Significance
This paper describes a major advance in genomic engineering, describing by far the largest genetic humanization of the mouse ever attempted. Six megabases of mouse immune genes were replaced in a precise manner and “in situ” (in the orthologous position) with the corresponding human immune genes using largechimeric bacterial artificial chromosome targeting vectors. The accompanying manuscript demonstrates that the engineered mice function with high efficiency and have already generated therapeutic candidates that have progressed into human trials.
Keywords: genome engineering, therapeutic antibody, immunoglobulin locus
Abstract
Genetic humanization, which involves replacing mouse genes with their human counterparts, can create powerful animal models for the study of human genes and diseases. One important example of genetic humanization involves mice humanized for their Ig genes, allowing for human antibody responses within a mouse background (HumAb mice) and also providing a valuable platform for the generation of fully human antibodies as therapeutics. However, existing HumAb mice do not have fully functional immune systems, perhaps because of the manner in which they were genetically humanized. Heretofore, most genetic humanizations have involved disruption of the endogenous mouse gene with simultaneous introduction of a human transgene at a new and random location (so-called KO-plus-transgenic humanization). More recent efforts have attempted to replace mouse genes with their human counterparts at the same genetic location (in situ humanization), but such efforts involved laborious procedures and were limited in size and precision. We describe a general and efficient method for very large, in situ, and precise genetic humanization using large compound bacterial artificial chromosome–based targeting vectors introduced into mouse ES cells. We applied this method to genetically humanize 3-Mb segments of both the mouse heavy and κ light chain Ig loci, by far the largest genetic humanizations ever described. This paper provides a detailed description of our genetic humanization approach, and the companion paper reports that the humoral immune systems of mice bearing these genetically humanized loci function as efficiently as those of WT mice.
The laboratory mouse is one of the premier model organisms used by biologists. As a mammal, the mouse is more genetically similar to humans and thus more relevant to human physiology and disease than many other model organisms. Its small size, short generation time, and the availability of a large variety of inbred strains have led to a robust body of classical genetic research on the mouse. The utility of mice as a genetic model is also greatly enhanced by powerful transgenic and knockout technologies, allowing researchers to study the effects of the directed overexpression or deletion of specific genes. However, despite all of its advantages, the mouse remains an imperfect model of human disease and an imperfect platform on which to test potential human therapeutics. One issue is that, although about 99% of human genes have a mouse homolog (1), potential therapeutic agents often do not cross-react, or cross-react only poorly, with the mouse ortholog of the intended human target. To overcome this problem, selected target genes can be humanized, that is, the mouse gene can be eliminated and replaced by the corresponding human orthologous gene sequence. Because of the difficulties of using conventional KO technologies to directly replace large mouse genes with their large human genomic counterparts, genetic humanization is currently most often accomplished by the so-called “KO-plus-transgenic humanization” strategy that involves crossing a KO of the mouse gene with a randomly integrated transgenic of the human gene (2–5). The conceptually more straightforward direct homologous replacement, in which a mouse gene is directly replaced by its human counterpart at the same genetic location (“in situ humanization”), is rarely attempted because of technological difficulties. Heretofore, such efforts have involved laborious procedures and were limited in size and precision; the largest such in situ genetic humanization described to date involved on the order of 100 kb and required four ES cell steps (6).
We have previously shown that VelociGene technology (7), which pioneered the use of large bacterial artificial chromosome (BAC)-based vectors for gene targeting in mouse ES cells, allowed much larger targeted deletions in ES cells than can normally generated with non–BAC-based targeting vectors. Recently, we found that large insertions can also be precisely targeted to specific genomic locations using VelociGene technology. Herein we combine these two features, targeting both large deletions and insertions, to humanize large regions in situ by direct homologous replacement of mouse genes with their human counterparts. Using this approach, more than 200 kb can be precisely humanized in a single step. We exploit this approach to create a mouse with a unique type of genetically humanized immune system.
Early studies showing that exogenously introduced Ig transgenes could successfully be rearranged in precursor B cells led to the suggestion that mice could be genetically humanized to produce fully human monoclonal antibodies (8). Such mice were ultimately engineered and provide an extreme example of the gene humanization strategy using the KO-plus-transgenic approach (9–12). To generate these mice, the endogenous mouse Ig heavy chain and κ light chain loci were inactivated by targeted deletion of small, but critical, portions of each locus and combined with human Ig gene loci introduced as randomly integrated large transgenes or minichromosomes (13). These engineered mice represented an important medical advance, and fully human monoclonal antibodies have been isolated from them with promising therapeutic potential for treating a number of human diseases (11, 12, 14–17). However, these mice display compromised B-cell development (see ref. 18), which may limit their ability to generate fully human antibodies against some antigens. We reasoned that the compromised B-cell development might arise from some combination of three possible sources of inefficiency. First, as with other transgenes, the random introduction of the human Ig transgenes might contribute to their incorrect expression due to a lack of some known mouse downstream enhancer elements (19, 20) and postulated upstream locus control elements (21). Second, inefficient interspecies interactions between the constant domains of human cell surface immunoglobulins and the additional mouse components of the B-cell, and pre-B-cell, receptors might impair signaling processes required for normal maturation, proliferation, and survival of this lineage. Finally, inefficient interspecies interactions between soluble human immunoglobulins and mouse Fc receptors might reduce affinity selection (22) and Ig serum concentrations (23, 24). All three of these possible sources of inefficiency would likely be corrected by in situ humanization of only the variable regions of the mouse Ig loci within their natural locations. Such a strategy would result in mice making “reverse chimeric” (i.e., human V: mouse C) antibodies that would presumably undergo normal interactions and selections with the mouse environment based on retaining mouse constant regions; however, these reverse chimeric antibodies should be readily reformattable into fully human antibodies for therapeutic purposes. Chimeric antibodies (i.e., mouse V: human C) have been shown to be easily formatted (25), and mice have been created that produce them (26, 27).
Thus, we performed precise large-scale in situ replacements of only the variable regions of the mouse heavy and light chain Ig loci (heavy chain V-D-J and κ chain V-J) with the corresponding human sequences. The flanking mouse sequences, including all mouse constant chain genes and locus transcriptional control regions, remained intact and functional within the hybrid loci. In all, 6 Mb of the mouse loci were replaced with 1.4 Mb of human genomic sequences, by far the largest genetic humanizations ever described. As described in the companion article (18), the humoral immune systems of the resulting mice function as efficiently as those of WT mice.
Results
Ig Heavy Chain Variable Locus Humanization.
Antibody genes are rearranged and expressed similarly in humans and mice. The genomic organizations of the heavy chain loci are also very similar between the two species. Therefore, humanization of the variable domain of the heavy chain locus can be accomplished by the conceptually simple, direct replacement of roughly 3 Mbp of contiguous mouse genomic sequence containing all VH, DH, and JH gene segments with roughly 1 Mbp of contiguous human genomic sequence containing the equivalent human gene segments (Fig. 1A). The intron between J segments and constant domains (the J-C intron) contains a transcriptional enhancer (28) followed by a region of simple repeats required for recombination during isotype switching (29). The junction between human V-D-J region and the mouse C region (the proximal junction) was chosen to maintain the mouse intronic enhancer and switch domain to give the best chance for efficient expression and class switching in the mouse. The rational choice for the exact nucleotide position of this and subsequent junctions is possible because the replacements were accomplished using the VelociGene method (7), which relies on bacterial homologous recombination driven by synthesized oligonucleotides. Thus, the proximal junction was placed about 200 bp downstream from the last J segment, and the ultimate distal junction was placed several hundred base pairs upstream of the most 5′ V gene of the human locus and about 9 kbp downstream from VH1-86, also known as J558.55. This most distal variable gene segment, which is a pseudogene in C57BL/6 mice, but potentially active, albeit with a poor RSS sequence, in the targeted 129 allele, was retained due to reports at the time (21) that elements at the distal end of the heavy chain locus might contain elements controlling locus expression and/or rearrangement. Although the elements 20–30 kb from the last VHJ558.55 have since been shown to be dispensable for IgH locus recombination (30), maintenance of mouse sequences at the most distal end of the locus may have buffered against the loss of other functional elements yet to be described.
Fig. 1.
Humanization of the mouse Ig heavy chain variable locus. Representations are not drawn to scale. The general scheme for direct genomic swap is depicted in A. The first three steps (B; steps A, B, and C) result in deletion of all mouse VH, DH, and JH gene segments and insertion of all human DH and JH segments and three human VH gene segments. The representative 3hVH BACvec shown for step A consists of a 67-kb mouse homology arm, a neo selection cassette, a 145-kb human insert, and an 8-kb mouse homology arm. The next six steps (C) result in the insertion of the remainder of the human V gene segments and the removal of the final neo selection cassette. The representative 18hVH BACvec for step D consists of a 20-kb mouse homology arm, a neo selection cassette, 196 kb of human sequence, and a 62-kb human homology arm. Human genomic sequences are dark blue, mouse genomic sequences are red, neo selection cassettes are light blue, hyg selection cassettes are orange, and loxP sites are yellow triangles.
Previous experiments (7) using the VelociGene method had shown that large BAC-based targeting vectors (BACvecs) could be precisely inserted using homologous recombination while simultaneously allowing deletions up to 75 kb, with frequencies (0.5–1%) only slightly below the overall average targeting frequency (3.8%) from 200 projects. The first test of the use of VelociGene for the insertion of large, BAC-sized segments of human Ig DNA sequence into the mouse was the insertion of 144 kb of the proximal end of the human heavy chain locus containing 3 VH, all 27 DH, and 9 JH human gene segments into the proximal end of the mouse IgH locus, with a concomitant 16.6-kb deletion of mouse sequence, using about 75 kb of mouse homology arms (Fig. 1B, step A, and Table 1, 3hVH). This large 144-kb insertion and accompanying 16.6-kb deletion was performed in a single step (step A) that occurred with a frequency of 0.2% (Table 1). Correctly targeted ES cells were scored by the loss-of-native-allele (LONA) assay (7) to determine the copy number of probes within and flanking the deleted mouse sequence and within the inserted human sequence (Fig. S1), and the integrity of the large human insert was verified using multiple probes spanning the entire insertion. Because many rounds of sequential ES cell targeting were anticipated, targeted ES cell clones at this, and all subsequent, steps were subjected to karyotypic analysis, and only those clones showing normal karyotypes in at least 17 of 20 spreads (about 90% of those tested) were used for subsequent steps.
Table 1.
Humanization of mouse Ig heavy chain locus
| Modified hybrid allele name | Amount human sequence (kb) | Size of targeting construct (kb) | Targeting efficiency (%) | Percentage of total hV use | Total V segments | No. of ES cell steps |
| 3hVH | 144 | 240 | 0.2 | 5 | 3 | 1 |
| 3hVH/DC | 144 | 110 | 0.1 | 5 | 3 | 2 |
| 3hVH-cre | 144 | 8 | 5 | 3 | 3 | |
| 18hVH | 340 | 272 | 0.1 | 25 | 18 | 4 |
| 39hVH | 550 | 282 | 0.2 | 60 | 39 | 5 |
| 53hVH | 655 | 186 | 0.4 | 65 | 53 | 6 |
| 70hVH | 850 | 238 | 0.5 | 90 | 70 | 7 |
| 80hVH | 940 | 124 | 0.2 | 100 | 80 | 8 |
| 80hVHdNeo | 940 | 2.6 | 100 | 80 | 9 |
The Ig heavy chain variable gene locus was humanized in nine sequential steps. Transient expression of CRE recombinase directed a 3-Mbp deletion to create the 3hVH-cre allele and the deletion of a neoR selection cassette to create the 80hVHdNeo allele. All other steps were LC-BACvec–driven homologous recombination. Amount of human sequence is the cumulative amount added for each allele. Total V segments includes pseudogenes.
Mice were generated from ES cells containing this initial heavy chain modification by recombination activating gene (RAG) complementation (31), and cDNA was prepared from splenocyte RNA. The cDNA was amplified using primer sets specific for the predicted chimeric heavy chain mRNA that would arise by V-D-J recombination within the inserted human segment and subsequent splicing to either mouse mu or gamma constant domains. Sequences derived from these cDNA clones demonstrated that proper V-D-J recombination had occurred within the human sequences, that the rearranged human V-D-J segments were properly spliced in-frame to mouse constant domains, and that class-switch recombination had occurred. Much more extensive sequence analyses of the mRNA products of subsequent hybrid Ig loci are described in the companion article (18).
Targeted ES cells from step A were retargeted with a BACvec that produced a 19-kb deletion at the distal end of the heavy chain locus (Fig. 1B, step B). The step B BACvec contained a hygromycin resistance gene (hygR) in contrast to the neomycin resistance gene (neoR) contained on the BACvec of step A. The resistance genes from the two BACvecs were also designed in such a way that, if the second targeting occurred on the same chromosome as the first, then an ∼3-Mb portion of the mouse heavy chain locus containing all of the mouse V gene segments other than VH1-86 and all of the D gene segments other than DQ52, as well as the two resistance genes, would be flanked by loxP sites; DQ52 and all of the mouse J chain gene segments had already been deleted in step A. ES cell clones doubly targeted on the same chromosome were identified by driving the 3hVH proximal cassette to homozygosity in high G418 (32) and following the fate of the distal hyg cassette. It has been shown that loxP-flanked segments of mouse chromomes up to 4 Mbp can be deleted in ES cells by transient expression of Cre recombinase with surprisingly high (up to ∼11%) efficiencies even in the absence of drug selection (33). Likewise, we observed the 3-Mbp deletion (Fig. 1B, step C) in 8% of ES cell clones following transient Cre expression (Table 1). The deletion was scored by the LONA assay using probes at either end of the deleted mouse sequence, as well as the loss of neoR and hygR genes and the appearance of a PCR product across the sole remaining loxP site. Finally, the deletion was confirmed by FISH (Fig. S2).
The remainder of the human heavy chain variable region was added to the 3hVH allele in a series of five steps using the Velocigene method (Fig. 1, steps E, F, G, and H), with each step involving precise insertion of up to 210 kb of human gene sequences. For each step, the proximal end of each new BACvec was designed to overlap the most distal human sequences of the previous step, and the distal end of each new BACvec contained the same distal region of mouse homology as used in step A. The BACvecs of steps D, F, and H contained neoR selection cassettes, whereas those of steps E and G contained hygR selection cassettes; thus, selections were alternated between G418 and hygromycin. Targeting in step D was assayed by the loss of the unique PCR product across the distal loxP site of 3hVH. Targeting for steps E through I was assayed by loss of the previous selection cassette. In the final step (I), the neo selection cassette, flanked by FRT sites (34), was removed by transient FLPe expression (35). The human sequences of the BACvecs for steps D, E, and G were derived from two parental human BACs each, whereas those from steps F and H were from single BACs. Retention of human sequences was confirmed at every step using multiple probes spanning the inserted human sequences. Only those clones with normal karyotype and germ-line potential were carried forward in each step. ES cells from the final step were still able to contribute to the germ line after nine sequential manipulations (Table 1). Mice homozygous for each of the heavy chain alleles described in Fig. 1 and Table 1 were viable and appeared healthy, indicating that no essential mouse gene or toxic human gene lies buried within the heavy chain variable region. Southern blot analysis of mice heterozygous and homozygous for the 3hVH allele and 3hVH ES cells compared with human and mouse fibroblasts confirmed that the proximal junction, human variable region, and distal mouse flanking regions remained intact.
Light Chain Variable Humanization.
The κ light chain variable region was humanized in a manner similar to that of the heavy chain (Fig. 2 and Table 2). The variable region of the human κ locus contains two nearly identical 400-kb repeats separated by an 800-kb spacer (36). Because the repeats are so similar, nearly all of the locus diversity can be captured without the distal repeat. In fact, a natural human allele of the κ locus is missing the distal repeat (37). Thus, we replaced about 3 Mbp of mouse sequence with about 0.5 Mbp of human sequence to replace all of the mouse VK and JK segments with the proximal human VK and all of the human JK gene segments (Fig. 2A). Unlike the heavy chain locus, the entire mouse variable gene region, containing all VK and JK gene segments, was deleted in a three-step process before any human sequence was added. First a neoR cassette was introduced at the proximal end of the variable region (Fig. 2B, step A). Next, a hygR cassette was inserted at the distal end of the κ locus (Fig. 2B, step B). LoxP sites were again situated within each selection cassette such that Cre treatment induced deletion of the remaining 3 Mbp of the mouse κ variable region along with both resistance genes (Fig. 2B, step C).
Fig. 2.
Humanization of the mouse Ig κ light chain locus. Representations are not drawn to scale. The general scheme for direct genomic swap is depicted in A. The first three steps (B; steps A, B, and C) result in deletion of all mouse VH, and JH gene segments, whereas the subsequent five steps (C) result in the insertion of all human JK gene segments and all human VK segments in the proximal repeat and deletion of the final hyg selection cassette. Human genomic sequences are dark blue, mouse genomic sequences are red, neo selection cassettes are light blue, hyg selection cassettes are orange, and loxP sites are yellow triangles.
Table 2.
Humanization of mouse Ig κ light chain locus
| Modified allele name | Amount human sequence (kb) | Size of targeting construct (kb) | Targeting efficiency (%) | Percentage of total hV use | Total V segments | No. of ES cell steps |
| IgK-PC | 0 | 132 | 1.1 | 1 | ||
| IgK-PC/DC | 0 | 90 | 0.4 | 2 | ||
| IgK-cre | 0 | 1 | 3 | |||
| hybrid 6hVK | 110 | 122 | 0.3 | 14 | 6 | 4 |
| 16hVK | 240 | 203 | 0.4 | 47 | 16 | 5 |
| 30hVK | 390 | 193 | 0.1 | 70 | 30 | 6 |
| 40hVK | 480 | 185 | 0.2 | 100 | 40 | 7 |
| 40hVKdHyg | 480 | 0.7 | 100 | 40 | 8 |
The Ig κ light chain variable gene locus was humanized in eight sequential steps. Transient expression of CRE recombinase directed a 3-Mbp deletion to create the IgK-cre allele and the deletion of a hygR selection cassette to create the 40hVKdHyg allele. All other steps were BACvec-driven homologous recombination. Amount of human sequence is the cumulative amount added for each allele. Total V segments includes pseudogenes.
A 480-kb contiguous stretch of human genomic DNA containing the entire κ variable region was inserted in four sequential steps (Fig. 2C and Table 2), with up to 150 kb of human DNA inserted in a single step, using methods similar to those used for the heavy chain. The final hygromycin resistance gene was removed by transient FLPe expression. As with the heavy chain, targeted ES cell clones were evaluated for integrity of the entire human insert, normal karyotype, and germ-line potential after every step. Mice homozygous for each of the κ chain alleles were generated and found to be healthy and of normal appearance.
Combining Heavy Chain and κ Light Chain Humanized Alleles.
Mice doubly homozygous for both heavy and κ light chain humanizations were generated from a subset of the alleles described. For convenience, we have named mice homozygous for both 18hVH and 16hVK alleles “VelocImmune I,” mice homozygous for both 39hVH and 30hVK alleles “VelocImmune II,” and mice homozygous for the complete set of 80hVH and 40hVK alleles “VelocImmune III.” All genotypes observed during the course of breeding to generate the doubly homozygous mice occurred in roughly Mendelian proportions. However, we observed that male mice homozygous for each of the heavy chain alleles showed reduced fertility. We recently identified and corrected the problem of reduced male fertility. Briefly, Adam6 genes required for mouse sperm function are embedded within the mouse IgH locus and are not conserved in humans; reinserting these mouse genes into the humanized locus restored mouse fertility.
BAC Recombineering to Generate Large Compound BAC-Based Targeting Vectors.
To perform the large genetic humanizations described above, we needed to generate a variation of the BAC-based targeting vectors (BACvecs) previously described (7), in which human and mouse BACs were combined to create large compound BACvecs (LC-BACvecs). A total of 13 different LC-BACvecs were used to humanize the variable region genes of both the heavy and κ light chain loci. For illustrative purposes, a brief description of the construction of the LC-BACvec used for step A of heavy chain humanization is provided here, whereas construction of the remaining 12 LC-BACvecs is described in Tables S2 and S4.
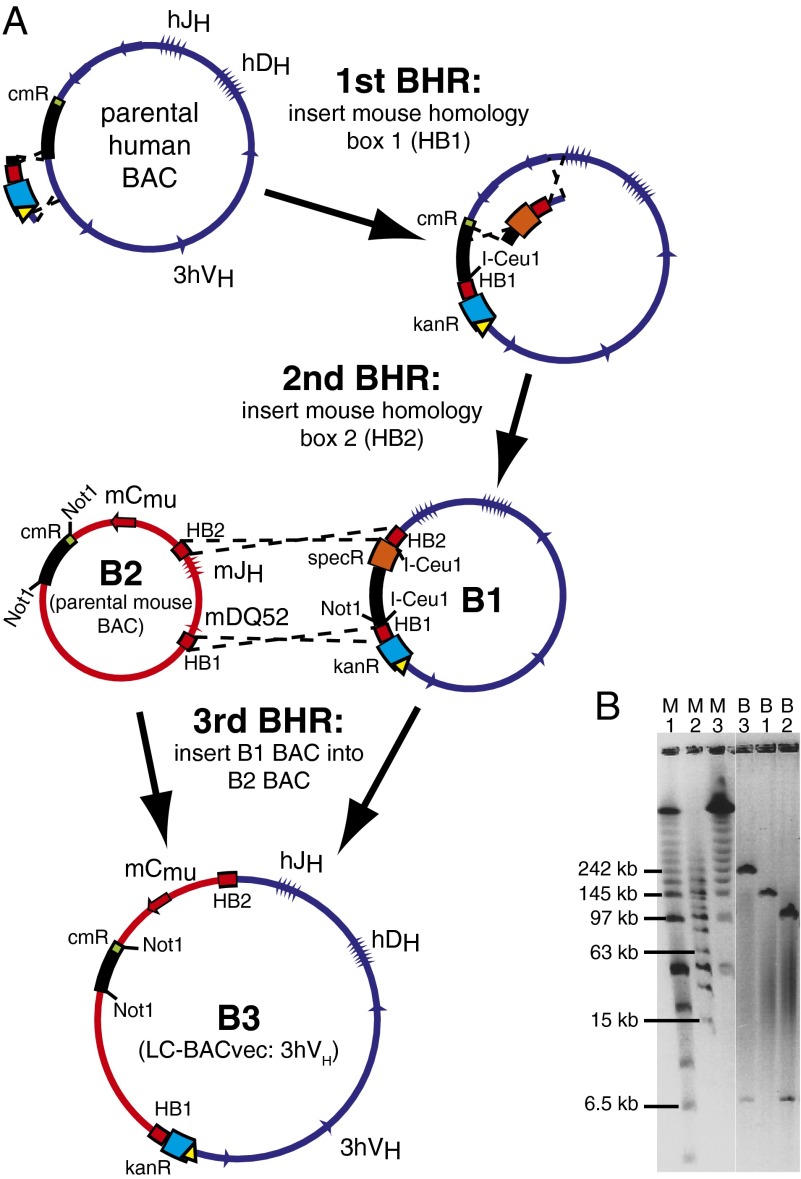
The 3hVH LC-BACvec was constructed using three sequential bacterial homologous recombination (BHR) steps (Fig. 3). In step 1, a cassette was introduced upstream from VH1–3 that contains a region of homology to the mouse IgH locus (HB1), a gene that confers kanamycin resistance in bacteria and G418 resistance in animals cells (kanR) and a loxP site. In step 2, a second cassette was introduced just downstream from the last JH segment that contains a second region of homology to the mouse IgH locus (HB2) and a gene that confers resistance in bacteria to spectinomycin (specR). Step 2 also deleted human IgH sequences downstream from JH6 and the BAC vector chloramphenicol resistance gene (cmR). In step 3, the doubly modified human BAC (B1) was then linearized using I-Ceu1 sites that had been added during steps 1 and 2 and integrated into a mouse BAC (B2) by BHR through the HB1 and HB2 regions of homology. The drug selections for step 1 [chloramphenicol/kanamycin (cm/kan)], step 2 (spectinomycin/kanamycin), and step 3 (cm/kan) were designed to be specific for the desired products.
Fig. 3.
Construction of LC-BACvec. (A) The three steps in the construction of the 3hVH BACvec. pBelo BAC vector is black, human genomic sequences are dark blue, mouse genomic sequences are red, neo/kan selection cassette is light blue, cmR gene is green, specR gene is dark orange, and loxP site is yellow triangle. PFG analysis (B) of three BACs, B1, B2, and B3, after digestion with Not1. Markers M1, M2, and M3 are low range, mid-range, and lambda ladder PFG markers (New England BioLabs), respectively.
Similar steps were performed in the creation of the other 12 LC-BACvecs used. A common variation was the use of ligation instead of BHR to conjoin two large BACs (SI Materials and Methods). In those cases, rare restriction sites were introduced into both BAC parents by BHR along with careful placement of selectable markers so that only the desired ligation product is able to survive on a certain combination of drugs. Another variation that we have begun to use for other humanization projects is the consolidation of the equivalent of steps 1 and 2 of Fig. 3 into a single step by using a “gap repair” fragment similar to methods described by Zhang et al. (38).
Discussion
The methodologies described here are broadly applicable for large-scale engineering of the mouse genome. They rely on robust established technologies of VelociGene (7), bacterial homologous recombination (39, 40), and Cre recombinase-mediated large deletions (33), together with a variation of the original VelociGene technology involving the creation of LC-BACvecs. Using our approach, we routinely inserted up to 210 kb of human sequences in a single step using LC-BACvecs and normal drug selections, allowing us to efficiently replace 6 Mb of the mouse genome with the corresponding human genetic sequences. An alternate strategy for large-scale in situ humanization was recently published (6) that used a variation of recombinase mediated cassette exchange (41, 42) to introduce a 117-kb segment into a specific location in the mouse genome. This method required three ES cell manipulations for a single 100-kb insertion that was obtained only under very stringent drug selection using a split HPRT gene and two negatively selected tk genes.
The ability to replace very large, megabase-sized regions of the mouse genome with very high nucleotide-level precision has allowed us to create superior mice for the generation of human monoclonal antibodies. The dramatically improved functioning of VelocImmune mice compared with the previous generation of humanized antibody mice that were created by random integration of human Ig transgenes (18) is a powerful indication of the importance of precise engineering for genetic humanization projects. On a less complex scale, it is highly desirable to generate mice in which the specific drug targets are humanized. This is especially true for the targets of monoclonal antibody therapeutics because, due to their high degree of specificity, antibodies to human targets rarely cross-react with their mouse orthologs. The limited preclinical testing of monoclonals that is currently performed in monkeys and that is often compromised due to poor cross-reactivity could be performed more cheaply, more humanely, more extensively, and with greater confidence in humanized mice. As we have done with the Ig loci, the most effective humanization of transmembrane targets will often be to generate a chimeric gene in which the genomic region encoding the extracellular domain is humanized and fused within an intron to mouse genomic sequences encoding the transmembrane and intracellular regions.
It should also now be possible to humanize other large loci, for instance, those encoding the T-cell receptor and major histocompatability loci, to better mimic human cell-based immunity. In addition, swaps of large regions of the genome of one inbred mouse strain with that of another inbred strain or a mutant strain may now be considered in the later stages of positional mapping of phenotype-associated strain-specific polymorphisms or chemically induced mutations that have been mapped to megabase-sized regions by standard breeding.
Materials and Methods
Identification of Human and Mouse BACs.
Mouse BACs that span the 5′ and 3′ ends of the IgH and Igk locus were identified by hybridization of filters spotted with the BAC library or by PCR screening mouse BAC library DNA pools. Filters were hybridized under standard conditions using probes that corresponded to the regions of interest. Library pools were screened by PCR using unique oligos pairs that flank the targeted region of interest. Additional PCR using the same oligos was done to deconvolute the well and isolate the corresponding BAC of interest. Both BAC filters and library pools were generated from 129 SvJ mouse ES cells (Incyte Genomics/Invitrogen). Human BACs that cover the entire IgH and Igk locus were identified either by hybridization of filters spotted with BAC library (Caltech B, C, or D Libraries and RPCI-11 Library; Research Genetics/Invitrogen), by screening human BAC library pools (Caltech Library; Invitrogen) by the PCR method as described above or by using a BAC end sequence database (Caltech D library; Tigr).
Construction of BACvecs by Bacterial Homologous Recombination and Ligation.
BHR was performed as described previously (7, 40). In most cases, linear fragments were generated by ligating PCR-derived homology boxes to cloned cassettes followed by gel isolation of ligation products and electroporation into BHR-competent bacteria harboring the target BAC. After selection on appropriate antibiotic plates, correctly recombined BACs were identified by PCR across both novel junctions followed by restriction analysis on pulsed-field gels (43) and spot checking by PCR using primers distributed across the human sequences (Table S1). Recombinant BACs obtained by ligation after digestion with rare restriction enzymes were identified and screened in a similar manner. A detailed description of all of the steps for constructing all LC-BACvecs is provided in Tables S2–S5.
Modification of ES Cells and Generation of Mice.
Targeting of ES cells (F1H4) was performed using the VelociGene method as previously described (7). Between 2 and 24 96-well plates were screened for each targeting step. Derivation of mice from modified ES cells by either blastocyst (7) or eight-cell morula (44) injection was as previously described. The sequences of probes and primers used to screen for targeted ES cells and mice are included in Tables S6 and S7. All mouse studies were overseen and approved by Regeneron’s Institutional Animal Care and Use Committee (IACUC).
Karyotyping.
Karyotyping was performed by Coriell Cell Repositories, Coriell Institute for Medical Research.
FISH.
FISH was performed on targeted ES cells as previously described (7). Probes corresponding to either mouse BAC (929d24) or human BAC (modified 2572o2) were labeled by nick translation (Invitrogen) with the fluorescently labeled dUTP nucleotides (Vysis) Spectrum Orange or Spectrum Green as indicated.
Supplementary Material
Acknowledgments
We thank Silvia Rivera, Diana Barber, Kevin Pobursky, Marylene Boucher, Amalia Dutra, Lakeisha Esau, Jennifer A. Griffiths, and Jinsop Om for technical assistance and Neil Stahl and Len Schleifer for advice and encouragement. F.W.A. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: G.D.Y. is a board member, employee, and shareholder of Regeneron Pharmaceuticals, Inc. Other Regeneron authors are employees and shareholders of Regeneron Pharmaceuticals, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323896111/-/DCSupplemental.
References
- 1.Waterston RH, et al. Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 2.Bril WS, et al. Tolerance to factor VIII in a transgenic mouse expressing human factor VIII cDNA carrying an Arg(593) to Cys substitution. Thromb Haemost. 2006;95(2):341–347. doi: 10.1160/TH05-08-0559. [DOI] [PubMed] [Google Scholar]
- 3.Homanics GE, Skvorak K, Ferguson C, Watkins S, Paul HS. Production and characterization of murine models of classic and intermediate maple syrup urine disease. BMC Med Genet. 2006;7:33. doi: 10.1186/1471-2350-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamsai D, et al. A humanized BAC transgenic/knockout mouse model for HbE/beta-thalassemia. Genomics. 2006;88(3):309–315. doi: 10.1016/j.ygeno.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Pan Q, Brodeur JF, Drbal K, Dave VP. Different role for mouse and human CD3delta/epsilon heterodimer in preT cell receptor (preTCR) function: Human CD3delta/epsilon heterodimer restores the defective preTCR function in CD3gamma- and CD3gammadelta-deficient mice. Mol Immunol. 2006;43(11):1741–1750. doi: 10.1016/j.molimm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Wallace HA, et al. Manipulating the mouse genome to engineer precise functional syntenic replacements with human sequence. Cell. 2007;128(1):197–209. doi: 10.1016/j.cell.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela DM, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21(6):652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 8.Alt FW, Blackwell TK, Yancopoulos GD. Immunoglobulin genes in transgenic mice. Trends Genet. 1985;1(1):231–236. [Google Scholar]
- 9.Green LL, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994;7(1):13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- 10.Lonberg N, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368(6474):856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- 11.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23(9):1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 12.Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol. 2007;25(10):1134–1143. doi: 10.1038/nbt1337. [DOI] [PubMed] [Google Scholar]
- 13.Tomizuka K, et al. Double trans-chromosomic mice: Maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc Natl Acad Sci USA. 2000;97(2):722–727. doi: 10.1073/pnas.97.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson TB, Ranganathan A, Grothey A. Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6(1):29–31. doi: 10.3816/CCC.2006.n.01. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, et al. Clinical efficacy of zanolimumab (HuMax-CD4): Two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109(11):4655–4662. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- 16.Maker AV, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: A phase I/II study. Ann Surg Oncol. 2005;12(12):1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClung MR, et al. AMG 162 Bone Loss Study Group Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AJ, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111:5153–5158. doi: 10.1073/pnas.1324022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrett FE, et al. Chromatin architecture near a potential 3′ end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol Cell Biol. 2005;25(4):1511–1525. doi: 10.1128/MCB.25.4.1511-1525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manis JP, Michaelson JS, Birshtein BK, Alt FW. Elucidation of a downstream boundary of the 3′ IgH regulatory region. Mol Immunol. 2003;39(12):753–760. doi: 10.1016/s0161-5890(02)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Pawlitzky I, et al. Identification of a candidate regulatory element within the 5′ flanking region of the mouse Igh locus defined by pro-B cell-specific hypersensitivity associated with binding of PU.1, Pax5, and E2A. J Immunol. 2006;176(11):6839–6851. doi: 10.4049/jimmunol.176.11.6839. [DOI] [PubMed] [Google Scholar]
- 22.Rao SP, Vora KA, Manser T. Differential expression of the inhibitory IgG Fc receptor FcgammaRIIB on germinal center cells: Implications for selection of high-affinity B cells. J Immunol. 2002;169(4):1859–1868. doi: 10.4049/jimmunol.169.4.1859. [DOI] [PubMed] [Google Scholar]
- 23.Brambell FW, Hemmings WA, Morris IG. A theoretical model of gamma-globulin catabolism. Nature. 1964;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- 24.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93(11):5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: Mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA. 1984;81(21):6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou YR, Gu H, Rajewsky K. Generation of a mouse strain that produces immunoglobulin kappa chains with human constant regions. Science. 1993;262(5137):1271–1274. doi: 10.1126/science.8235658. [DOI] [PubMed] [Google Scholar]
- 27.Zou YR, Müller W, Gu H, Rajewsky K. Cre-loxP-mediated gene replacement: A mouse strain producing humanized antibodies. Curr Biol. 1994;4(12):1099–1103. doi: 10.1016/s0960-9822(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 28.Neuberger MS. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka T, Kawakami T, Takahashi N, Honjo T. Rearrangement of immunoglobulin gamma 1-chain gene and mechanism for heavy-chain class switch. Proc Natl Acad Sci USA. 1980;77(2):919–923. doi: 10.1073/pnas.77.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlot T, et al. Analysis of mice lacking DNaseI hypersensitive sites at the 5′ end of the IgH locus. PLoS ONE. 2010;5(11):e13992. doi: 10.1371/journal.pone.0013992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: An assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90(10):4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AA, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng B, Sage M, Sheppeard EA, Jurecic V, Bradley A. Engineering mouse chromosomes with Cre-loxP: Range, efficiency, and somatic applications. Mol Cell Biol. 2000;20(2):648–655. doi: 10.1128/mcb.20.2.648-655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLeod M, Craft S, Broach JR. Identification of the crossover site during FLP-mediated recombination in the Saccharomyces cerevisiae plasmid 2 microns circle. Mol Cell Biol. 1986;6(10):3357–3367. doi: 10.1128/mcb.6.10.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchholz F, Angrand PO, Stewart AF. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat Biotechnol. 1998;16(7):657–662. doi: 10.1038/nbt0798-657. [DOI] [PubMed] [Google Scholar]
- 36.Weichhold GM, Ohnheiser R, Zachau HG. The human immunoglobulin kappa locus consists of two copies that are organized in opposite polarity. Genomics. 1993;16(2):503–511. doi: 10.1006/geno.1993.1217. [DOI] [PubMed] [Google Scholar]
- 37.Schaible G, Rappold GA, Pargent W, Zachau HG. The immunoglobulin kappa locus: Polymorphism and haplotypes of Caucasoid and non-Caucasoid individuals. Hum Genet. 1993;91(3):261–267. doi: 10.1007/BF00218268. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Muyrers JP, Testa G, Stewart AF. DNA cloning by homologous recombination in Escherichia coli. Nat Biotechnol. 2000;18(12):1314–1317. doi: 10.1038/82449. [DOI] [PubMed] [Google Scholar]
- 39.Muyrers JP, Zhang Y, Testa G, Stewart AF. Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res. 1999;27(6):1555–1557. doi: 10.1093/nar/27.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet. 1998;20(2):123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 41.Bouhassira EE, Westerman K, Leboulch P. Transcriptional behavior of LCR enhancer elements integrated at the same chromosomal locus by recombinase-mediated cassette exchange. Blood. 1997;90(9):3332–3344. [PubMed] [Google Scholar]
- 42.Feng YQ, et al. Site-specific chromosomal integration in mammalian cells: Highly efficient CRE recombinase-mediated cassette exchange. J Mol Biol. 1999;292(4):779–785. doi: 10.1006/jmbi.1999.3113. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz DC, Cantor CR. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 44.Poueymirou WT, et al. F0 generation mice fully derived from gene-targeted embryonic stem cells allowing immediate phenotypic analyses. Nat Biotechnol. 2007;25(1):91–99. doi: 10.1038/nbt1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.