Significance
Dysregulation of emotion is central to the etiology of mood disorders, such as depression. A causal understanding of how neural structures regulate emotion and arousal could help to improve treatments for these psychiatric disorders. Studies of patients with depression indicate that a particular part of the frontal lobe, the subgenual cingulate cortex, plays an important role in affective processing, though its precise contribution remains unclear. Here we show that, in macaque monkeys, this small part of the frontal cortex is necessary for sustaining elevated arousal in anticipation of positive emotional events. This finding suggests a mechanism for the contribution of this area to affective regulation, including an account for the lack of pleasure and passivity that characterizes mood disorders.
Keywords: Area 25, infralimbic, pupil size, anticipatory arousal
Abstract
The subgenual anterior cingulate cortex (subgenual ACC) plays an important role in regulating emotion, and degeneration in this area correlates with depressed mood and anhedonia. Despite this understanding, it remains unknown how this part of the prefrontal cortex causally contributes to emotion, especially positive emotions. Using Pavlovian conditioning procedures in macaque monkeys, we examined the contribution of the subgenual ACC to autonomic arousal associated with positive emotional events. After such conditioning, autonomic arousal increases in response to cues that predict rewards, and monkeys maintain this heightened state of arousal during an interval before reward delivery. Here we show that although monkeys with lesions of the subgenual ACC show the initial, cue-evoked arousal, they fail to sustain a high level of arousal until the anticipated reward is delivered. Control procedures showed that this impairment did not result from differences in autonomic responses to reward delivery alone, an inability to learn the association between cues and rewards, or to alterations in the light reflex. Our data indicate that the subgenual ACC may contribute to positive affect by sustaining arousal in anticipation of positive emotional events. A failure to maintain positive affect for expected pleasurable events could provide insight into the pathophysiology of psychological disorders in which negative emotions dominate a patient’s affective experience.
The ability to regulate emotion and arousal in response to pleasurable and aversive situations is essential for adapting to our environment and, ultimately, for our mental health. The anterior cingulate cortex (ACC), specifically its subgenual part, has been implicated in a number of psychiatric disorders, including major depressive disorder (1). Dysfunction and degeneration in the subgenual ACC have been reported in patients suffering from depression (2, 3), and the degree of activation in this area correlates with anhedonia, the loss of positive emotions (4). Based on these findings, new approaches for treatment-resistant depression target the subgenual ACC with deep brain stimulation (5). Determining the causal role of subgenual ACC in the regulation of affect and arousal would advance our understanding of emotional regulation and could provide insight into the pathophysiology of depression.
A long history of research implicates the ACC as a whole in the control of autonomic arousal, emotional responses, and behavior (6–8). Much of what is known about the function of the ACC, however, relates to the more dorsal parts of the ACC and its role in higher cognition and arousal (9–11). Less is known about the function of the ventral ACC, especially the subgenual ACC, in part because lesions of the ventromedial prefrontal cortex often include the subgenual ACC as well as adjacent portions of orbitofrontal cortex and the dorsal ACC (12–14). Where research has focused on the primate subgenual ACC, it has emphasized its role in mediating responses to threatening or fear-inducing situations, as work in rodents has (15–17). The contribution of the subgenual ACC to regulating positive emotion and arousal, by comparison, remains unclear. We addressed this imbalance by assessing the effect of subgenual ACC lesions on autonomic arousal in response to a positive emotional event: the receipt of a reward.
Results
Task.
We recorded pupil size, a measure of autonomic and emotional arousal (18), in six monkeys as they performed a task in which they could anticipate fluid rewards. Three monkeys sustained bilateral aspiration lesions of the subgenual ACC. The remaining three monkeys served as unoperated controls (CON). The task involved Pavlovian conditioning of stimulus–reward associations superimposed on instrumental conditioning of active visual fixation (see Methods, Fig. 1A, and SI Methods). We chose to examine pupil size because the relatively fast (<0.5 s) response of the pupil to external stimuli allows alterations in autonomic arousal to be correlated with behavioral events on a trial-by-trial basis with good temporal resolution.
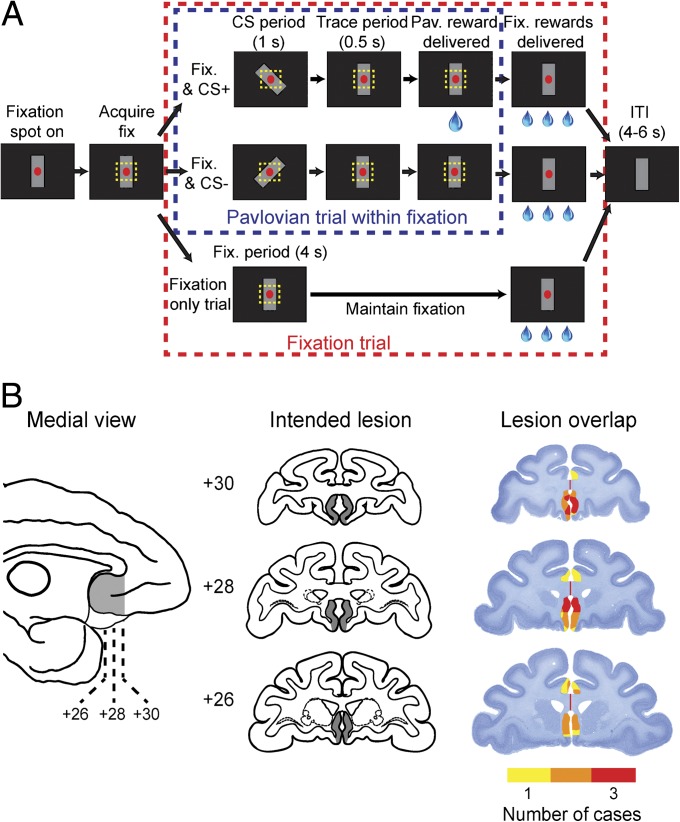
Fig. 1.
Trial sequence, stimuli, and lesions. (A) On each trial, monkeys had to hold their gaze within 3.0° (visual angle) of the red fixation spot for 4 s to receive three small drops of water. On 50% of trials, conditioned stimuli, either CS+ or CS−, were presented for 1 s (CS period) during either the 4-s fixation period or the ITI. If the CS+ was presented, a large drop of water was delivered 0.5 s after the offset of the stimulus (trace period). (B) Intended extent of the subgenual ACC lesion (shaded region) shown on a medial view of a macaque brain (Left) and coronal sections through the frontal lobe (Center). Nissl-stained sections from corresponding levels show the location and extent of the subgenual ACC lesions in cases 1–3 (Right). Colors represent the degree of overlap of the lesions, as indicated in the legend. Numerals indicate the distance in millimeters from the interaural plane.
On each trial, monkeys were operantly conditioned to maintain gaze on a central fixation spot for 4 s. If they did so, they received three small drops of water (3 × 0.1 mL) as a reward. A variable 4- to 6-s intertrial interval (ITI) followed immediately. On half of the fixation trials, randomly selected, we superimposed a Pavlovian conditioning procedure. One stimulus, the positive conditioned stimulus (CS+), always predicted a reward; another stimulus (CS–) never did so. On Pavlovian trials, either a CS+ or CS– was presented for 1.0 s (CS period) during either the 4-s fixation period (38% of fixation trials, randomly selected) or during the 4- to 6-s ITI (12% fixation trials, randomly selected). Conditioned stimuli, either CS+ or CS–, were subtle alterations in the gray mask stimulus that was present on the screen throughout testing (Fig. 1A). The CS+ was followed by the delivery of a large drop of water (0.5 mL), 0.5 s after the stimulus was turned off (trace period). Monkeys were initially tested with one pair of conditioned stimuli (set 1) before being tested on a second pair (set 2) to replicate the observations (Fig. S1).
The experimental design ensured that the instrumental fixation task and Pavlovian conditioning procedure were independent. Accordingly, autonomic responses to the Pavlovian conditioned stimuli were not under instrumental control. For example, if a monkey broke fixation during the 4-s fixation period (while no conditioned stimulus was present), the fixation spot was extinguished, no reward was delivered, and a penalty ITI was enforced. If, however, the monkey broke fixation when a Pavlovian-conditioned stimulus was presented during the fixation period, the fixation spot was extinguished but the Pavlovian trial continued, unaffected by the oculomotor behavior.
The subgenual ACC lesion did not affect the ability of the monkeys to perform the fixation task. Monkeys in both the lesion and control groups successfully maintained fixation during the task, completing more than 70% of the fixation trials [proportion correct per testing session: effect of lesion, F(1, 139) = 0.02, P > 0.8]. Toward the end of training (final day of acquisition), the monkeys aborted fixation during trials with a CS+ stimulus more frequently than trials with a CS–, but this difference failed to reach statistical significance [effect of CS, F(1, 35) = 3.35, P = 0.07]. Nevertheless, this trend served as one indication that the monkeys had learned the association between the CS+ and reward. Though this might seem counterintuitive, we believe the monkeys were more likely to break fixation during CS+ trials because they had learned that delivery of the 0.5-mL reward associated with the Pavlovian stimulus was not contingent upon continued fixation. Evidently, after CS+ presentations, the cost of maintaining effortful fixation was not always worth the additional rewards (3 × 0.1 mL) to be obtained for successfully completing the fixation trial. Indeed, one control monkey had to be excluded from the study because it would immediately break fixation upon presentation of the CS+ (see SI Methods).
Conditioned Autonomic Responses.
Both groups of monkeys showed differential autonomic responses to the CS+ and CS–; they exhibited an increased pupil size to the CS+, the stimulus that predicted a reward, but not to the CS– (Fig. 2A). These differences appeared after a similar number of testing sessions in the two groups (mean ± SEM, CON = 18.2 ± 1.9 sessions; subgenual ACC = 14.2 ± 0.9; Wilcoxon test, χ2 = 1.76, P = 0.18). Thus, monkeys with lesions of the subgenual ACC acquired a conditioned autonomic response at the same rate as unoperated controls. Pupillary responses to the two sets of conditioned stimuli were highly similar [effect of set, F(1, 2,559) < 0.5, P > 0.9], and data from the two sets were therefore combined for subsequent analyses.
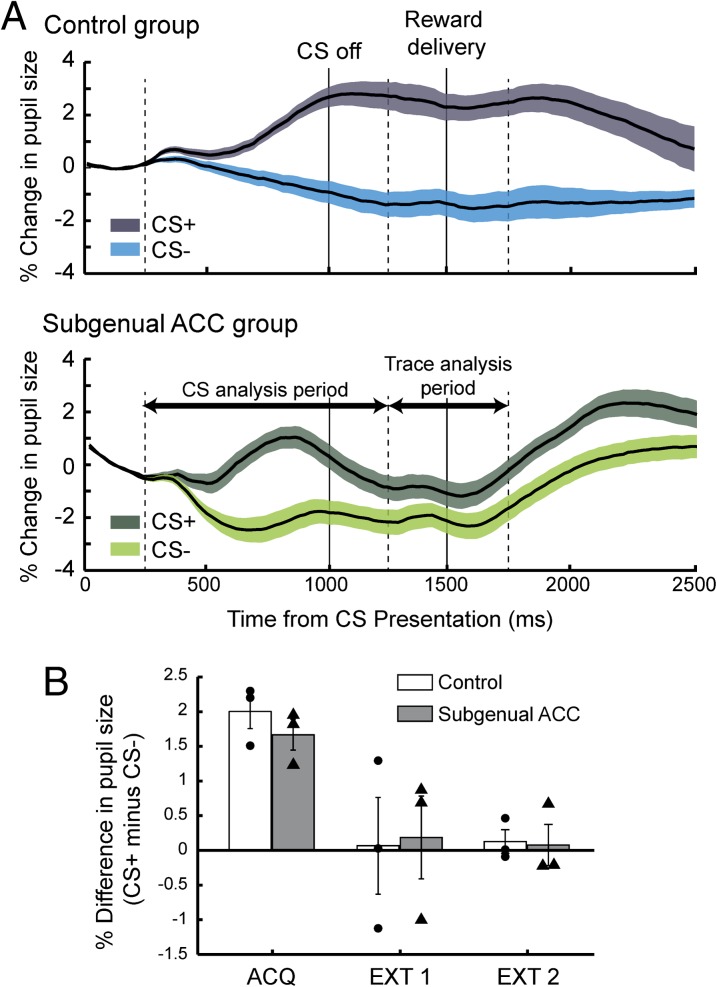
Fig. 2.
Conditioned responses. (A) Pupillary dilation as a function of time (percent change, mean ± SEM). Control (Upper, n = 3) and lesion group (Lower, n = 3). Data from stimulus sets 1 and 2, combined. Because the pupil response lags the trial events by ∼250 ms, each analysis period is shifted 250 ms relative to CS onset and offset (dashed vertical lines). Shaded regions show SEM. (B) Mean (±SEM) difference in pupillary responses during the CS period, CS+ minus CS–, during the final day of acquisition (ACQ), and two extinction sessions (EXT 1 and 2). Symbols represent the responses of individual monkeys. Negative values indicate pupillary contraction.
To examine the time course of the conditioned change in pupil size we divided the period after the presentation of the conditioned stimuli into two parts, taking into account the response latency for changes in pupil size (see SI Methods, Data Processing and Analysis): (i) the CS period, the time that conditioned stimuli were present and (ii) the trace period, the interval between CS offset and reward delivery (Fig. 1A). Our initial analysis of monkeys’ pupil responses during these two periods revealed that controls and monkeys with subgenual ACC lesions differed in their responses (cue × lesion × period interaction, F(1, 4316) = 36.87, P < 0.0001). Subsequent analyses were then conducted on these two periods of the trial separately. During the CS period, both groups exhibited increased pupil size to the CS+ relative to the CS– [for sample responses from individual monkeys, see Fig. S1B; CS period: effect of cue, F(1, 36) = 614.78, P < 0.00001; effect of lesion, F(1, 4) = 0.81, P > 0.3]. Although there was a marginally significant difference between the groups during this period [cue × lesion interaction, F(1, 26) = 3.87, P = 0.06], additional tests revealed that both groups exhibited robust differential responses to the conditioned stimuli (Fs > 200, P < 0.0001). During the trace period, by comparison, the lesion had a marked effect. Monkeys with lesions of subgenual ACC did not show the same sustained increase in autonomic arousal that the control group exhibited [trace period: lesion × cue interaction: F(1, 486) = 121.78, P < 0.00001]. These findings indicate that the subgenual ACC may be critical for maintaining heightened autonomic arousal in anticipation of rewards.
Extinction of Conditioned Autonomic Responses.
To confirm that the increase in pupil size was related to the positive emotional nature of the reward associated with CS+ and not other variables, such as stimulus identity, we tested monkeys under extinction conditions. Monkeys were tested for an additional 2 d using the same task parameters as before, but now no reward followed CS+ presentation.
Compared with the final acquisition session, pupillary responses decreased during extinction sessions, as judged by the difference between CS+ and CS– trials during the two extinction sessions [CS period: cue × session interaction, F(1, 586) = 26.53, P < 0.001; Fig. 2B]. Monkeys in both groups showed this decrease to the same extent [effect of lesion or lesion × cue interaction, F(1, 4/586) < 0.5, P > 0.7]. Extinction abolished the difference in pupillary response to the conditioned stimuli by the second session (Fig. 2B). That both groups extinguished responding to the CS+ suggests that the previously observed increases in pupil size were directly related to the positive nature of the reward associated with the CS+.
Previously, deficits in retention of extinction, characterized by higher rates of spontaneous recovery of responding during the second extinction session, have been reported in rats with lesions of the area homologous to the macaque subgenual ACC, infralimbic cortex (19, 20). In light of this work, we looked for heightened autonomic arousal at the start of the second compared with the end of the first extinction session. If anything, monkeys in both groups exhibited slightly decreased pupil responses to the CS+ early in the second compared with the end of the first extinction session [Fig. S2; effect of session, F(2, 189) = 13.7, P < 0.001]. Monkeys with subgenual ACC lesions were no different to controls [effect of lesion or interaction involving lesion, F(1, 4/189) < 0.75, P > 0.4]. At least in the present paradigm, subgenual ACC lesions were not associated with decreased retention of extinction.
Unconditioned Autonomic Responses.
The findings from the extinction sessions strongly suggest that group differences in the sustained autonomic arousal are indeed related to the anticipation of reward. It is possible, however, that the difference between the groups could arise if the monkeys with subgenual ACC lesions showed diminished autonomic arousal to the rewards per se; to explore this, we tested monkeys in the same task as before, but without conditioned stimuli. In this setting, the delivery of large drops of water (0.5 mL) on 50 randomly selected trials was unsignaled (see Methods, Fig. 3A and SI Methods).
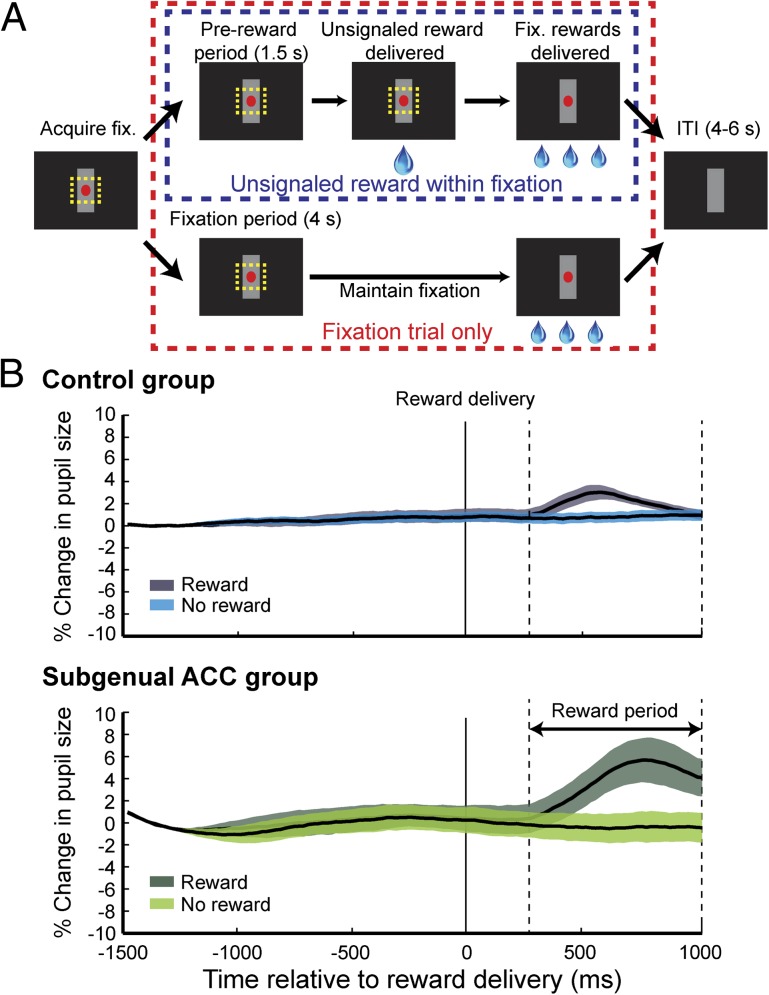
Fig. 3.
Autonomic responses to unsignaled reward. (A) On 25% of trials, a large drop of water (0.5 mL) was delivered either during the fixation period or the ITI, with no prior indication. (B) Pupillary dilation as a function of time (percent change, mean ± SEM) for the control (Upper) and lesion (Lower) groups. Format as in Fig. 2A.
Both groups showed increases in pupil size after the delivery of unsignaled rewards, as contrasted with trials in which no reward was delivered [reward period: effect of reward F(1, 420) = 127.7, P < 0.0001; Fig. 3B]. Along with the CS+ response during the CS period (Fig. 2A), this finding rules out a diminished autonomic arousal to positive emotional events as an explanation of the impairment observed in anticipatory autonomic arousal. In fact, monkeys with a lesion of the subgenual ACC exhibited greater increases in pupil size following unsignaled reward than did monkeys in the control group [reward × lesion interaction, F(1, 420) = 12.37, P = 0.0005]. We interpret this group difference with caution, however, because slight differences in the dynamic range of the pupil among monkeys could have accentuated the response to unsignaled rewards.
Pupil Size Changes in Response to Varying Luminance.
Modulation of pupil size is dominated by the amount of light on the retina, known as the light reflex (21). We controlled for the light reflex by using a stimulus mask (vertical gray bar presented before and after CS+/− onset; Fig. 1A) and conditioned stimuli of equal luminance. Still, it is possible that the subgenual ACC lesions produced alterations in the basic physiology of the pupil, generally, or the light reflex in particular, changes that led to group differences in the pupillary response. Indeed, one might posit that monkeys with subgenual ACC lesions are unable to maintain increases in pupil size, even to increases in luminance, and that this might account for our results.
To rule out effects due to the light reflex, we tested monkeys in settings nearly identical to those of the main task. Instead of presenting either the CS+ or CS–, however, we either extinguished or brightened the mask stimulus (see Methods and Fig. S3A). Changes in the luminance of the mask stimulus were not associated with the delivery of an additional reward.
Extinguishing or brightening the mask stimulus led to a sustained dilation or contraction of the pupil, respectively, in all monkeys [CS period: effect of cue, F(1, 221) = 1,186.26, P < 0.0001; Fig. S3B]. Comparing the magnitude of pupil size responses during the period where the luminance of the mask was altered did not reveal any differences between the two groups [effect of lesion or lesion by cue interaction, F(1, 4/221) < 1.64, P > 0.25]. Similarly, the latency, defined as the first 20-ms bin in which there was a significant difference between extinguishing or brightening the mask, was comparable between controls and lesioned monkeys [mean ± SEM, CON = 16.67 ± 2.19; subgenual ACC = 14.67 ± 0.33]. Thus, alterations in the light reflex cannot account for the effect of subgenual ACC lesions on autonomic arousal in anticipation of reward.
Acquisition and Extinction of Object–Reward Associations.
To confirm that lesions of the subgenual ACC did not affect the ability of monkeys to learn stimulus–reward associations and then subsequently modify such learned associations and behavior, monkeys were tested on an object–reward association and extinction task. This task has been shown to be sensitive to lesions of both the orbitofrontal cortex (OFC) and amygdala (22). During acquisition sessions consisting of 30 trials each, monkeys were allowed to displace a single object and to obtain a food reward hidden underneath. Monkeys were tested until they displaced the object on nearly every trial. Following acquisition, two extinction sessions were conducted. Extinction sessions were identical to those in acquisition with the exception that no food reward was available under the object. Omissions, i.e., trials on which monkeys failed to displace the object, were counted as a measure of extinction.
All monkeys readily displaced the object for food reward. During acquisition, controls and monkeys with lesions of the subgenual ACC learned at a similar rate (mean omissions ± SEM: CON = 1.75 ± 1.75; subgenual ACC = 5 ± 5; Kruskal–Wallis test χ2 = 0.19, P = 0.66). During extinction, displacement of the unrewarded object decreased markedly across sessions [effect of session, F(2, 10) = 31.15, P < 0.001; Fig. S4A]. This change in behavior was similar in the two groups [F(1, 5) = 0.005, P = 0.95; all interactions involving group, F < 1.74, P > 0.1]. We also investigated whether monkeys with subgenual ACC lesions exhibited spontaneous recovery of responding at the start of the second extinction session. Though both groups exhibited elevated responding early in the second session by comparison with the end of the first session [Fig. S4B; effect of session, F(2, 10) = 13.08, P < 0.002], monkeys with subgenual ACC lesions performed similarly to controls [effect of lesion, F(1, 5) = 0.03, P > 0.8]. Thus, lesions of subgenual ACC failed to disrupt the acquisition, extinction, and retention of extinction of object–reward associations.
Discussion
We found that lesions of the subgenual ACC caused a specific impairment in autonomic arousal during the anticipation of rewards, as measured by pupillary dilation. In contrast to the sustained prereward arousal observed in intact monkeys, monkeys with lesions of the subgenual ACC showed only transient arousal at the time of the CS+ (Fig. 2A). The lesions did not affect the extinction of conditioned autonomic arousal (Fig. 2B), autonomic arousal to unsignaled rewards (Fig. 3B), pupillary responses to light (Fig. S3), or the acquisition and extinction of object–reward associations (Fig. S4). Our findings suggest that the subgenual ACC is necessary for sustaining elevated autonomic arousal in anticipation of rewards, and implicate this area in the regulation of positive affect.
Interpretational Issues.
This study sought to identify the previously uncharacterized effects of lesions specifically targeting the subgenual ACC, and is one of only a handful to assess the effects of cortical lesions on autonomic arousal in primates (23–26). The subgenual ACC is one of the least accessible parts of the frontal cortex. To investigate its function, we chose to make lesions by aspiration because it allowed direct visualization of landmarks in this region and had a better chance of success; it also had the potential to cause less damage to other parts of the medial prefrontal cortex relative to other approaches, such as stereotaxically placed injections of excitotoxins or pharmacological inactivation, either of which could have caused inadvertent damage to other parts of the ACC. Our lesions involved the transection of the genu of the corpus callosum, which was required to gain access to the subgenual ACC. Consequently, we cannot rule out a contribution by the genu. In addition, it is possible that inadvertent damage to the white matter subjacent to subgenual ACC might have contributed to our results. One possible site of termination for these fibers is the OFC, an adjacent part of the prefrontal cortex that has been associated with autonomic control and emotion regulation (6). In marmoset monkeys, lesions of the OFC cause alterations in autonomic arousal that differ from those reported here. Specifically, OFC lesions render monkeys less able to suppress arousal when the contingency between a CS+ and reward is abolished. By contrast, lesions of this area spare acquisition and expression of conditioned autonomic arousal (24). It therefore seems unlikely that damaging fibers running to or from the OFC could account for the present pattern of results. An alternative possibility is that the lesions might have damaged fibers coursing to or from the more dorsal parts of the ACC. We are not aware of any lesion studies that have assessed the role of the dorsal ACC in autonomic arousal in monkeys. Lesion and functional studies in humans indicate that the dorsal ACC plays a role in the control of autonomic arousal, but these studies often emphasize a role for this area in arousal in relation to effortful responding and action as opposed to subjective emotional experience (9, 27). Either way, delineating the effects on arousal of damage limited to cell bodies within the subgenual ACC, potentially by using excitotoxic lesions, is an important avenue for future research.
Subgenual ACC, Emotion, and Arousal.
Fundamentally, the autonomic nervous system is part of the motor system in the broad sense, in that it is one way that the central nervous system controls the body (28). It has long been recognized that the subgenual ACC contributes to autonomic control (for a review, see ref. 9). Electrical stimulation of this area produces changes in breathing and heart rate (6, 8, 29), and the subgenual ACC is densely interconnected with structures that play a central role in visceromotor control, such as the hypothalamus (30).
Despite this understanding, the role of the primate subgenual ACC in positive affect has remained unclear; in part, this is because lesions of the ventromedial frontal cortex often include parts of the dorsal ACC and/or medial OFC as well as subgenual ACC (12–14). Likewise, there are few neurophysiology studies of the subgenual ACC. Though one study found slight changes in tonic firing rates in response to emotional events, such as rewards (31), another suggested that the subgenual ACC might play a role in internally driven motivational behavior (32), an issue we take up later.
Here we provide evidence that the role of the subgenual ACC in visceromotor behavior is specific to regulating conditioned autonomic arousal (Fig. 2), as opposed to unconditioned arousal (Fig. 3), in anticipation of positive emotional events—specifically, fluid rewards. We note that the pattern of effects is subtly different from those reported after lesions of the amygdala in marmoset monkeys (25). Whereas lesions of the subgenual ACC, like amygdala lesions, diminished arousal in anticipation of rewards, in the case of the subgenual ACC this effect was limited to the trace interval (i.e., the period between the offset of the CS and reward delivery). Furthermore, in the marmoset experiments, the CS+ (food) was visible throughout the anticipatory period. Thus, there was no possibility to examine sustained autonomic arousal in the absence of the CS+.
An indirect, as opposed to a direct, role for the primate subgenual ACC in the control of autonomic arousal is underscored by tract-tracing studies that show that the subgenual ACC in primates, unlike rodents (33), is not directly connected to autonomic effector regions in the brainstem (30). Such a pattern of connections in primates could account for the differences between the present findings and those reported in rodents after lesions of the homologous area, infralimbic cortex (29, 34). In rodents, lesions of the infralimbic cortex have been reported to lead to heightened respiration (34), but also attenuated heart rate changes in response to stimuli that predict aversive foot shock (35). It is possible, however, that other factors, such as the valence of the stimuli used, might have contributed, and this is one clear difference between the present study and those in rodents. Indeed, whether our results would hold for negative emotional events invites empirical investigation. Based on studies in rats (19, 20), we would predict that the subgenual ACC would play a similar role in the control of emotion and arousal in anticipation of aversive events, as it does in anticipation of positive events.
Lesion studies in rodents and functional MRI investigations in humans suggest that subgenual ACC is important for retention of extinction (15–17, 20). Here, we did not see evidence for decreased extinction retention in the form of increased spontaneous recovery of either autonomic or behavioral responding during the second extinction session in monkeys with subgenual ACC lesions (SI Methods and Figs. S2 and S4B). It is possible that the task we used to assess conditioned autonomic responding, which used concurrent Pavlovian and fixation trials, might have contributed to these negative findings. In addition, it is possible that the potential for the object–reward association task to be solved using either Pavlovian or instrumental strategies might have been a factor.
Subgenual ACC and Mental Health.
Patients with psychiatric disorders involving mood dysregulation, as a group, display both functional and architectonic changes within the subgenual ACC (2, 3). Based on these and related findings, clinicians have used deep brain stimulation techniques targeting this portion of the ACC as a potential treatment for depression (5). Here we provide evidence that the subgenual ACC contributes to maintaining heightened arousal in anticipation of positive emotional events, especially in the absence of predictive cues. Our findings present a starting point for future studies seeking to determine the role of the subgenual ACC in affect by providing an initial autonomic fingerprint of the effects of damage within this area. They might also inform studies of autonomic function in patients with depression (36) and potentially contribute to efforts to understand the neural mechanisms underlying deep brain stimulation techniques for mood disorders such as depression.
Anhedonia, characterized by a loss of pleasure from previously rewarding events, is associated with depression and dysfunction within the subgenual ACC (4). From one perspective, the present results would appear inconsistent with a role for the subgenual ACC dysfunction in anhedonia. Monkeys with subgenual ACC lesions showed normal patterns of arousal following the receipt of rewards and also in response to stimuli (CS+) that predicted rewards. Anhedonia, however, is thought to incorporate a motivational or anticipatory component, related to maintaining positive affect in advance of appetitive stimuli, as well as a “consummatory” component related to the experience of primary rewards (37, 38). Our findings indicate that the subgenual ACC may play a specific role in the anticipatory aspects of positive affect. Indeed, a recent functional neuroimaging study has reported that depressed individuals are unable to sustain neural activation associated with positive emotional affect in the ventral striatum (39), a portion of the basal ganglia directly connected with the subgenual ACC (40).
Using measures of autonomic arousal in conjunction with manipulations of subgenual ACC function potentially provides a means of modulating the motivational and anticipatory aspects of anhedonia. Our results also demonstrate the value of establishing causal links between specific neural structures and the regulation of emotion and arousal.
Methods
Subjects.
Seven adult rhesus monkeys (Macaca mulatta, six male and one female) served as subjects. Three monkeys sustained bilateral aspiration lesions of the subgenual ACC, and the remaining four were retained as unoperated controls. Six monkeys (three controls and three subgenual ACC lesion) were tested on the tasks assessing autonomic function. The remaining control monkey was not tested on the tasks assessing autonomic function due to an inability to meet behavioral performance requirements (SI Methods). All seven monkeys were tested on the object–reward and extinction task. Monkeys were at least 4.5 y old at the start of testing. Each animal was individually or pair housed, and kept on a 12-h light-dark cycle. Monkeys’ access to food and water was controlled for 6 d a week. All procedures were reviewed and approved by the National Institute of Mental Health Animal Care and Use Committee.
Surgical Procedures.
Surgery was conducted using standard aseptic procedures. For full a description of the surgical methods, see SI Methods. Bilateral removal of subgenual ACC was carried out in a single operation. A large, symmetrical bone flap was turned over the dorsal aspect of the cranium and the dura mater opened over the frontal lobe. With the aid of an operating microscope, key landmarks on the medial surface of the hemisphere and along the midline (e.g., the cingulate sulcus, corpus callosum, and anterior cerebral artery) were identified. The subgenual ACC was then removed by subpial aspiration, i.e., the cortex ventral to the genu and rostrum of the corpus callosum was removed. The cortical removal was carried out in the same manner in the other hemisphere via the same opening. Thus, the intended lesion includes both areas 25 and anterior area 24 ventral to the genu of the corpus callosum. Approximately 1 mo before training, all monkeys were implanted with a titanium head post using asceptic procedures.
Lesion Assessment.
Lesions of the subgenual ACC were assessed using T1-weighted MRI scans acquired shortly after surgery and at the conclusion of the experiment by histological examination (Fig. 1B). In all three cases, lesions encompassed the intended parts of the cortex, with damage centering on areas 25 and area 24 ventral to the genu of the corpus callosum. There was systematic sparing of the cortex across the two hemispheres in the most caudal parts of area 25. Inadvertent damage was limited to parts of areas 14, 24a/b and 32.
Pavlovian and Fixation Task: Acquisition.
Previously, monkeys had been trained to fixate the central red spot within 3.0° of visual angle (dva) for 4 s on every trial (for full details, see SI Methods). A Pavlovian trace-conditioning procedure was then superimposed, and both tasks were conducted at the same time (Fig. 1A). There was a random delay of between 200 and 600 ms after fixation or ITI onset before the presentation of either of the conditioned stimuli. The CS+ was followed by a 0.5-mL fluid reward, delivered 500 ms (trace period) after the stimulus was turned off, whereas the CS– was followed by an unfilled interval and no reward was delivered.
Monkeys were first tested with stimulus set 1, in which the CS+ and CS– consisted of the gray mask stimulus rotated 45° clockwise and counterclockwise, respectively (Fig. S1). To replicate the effects, we repeated the task with a second pair of conditioned stimuli (stimuli: set 2; Fig. S1). At least 6 d intervened between training with stimulus set 1 and 2. Altering the mask stimulus to present either the CS+ or CS– meant that the same number of gray pixels were physically present on the screen throughout Pavlovian conditioning trials, minimizing differences in luminance; this was done so that any within-trial alterations in pupil size would most likely reflect changes in autonomic arousal and not simply changes associated with turning on or off the conditioned stimuli or differences between the CS+ and CS–. The assignment of CS+ and CS– was counterbalanced across lesion groups. Monkeys received 50 CS+ and 50 CS– presentations per session over the course of ∼200 fixation trials. One session was conducted per day, until monkeys exhibited a stable difference in pupil size during the CS+ and CS– presentation periods for four consecutive sessions, referred to from here onward as criterion sessions (SI Methods, Data Processing and Analysis).
Pavlovian and Fixation Task: Extinction.
At the conclusion of the four criterion sessions, we conducted an extinction procedure. Monkeys were tested for two additional sessions, one per day, using the same task as before, but now neither the CS+ nor CS– led to reward delivery. There were 50 presentations of each of the conditioned stimuli per session, and monkeys completed ∼200 fixation trials per session. Extinction sessions were conducted after the monkeys reached criteria for both stimulus sets 1 and 2. Data from extinction sessions with stimulus set 1 from one monkey, subgenual ACC case 1, was not available for analysis due to experimenter error.
Unsignaled Reward Task.
The same general task design and trial structure as in the main task was used to provide unsignaled rewards, but neither the CS+ nor CS– stimuli were presented (SI Methods). Monkeys were required to fixate a central spot for fluid rewards (3 × 0.1 mL). On 50 randomly chosen trials, large rewards (1 × 0.5 mL of fluid) were delivered either during the fixation period or during the ITI. Thus, by design, the frequency and timing of unsignaled reward delivery matched the parameters used in the main task. Monkeys were tested for four consecutive days, ∼200 fixation trials per daily session. As in the Pavlovian and fixation task, monkeys had to maintain a criterion of successfully completing >80% of fixation trials during each of the 4 d of testing.
Luminance Test.
The effect of varying luminance on extent and latency of monkey’s pupil size responses was assessed in a separate task (Fig. S3A). This procedure differed from the main task in several ways. Monkeys were required to fixate a central spot for fluid rewards (3 × 0.1 mL). Over the course of a session, instead of introducing a CS+/CS–, we either extinguished the mask stimulus (mask stimulus removed, 50 trials) or brightened the mask stimulus (mask turned white, 50 trials) for 1 s. Extinguishing or brightening the mask stimulus occurred randomly on 50% of trials and was not associated with the delivery of additional fluid reward. Matching the previous tasks, either extinguishing or brightening of the mask stimulus could occur either during the fixation period or the ITI. Monkeys were tested for three consecutive days, ∼200 fixation trials per daily session.
Object–Reward and Extinction Task.
We used highly similar methods to those used previously in the laboratory (22). Two separate tests, acquisition followed by extinction, were conducted.
Acquisition phase.
On each trial, the monkey was presented with a single object, novel at the start of acquisition, covering the central well of the test tray. Monkeys were given 30 s to displace the object and retrieve the food reward hidden underneath. If the monkey retrieved the food, the trial was scored as correct; however, the trial ended only after the full 30 s had elapsed. If the monkey failed to retrieve the food within the limit of 30 s, it was scored as an omission. At the end of each 30-s trial, the screen was lowered. Trials were separated by 15 s. Monkeys were tested at the rate of 30 trials per session, one session per day. The criterion for acquisition was set at 93% over five consecutive days i.e., 140/150.
Extinction phase.
At the conclusion of the acquisition phase, monkeys received two consecutive extinction sessions. Trials were conducted exactly as in acquisition with the exception that no food rewards were provided.
Supplementary Material
Acknowledgments
We thank Vincent Costa, Bruno Averbeck, and Steven Wise for advice on analyses and comments on an earlier version of the manuscript. We also thank Yogita Chudasama for assistance with surgery and John Kakareka, Randal Pursely, and Tom Pohida for their assistance in processing physiological signals. This work was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317695111/-/DCSupplemental.
References
- 1.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 3.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J Neurophysiol. 1949;12(5):347–356. doi: 10.1152/jn.1949.12.5.347. [DOI] [PubMed] [Google Scholar]
- 7.Papez J. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;79:217–224. [Google Scholar]
- 8.Smith WK. The functional significance of the rostral cingular cortex as revealed by its responses to electrical stimulation. J Neurophysiol. 1945;8:241–255. [Google Scholar]
- 9.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 10.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8(9):410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 12.Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41(8):919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 13.Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 14.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11(5):611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowenstein O, Loewenfeld IE (1969) The pupil. The Eye, ed Davson H (Academic, New York), 2nd Ed, Vol 3.
- 22.Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22(9):2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- 23.Marshall LB, Smith OA. Prefrontal control of conditioned suppression and associated cardiovascular variables in the monkey (Macaca mulatta) J Comp Physiol Psychol. 1975;88(1):21–35. doi: 10.1037/h0076197. [DOI] [PubMed] [Google Scholar]
- 24.Reekie YL, Braesicke K, Man MS, Roberts AC. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci USA. 2008;105(28):9787–9792. doi: 10.1073/pnas.0800417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braesicke K, et al. Autonomic arousal in an appetitive context in primates: A behavioural and neural analysis. Eur J Neurosci. 2005;21(6):1733–1740. doi: 10.1111/j.1460-9568.2005.03987.x. [DOI] [PubMed] [Google Scholar]
- 26.Kimble D, Bagshaw MH, Pribram KH. The GSR of monkeys during orienting and habituation after selective partial ablations of the cingulate and frontal cortex. Neuropsychologia. 1965;3(2):121–128. [Google Scholar]
- 27.Critchley HD, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 28.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1-2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan SL, Powell DA. Cingulothalamic and prefrontal control of autonomic function. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Cambridge, MA: Birkhauser; 1993. pp. 1381–1414. [Google Scholar]
- 30.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421(2):172–188. [PubMed] [Google Scholar]
- 31.Monosov IE, Hikosaka O. Regionally distinct processing of rewards and punishments by the primate ventromedial prefrontal cortex. J Neurosci. 2012;32(30):10318–10330. doi: 10.1523/JNEUROSCI.1801-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouret S, Richmond BJ. Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys. J Neurosci. 2010;30(25):8591–8601. doi: 10.1523/JNEUROSCI.0049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308(2):249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 34.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex. 1991;1(5):418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- 35.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643(1-2):181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 36.Lane RD, et al. Subgenual anterior cingulate cortex activity covariation with cardiac vagal control is altered in depression. J Affect Disord. 2013;150(2):565–570. doi: 10.1016/j.jad.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomarkenand A, Keener A. Frontal brain asymmetry and depression: A self-regulatory perspective. Cogn Emotion. 1998;12(3):387–420. [Google Scholar]
- 39.Heller AS, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15(7 Pt 1):4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.