin (21, 22). However, inhibition of GSK3 can also lead to activation of multiple downstream pathways, including mammalian target of rapamycin (mTOR)-mediated signaling, independent of β-catenin (23). BIO treatment of RD cells paradoxically resulted in decreased mTOR signaling as assessed by phosphorylation of S6 kinase (Fig. S3C). In addition, treatment of RD cells with rapamycin, an mTOR inhibitor, at doses that reduced phosphorylation of S6 kinase, did not affect the differentiation status of the tumor cells (Fig. S3D). Thus, GSK3 inhibitors are not acting through suppression of the mTOR signaling axis. By contrast, both BIO and WNT3A induced expression of AXIN2 (Fig. 3B, Fig. S3E, and Table S2) and nuclear localization of β-catenin—both of which are hallmarks of canonical WNT/β-catenin pathway activation (Fig. 3 C and D). BIO also activated T-cell factor/lymphoid enhancer factor (TCF/LEF) reporter expression in a dose-dependent manner, mimicking the effect of WNT3A ligand (Fig. S3F). These data show that BIO activates canonical WNT/β-catenin signaling rather than stimulating the mTORC1 pathway.
To validate the effect of canonical WNT/β-catenin signaling on inducing differentiation of human ERMS cells, RD and 381T cell lines were used in canonical WNT/ β-catenin gain-of-function and loss-of-function studies. Recombinant WNT3A treatment resulted in a significant increase in MF20-positive cells after 72 h (Fig. 3 E and F and Fig. S4 A and B; P = 0.0002), which could be reversed by β-catenin (CTNNB1) siRNA knockdown (Fig. 3 E and F and Fig. S4 C, D, G, and I). To assess gain-of-function phenotypes, stable RD and 381T cell lines were generated that express a doxycycline-inducible stabilized, constitutively active form of β-catenin (CTNNB1S33Y). Upon induction by doxycycline, ERMS cells expressing CTNNB1S33Y significantly increased the number of MF20-positive cells compared with uninduced control cells (Fig. 3 E and F and Fig. S4; P < 0.0001). Taken together, our data show that GSK3 inhibitors stimulate the WNT/β-catenin signaling pathway and are sufficient to induce differentiation in a large subset of human ERMS.
Canonical WNT/β-Catenin Pathway Activation Depletes Self-Renewing TPCs in Zebrafish ERMS.
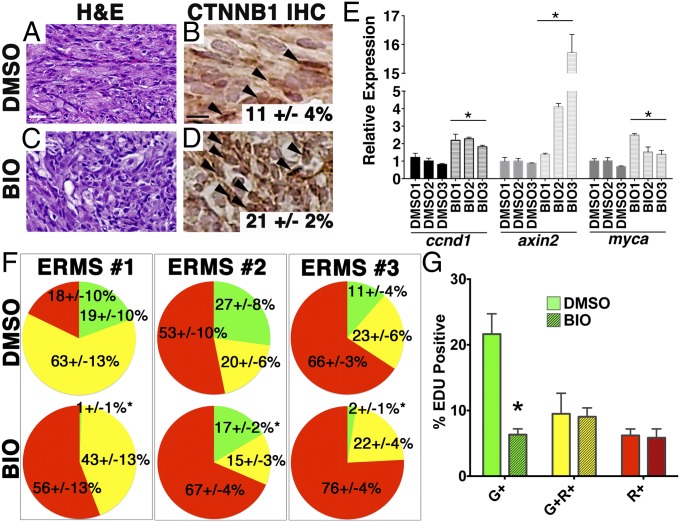
We hypothesized that activation of the canonical WNT/β-catenin pathway could result in either depletion of the preexisting TPCs by driving the cells into differentiation and/or specifically killing the TPCs. To distinguish between the two possibilities, we took advantage of our myf5:GFP/mylz2:mCherry transgenic zebrafish line to label distinct subpopulations of ERMS cells according to their differentiation status in vivo. Our previous studies have shown that the myf5:GFP+/mylz2:mCherry− TPC population exclusively retains self-renewal capacity, whereas late-differentiating myf5:GFP−/mylz2:mCherry+ tumor cells lack both the capacity to self-renew and to metastasize (13, 14). After 5-d drug treatment of animals engrafted with myf5:GFP/mylz2:mCherry expressing ERMS, relative proportions of ERMS cell subpopulations were assessed by FACS (n = 3 independent primary tumors). BIO- and CHIR99021-treated fish had reduced tumor growth (Fig. S5 A and B) and exhibited canonical WNT pathway activation as assessed by nuclear localization of β-catenin (Fig. 4 A–D and Fig. S5 C–F). Drug treatment also increased expression of downstream target genes, including axin2, ccnd1, and myca (Fig. 4E, Fig. S5J and Table S2). Both BIO and CHIR99021 treatment resulted in significant depletion in the myf5:GFP+/mylz2:mCherry− TPCs and a concomitant expansion of the myf5:GFP−/mylz2:mCherry+ late-differentiating ERMS cells (Fig. 4F and Fig. S5 H and I), validating the broad utility of GSK3 inhibitors in inducing differentiation of TPCs in vivo. The expansion of the late-differentiating ERMS cells was further validated by quantification of MF20-expressing cells (P = 0.002) (Fig. S6 A and B) and quantitative RT-PCR for myosin subunits (mylz2 and mhc6) (Figs. S5J and S6C). Importantly, BIO- and CHIR99021-treated ERMS also exhibited a significant reduction in the overall tumor proliferation rate by phospho-H3 staining (Figs. S5 K and L and S6 D and E). The reduction in 5-ethynyl-2′-deoxyuridine (EdU) incorporation was largely confined to the myf5:GFP+/mylz2:mCherry− TPCs (P < 0.05; Fig. 4G and Figs. S5 M–W and S6 F–O). GSK3 inhibitors did not induce apoptosis of tumor cells regardless of differentiation status (P = 0.9; Fig. S6 P and Q), suggesting that GSK3 inhibitors specifically suppress proliferation and induce differentiation of the TPCs rather than kill the TPCs.
Fig. 4.
BIO activates canonical WNT/β-catenin signaling and induces differentiation of ERMS cells in vivo. (A–D) H&E-stained sections (A and C) and immunohistochemistry for β-catenin (B and D) of ERMS-bearing fish after 7 d of drug treatment with BIO (300 nM) or DMSO (vehicle control). (Scale bar, 25 μM.) (E) Quantitative RT-PCR analysis. (F) Pie charts showing the relative percentage of myf5:GFP+/mylz2:mCherry− TPCs (green), myf5:GFP+/mylz2:mCherry+ (yellow), and late-differentiating myf5:GFP−/mylz2:mCherry+ cells (red) for three independent ERMS as assessed by FACS (n > 3 animals per experimental arm and ±SD). *Statistically significant differences between DMSO and BIO treatments (Student t test, P < 0.05). (G) Summary of EDU staining of DMSO- or BIO-treated tumors. Each error bar indicates SEM of three tumors from each treatment group. *P < 0.005, Student t test.
To directly assess whether GSK3 inhibitors would reduce the overall self-renewal potential of ERMS in vivo, zebrafish ERMS cells were isolated by FACS and transplanted into syngeneic larval fish at limiting dilution. Transplanted fish were treated with either DMSO or BIO for 5 d. Tumor engraftment was assessed every 10 d until 120 d after treatment. Remarkably, short-term BIO treatment significantly decreased TPC frequency by ≥fourfold in animals transplanted with either bulk tumor cells or sorted myf5:GFP+/mylz2:mCherry− TPCs (Table 1; n = 3 ERMS), validating that BIO treatment directly depletes the pool of self-renewing TPCs by inducing their differentiation.
Table 1.
Summary of limiting dilution transplantation experiments
| Cell no. | ERMS #1 (bulk tumor) | ERMS #2 (bulk tumor) | ERMS #3 (myf5-GFP sorted) | |||
| DMSO | BIO | DMSO | BIO | DMSO | BIO | |
| 104 | 6 of 11 | 3 of 11 | 7 of 8 | 9 of 11 | NA | NA |
| 103 | 2 of 10 | 0 of 12 | 16 of 17 | 9 of 19 | 3 of 5 | 1 of 5 |
| 102 | 1 of 11 | 0 of 13 | 9 of 20 | 2 of 14 | 1 of 5 | 0 of 5 |
| 10 | NA | NA | NA | NA | 2 of 10 | 0 of 10 |
| TPC | 1 in 9,425 | 1 in 35,868* | 1 in 753 | 1 in 2,947† | 1 in 608 | 1 in 5,084‡ |
| 95% CI | 4,646–19,121 | 11,624–110,687 | 440–1,289 | 1,600–5,430 | 233–1,589 | 722–35,843 |
CI, confidence interval; NA, not applicable.
P = 0.03.
P < 0.0001.
P = 0.02.
WNT/β-Catenin Activation Reduces Self-Renewal of Human ERMS.
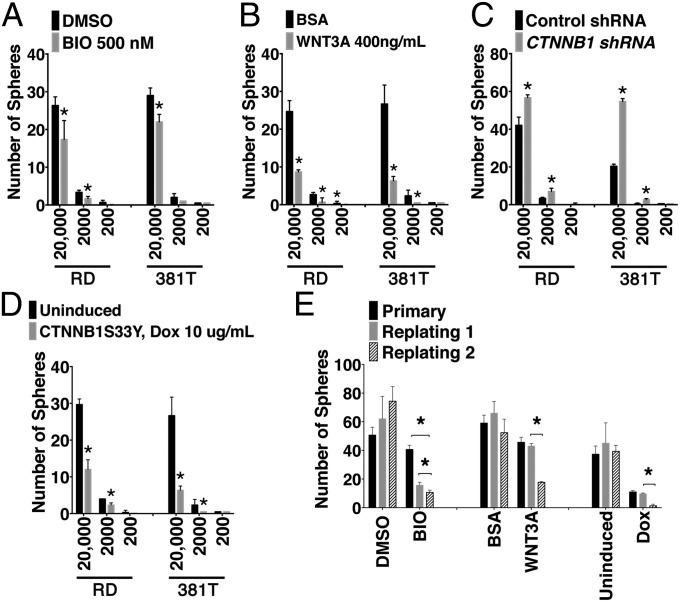
Sphere-forming assays are widely used as an in vitro measure of tumor cell self-renewal (24) and have been recently used to assess self-renewal in human ERMS cell lines (25). To assess the effect of activating canonical WNT/ β-catenin pathway on the rhabdosphere forming potential, RD and 381T cells were seeded at limiting dilution and treated with BIO and WNT3A. BIO and WNT3A treatment resulted in stabilization of β-catenin (Fig. S7A) and activation of canonical WNT pathway target gene, AXIN2 (Fig. S7B). Treated cells exhibited significantly decreased sphere-forming potential (Fig. 5 A and B). Both WNT3A and BIO treatment resulted in progressive decline in sphere-forming capacity upon serial replating (P < 0.05; Fig. 5E), showing that WNT/β-catenin pathway activation resulted in suppression of long-term self-renewal capacity. Human RD and 381T cells that stably express CTNNB1S33Y exhibited both elevated WNT/β-catenin pathway activation and reduced self-renewal potential after serial passaging (Fig. 5 D and E and Fig. S7A). By contrast, shRNA knockdown of β-catenin (CTNNB1) significantly reduced protein expression but increased sphere-forming potential (Fig. 5C and Fig. S7A). Together, our data confirmed that GSK3 inhibitors and WNT/β-catenin pathway activation suppress self-renewal in human ERMS.
Fig. 5.
Activation of canonical WNT/β-catenin pathway reduces self-renewal in human ERMS cells. (A–D) Quantitation of spheres in RD cells plated at limiting dilution in the presence of DMSO and BIO (A), BSA, and WNT3A (B). RD cells were also engineered to stably express control shRNA and CTNNB1-specific shRNA (C) and inducible CTNN1S33Y in the absence or presence of 10 μg/mL doxycycline treatment (D). *P < 0.05, Student t test. Each error bars denote ±1 SD. (E) Summary of serial replating self-renewal assays in the presence of BIO and WNT3A (Left) and of spheres expressing CTNNB1S33Y (Right).
Discussion
Activated WNT/β-catenin signaling has been predominantly viewed as an oncogenic driver in cancer, best demonstrated in colorectal cancer, where inactivating mutations in the adenomatous polyposis coli (APC) gene resulted in constitutive transcriptional activation of β-catenin/TCF complex (26). Aberrant WNT/β-catenin has also been shown in a variety of solid tumors, such as breast, pancreas, ovary, stomach, and prostate, as well as hematologic malignancies (27). However, recent studies have suggested that canonical WNT/β-catenin signaling can also elicit tumor-suppressive effects within a subset of cancers. For example, activation of WNT/β-catenin signaling suppressed melanoma tumor cell growth in mouse xenografts and was associated with increased survival in a subset of medulloblastoma, prostate, and ovarian cancers (8, 27). Moreover, WNT/β-catenin activation also induces differentiation of melanoma and glioblastoma cells in vitro (8, 28). In ERMS, APC mutation and nuclear β-catenin localization are rather uncommon, suggesting that this pathway is inactive in most ERMS (29). This finding is further supported by work in the mouse p53−/− Fos−/− ERMS model, in which the canonical WNT/β-catenin pathway was actively suppressed by DKK1 and sFRP4 (30). Despite the active suppression of WNT/β-catenin pathway in RMS, Annavarapu et al. (31) recently showed that human ERMS and ARMS cell lines are responsive to exogenous WNT3A, inducing stabilization of β-catenin, altering proliferation, and enhancing differentiation in a subset of RMS cell lines. However, these studies failed to identify a role for the WNT/β-catenin pathway in modulating ERMS self-renewal or in suppression of tumor growth in vivo. Our studies confirm that ERMS cells retain an intact canonical WNT/β-catenin signaling axis, with forced activation resulting in both reduced tumor growth and decreased self-renewal by inducing myogenic differentiation of TPCs.
GSK3 inhibitors and drugs that enhance canonical WNT/β-catenin pathway will likely provide therapeutic approaches for the treatment of ERMS. GSK3 inhibitors have rarely been considered as a cancer therapy owing to the predominantly tumor-suppressive role GSK3 plays in many cancer types. However, lithium chloride has long been used clinically for the treatment of bipolar disorder, and its chronic administration has not been associated with increased cancer risk (32). GSK3 inhibitors have also been shown to be promising therapeutic agents in a number of preclinical studies for Alzheimer’s disease and diabetes (33, 34). Inhibition of GSK3 in a preclinical model of mixed lymphocytic leukemia effectively suppressed tumor growth and prolonged survival (35). The therapeutic benefit of GSK3 inhibitors for sarcomas and pediatric malignancies has yet to be reported. However, our data lend strong support for additional preclinical studies to develop GSK3 inhibitors as differentiation therapy in ERMS, likely to be combined with currently available cytotoxic therapies.
Our data also show that common molecular pathways regulate differentiation and self-renewal of satellite cells and TPCs found in ERMS. For example, activation of the noncanonical WNT/planar cell polarity pathway promotes the expansion of satellite cells by stimulating symmetric cell divisions to produce two daughter satellite cells during the self-renewal process (36). By contrast, injury-induced muscle regeneration results in canonical WNT/β-catenin pathway activation and myogenic differentiation of satellite cells (18). These studies suggest that canonical and noncanonical pathways play antagonistic roles in regulating satellite cell function. Additional molecular pathways that regulate satellite cell self-renewal also have important roles in ERMS. For example, the Notch pathway promotes activation and proliferation of satellite cells during regeneration (18). In human ERMS cells, knockdown of the Notch1-Hey1 axis or Notch3 resulted in reduced tumor cell growth and enhanced myogenic differentiation (37, 38), suggesting that the Notch pathway may regulate self-renewal and differentiation of human ERMS. In total, our data confirm that common core developmental pathways regulate both satellite cell and ERMS self-renewal programs and may provide new therapeutic avenues for regenerative medicine, muscle dystrophies, and rhabdomyosarcoma.
Finally, we have established high-throughput methods and cross-species validation studies to identify drugs that inhibit tumor growth by altering the differentiation status of TPCs. Our differentiation screen completed in human ERMS cells identified a distinct group of compounds that was largely different from those identified in the in vivo Ras activity screen completed in zebrafish. These data suggest that essential pathways that regulate ERMS differentiation and self-renewal likely function largely independently from RAS-driven pathways that drive tumor progression. The approaches described in this study will likely be useful to a large range of tumor types that can be modeled in the zebrafish and for which large collections of human cell lines exist. The power of this approach lies in the large-scale cell transplantation assays possible in the zebrafish model, the ease of imaging tumor growth in vivo, and the ability to deliver drugs in a cost-effective manner. The imposed high stringency of our chemical screen identified lead compounds that affect both zebrafish and human tumor growth, identifying evolutionarily conserved and essential genetic pathways required for ERMS growth. Finally, our work identifies additional druggable pathways that suppress ERMS growth and self-renewal, providing a rich resource for preclinical studies focused on developing new treatment regimens for ERMS.
Methods
A Large-Scale Chemical Screen Using Human ERMS Cells.
After initial seeding of human RD cells into 384-well plates, chemical libraries consisting of ∼40,000 compounds from the Massachusetts General Hospital and the Genomics Institute of the Novartis Research Foundation were added with a final concentration of 10 μM and incubated for 5 d. Subsequently, cells were fixed and stained with the MF20 antibody (1:4,000; Developmental Studies Hybridoma Bank), Alexa Fluor 488-conjugated, anti-mouse secondary antibody (at 1:1,000; Life Technologies), and TOPRO3 (1:1,000; Life Technologies). Stained plates were scanned at 100× resolution and analyzed using Image Express Ultra (IXU, Molecular Devices). Wells that exhibited a ≥2.5-fold increase in MF20-positive cells were considered hits (3 SDs above the mean). Secondary reconfirmation was completed using additional drugs that target the top six classes of biologically active compounds.
Transplantation and Chemical Treatment of Zebrafish.
The animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care under protocol #2012N000127 (zebrafish).
Fluorescence-labeled ERMS were created by microinjecting the rag2:kRASG12D transgene into mylz2-mCherry transgenic, one-cell stage syngeneic zebrafish (13) and transplanted into 5- to 6-wk-old nonfluorescent, CG1-strain recipient fish (2.5 × 104 unsorted cells per fish, 2-μL volume). ERMS-engrafted fish were imaged and then exposed to drug or DMSO vehicle in six-well plates (two fish per well, n = 4–8 animals per drug treatment). Posttreatment imaging was performed after 7 d of drug treatment and tumor volume calculated by multiplying tumor area by red fluorescent intensity in ImageJ. Relative tumor growth was calculated by dividing final tumor volume by pretreatment tumor volume.
For limiting dilution experiments, ERMS cells were engineered to express rag2:kRASG12D and either rag2:dsRED or both the myf5:GFP and mylz2:mCherry transgenes (13). FACS-sorted cells were transplanted at limiting dilution using fluorescent, bulk-sorted tumor or myf5:GFP+/mylz2:mCherry− FACS-sorted cells. Transplanted fish were treated with BIO (300 nM), CHIR99021 (600 nM), or DMSO (1:30,000) for 5 d and engraftment monitored from 10 to 120 d after treatment.
Cell Lines, siRNA, and shRNA Knockdown and Western Blot Analysis.
Human RD and 381T cell lines were obtained from the ATCC cell biology collection. CTNNB1 siRNA was obtained from Santa Cruz Biotechnology and CTNNB1 shRNA from Addgene (39). The doxycycline-inducible lentiviral vector expressing CTNNB1 S33Y contiguous with an internal ribosome entry site-driven GFP was obtained from Hector Palmer (Barcelona). Lentiviral transduction was performed as previously described (16). Total cell lysates were immunoblotted using primary antibodies against β-catenin/CTNNB1 (1:2,500; Sigma) and GAPDH (1:2,500; Cell Signaling).
Immunofluorescence of Human ERMS Cells.
Human ERMS cells were seeded in 10% FBS in DMEM at 5 × 103 cells per well in 96-well plates. The next day cells were treated with BIO (200–500 nM), CHIR99021 (500–1,000 nM), DMSO (0.1%), WNT3A (400 μg/mL; R&D Systems), or BSA (100 μg/mL) and cultured in differentiation media (2% horse serum in DMEM) for 72 h. MF20 staining was performed as previously described (16). β-Catenin (CTNNB1) antibody (Sigma) was used at 1:1,000 dilution.
Human ERMS Sphere Assays.
Sphere and replating assays using RD and 381T cells were completed essentially as previously described (25).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health including R01CA154923 (to D.M.L.), R01CA143082 (to C.K.), R21CA156056 (to D.M.L.), U54CA168512, K08AR063165 (to E.Y.C.), and K99CA175184 (to M.S.I.). Additional support was provided by the Alex's Lemonade Stand Foundation (D.M.L. and M.S.I.), the Sarcoma Foundation of America (D.M.L.), the Massachusetts General Hospital Howard Goodman Fellowship (D.M.L.), the Harvard Stem Cell Institute (D.M.L. and X.W.), the Steward Rahl-Melanoma Research Alliance Young Investigator Award (X.W.), the American Cancer Society Research Scholar Award (X.W.), the St. Baldrick's Foundation Scholar Award (E.Y.C.), the Genomics Institute of the Novartis Research Foundation postdoctoral fellowship (K.B.G.), and the Massachusetts General Hospital Toteson Fund for Medical Discovery (M.S.I.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317731111/-/DCSupplemental.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 5.Uckun FM, et al. Leukemic cell growth in SCID mice as a predictor of relapse in high-risk B-lineage acute lymphoblastic leukemia. Blood. 1995;85(4):873–878. [PubMed] [Google Scholar]
- 6.Stone RM, et al. Complete remission in acute promyelocytic leukemia despite persistence of abnormal bone marrow promyelocytes during induction therapy: Experience in 34 patients. Blood. 1988;71(3):690–696. [PubMed] [Google Scholar]
- 7.Azzi S, et al. Differentiation therapy: Targeting human renal cancer stem cells with interleukin 15. J Natl Cancer Inst. 2011;103(24):1884–1898. doi: 10.1093/jnci/djr451. [DOI] [PubMed] [Google Scholar]
- 8.Chien AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci USA. 2009;106(4):1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rephaeli A, et al. In vivo and in vitro antitumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, in human prostate cancer. Int J Cancer. 2005;116(2):226–235. doi: 10.1002/ijc.21030. [DOI] [PubMed] [Google Scholar]
- 10.Sato A, et al. Resveratrol promotes proteasome-dependent degradation of Nanog via p53 activation and induces differentiation of glioma stem cells. Stem Cell Res (Amst) 2013;11(1):601–610. doi: 10.1016/j.scr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Charytonowicz E, et al. PPARγ agonists enhance ET-743-induced adipogenic differentiation in a transgenic mouse model of myxoid round cell liposarcoma. J Clin Invest. 2012;122(3):886–898. doi: 10.1172/JCI60015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demetri GD, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA. 1999;96(7):3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ignatius MS, et al. In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell. 2012;21(5):680–693. doi: 10.1016/j.ccr.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langenau DM, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21(11):1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, et al. St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24(6):710–724. doi: 10.1016/j.ccr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen EY, et al. Cross-species array comparative genomic hybridization identifies novel oncogenic events in zebrafish and human embryonal rhabdomyosarcoma. PLoS Genet. 2013;9(8):e1003727. doi: 10.1371/journal.pgen.1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le X, et al. A novel chemical screening strategy in zebrafish identifies common pathways in embryogenesis and rhabdomyosarcoma development. Development. 2013;140(11):2354–2364. doi: 10.1242/dev.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2(1):50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Blattmann C, et al. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2010;78(1):237–245. doi: 10.1016/j.ijrobp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Kutko MC, et al. Histone deacetylase inhibitors induce growth suppression and cell death in human rhabdomyosarcoma in vitro. Clin Cancer Res. 2003;9(15):5749–5755. [PubMed] [Google Scholar]
- 21.Lian X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8(1):162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 23.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 24.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8(5):486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter D, et al. CWS Study Group CD133 positive embryonal rhabdomyosarcoma stem-like cell population is enriched in rhabdospheres. PLoS ONE. 2011;6(5):e19506. doi: 10.1371/journal.pone.0019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 27.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 28.Rampazzo E, et al. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis. 2013;4:e500. doi: 10.1038/cddis.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouron-Dal Soglio D, et al. Beta-catenin mutation does not seem to have an effect on the tumorigenesis of pediatric rhabdomyosarcomas. Pediatr Dev Pathol. 2009;12(5):371–373. doi: 10.2350/08-11-0553.1. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, et al. Impaired Wnt signaling in embryonal rhabdomyosarcoma cells from p53/c-fos double mutant mice. Am J Pathol. 2010;177(4):2055–2066. doi: 10.2353/ajpath.2010.091195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annavarapu SR, et al. Characterization of Wnt/beta-catenin signaling in rhabdomyosarcoma. Lab Invest. 2013;93(10):1090–1099. doi: 10.1038/labinvest.2013.97. [DOI] [PubMed] [Google Scholar]
- 32.Gould TD, Manji HK. The Wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8(5):497–511. doi: 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- 33.Dokken BB, Sloniger JA, Henriksen EJ. Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288(6):E1188–E1194. doi: 10.1152/ajpendo.00547.2004. [DOI] [PubMed] [Google Scholar]
- 34.Kremer A, Louis JV, Jaworski T, Van Leuven F. GSK3 and Alzheimer’s disease: Facts and fiction…. Front Mol Neurosci. 2011;4:17. doi: 10.3389/fnmol.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, et al. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008;455(7217):1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Grand F, Jones AE, Seale V, Scimè A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4(6):535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belyea BC, Naini S, Bentley RC, Linardic CM. Inhibition of the Notch-Hey1 axis blocks embryonal rhabdomyosarcoma tumorigenesis. Clin Cancer Res. 2011;17(23):7324–7336. doi: 10.1158/1078-0432.CCR-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raimondi L, et al. Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21(Cip1) expression and hampers tumour cell growth in vitro and in vivo. Cell Death Differ. 2012;19(5):871–881. doi: 10.1038/cdd.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onder TT, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.