Allouch et al. (1) have shown that CDKN1A (p21) restricts HIV-1 replication in monocyte-derived macrophages (MDM) by controlling the expression of the ribonucleotide reductase subunit R2 (RNR2) of the ribonucleotide reductase enzyme that, in turn, controls the intracellular deoxynucleotide (dNTP) pool required for HIV-1 reverse transcription. dNTP levels are also tightly controlled by the dNTP triphosphohydrolase SAM domain and HD domain-containing protein 1 (SAMHD1), which is constitutively expressed in myeloid and lymphoid cells and is counteracted by the lentiviral virus protein x (Vpx) (reviewed in ref. 2). SAMHD1 is deactivated in proliferating cells by a mechanism that requires phosphorylation of SAMHD1 (3). Allouch et al. (1) conclude that p21-driven HIV-1 restriction in macrophages is independent of SAMHD1 because (i) p21 did not affect SAMHD1 expression and (ii) Vpx did not affect p21 expression.
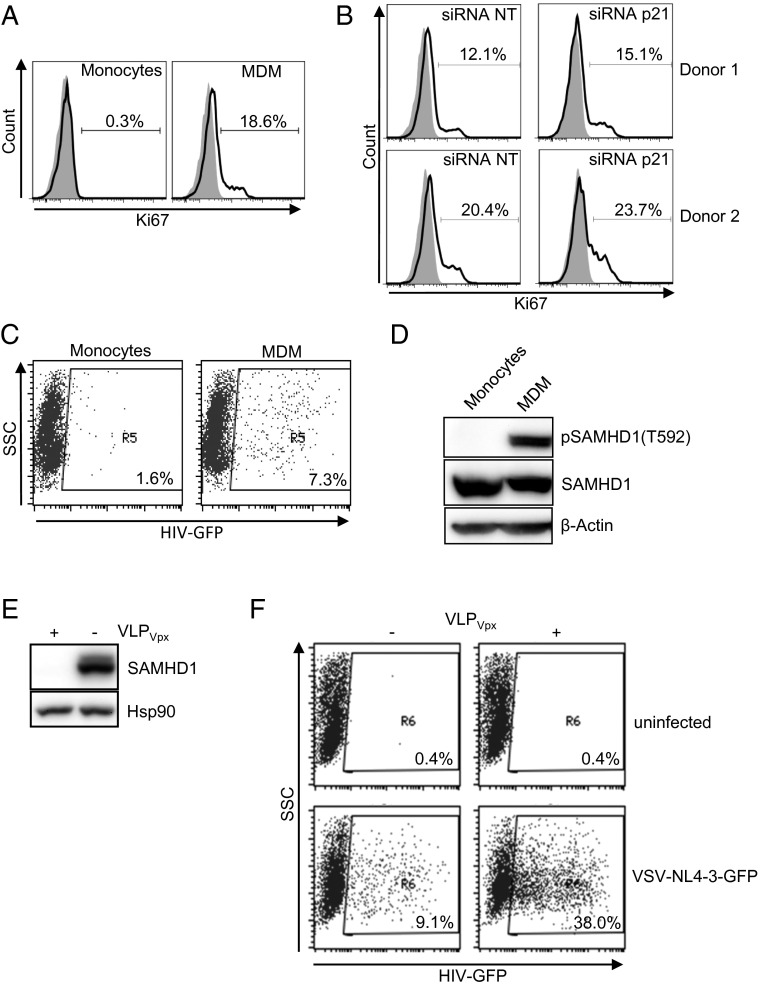
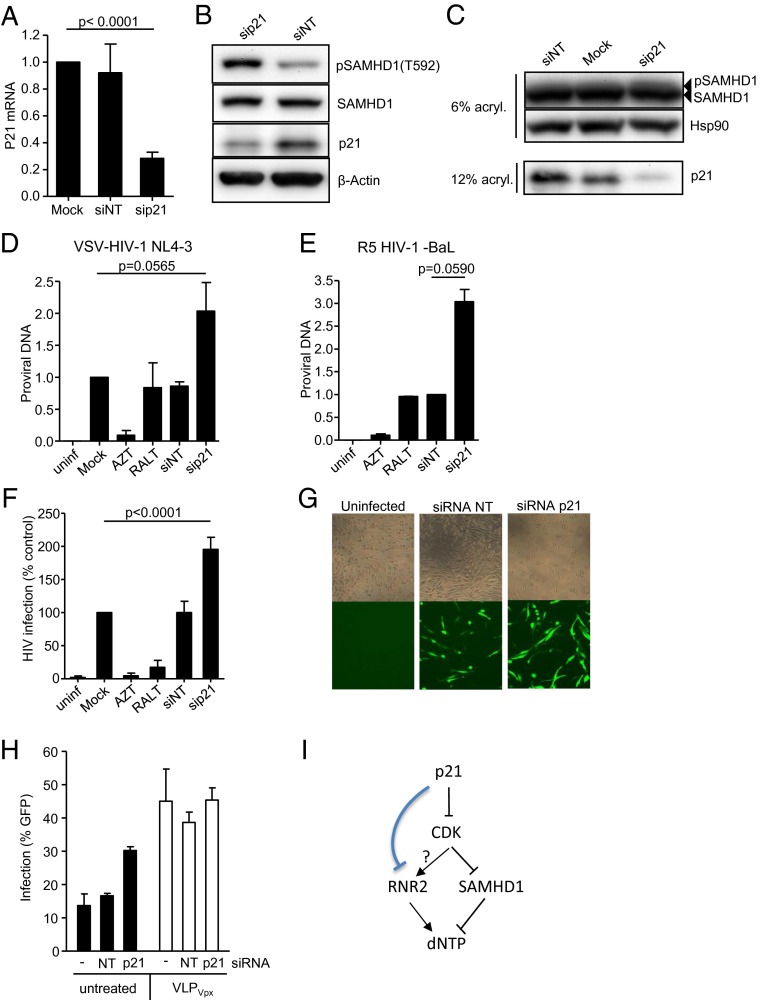
Here, we show that M-CSF induces monocyte differentiation into macrophages and cell proliferation (Fig. 1A), and RNA interference of p21 leads to an increase in the number of proliferating cells (Fig. 1B). Macrophages become susceptible to HIV-1 replication (Fig. 1C) because SAMHD1 is inactivated as measured by specific SAMHD1 phosphorylation at residue T592 (Fig. 1D). Delivery of simian immunodeficiency virus (SIV) mac Vpx-induced SAMHD1 degradation (Fig. 1E) and subsequently increased virus infection (Fig. 1F) (2, 4, 5). Of importance, siRNA-induced down-regulation of p21 (Fig. 2A) strongly enhanced the phosphorylation of SAMHD1 (Fig. 2 B and C), followed by an increase in HIV-1 proviral DNA formation (Fig. 2 D and E) and virus infection (Fig. 2 F and G), without affecting the overall SAMHD1 expression (Fig. 2 B and C). Our results strongly suggest that p21 positively correlates with the degree of SAMHD1-mediated HIV-1 restriction. Allouch et al. (1) did not assess SAMHD1 deactivation by phosphorylation and, therefore, were unable to exclude a role for SAMHD1 in p21-mediated HIV-1 restriction. In their study, up-regulation of p21 by immobilized Ig (IVIg) and siRNA down-regulation of RNR2 in the presence or absence of Vpx were also used to suggest that p21-mediated regulation of HIV-1 was independent of SAMHD1 expression. However, Allouch et al. did not evaluate the effect of p21 down-regulation in the presence or absence of SAMHD1. Here, we show that the increased HIV-1 replication observed after SAMHD1 degradation is not affected or further enhanced by down-regulation of p21 (Fig. 2H), suggesting that the effect of p21 was indeed dependent on SAMHD1 expression. Nevertheless, these results do not formally exclude the possibility of a dual effect of p21 through RNR2 and through SAMHD1.
Fig. 1.
SAMHD1 is phosphorylated in MDM that are susceptible to HIV-1 infection. (A) Ki67 expression in monocytes isolated from buffy-coats from healthy (seronegative) donors before and after stimulation with M-CSF as described in ref. 5. (B) MDM were transfected with a siRNA pool (siRNA; siGENOME SMARTpool, Dharmacon) as described in ref. 5, targeting p21 or a nontargeting siRNA control (siRNA NT) and Ki67+ cells were evaluated by flow cytometry. Histograms corresponding to two representative donors are shown. (C) Monocytes before and after 3-d stimulation with M-CSF were infected with a GFP-expressing, VSV-pseudotyped NL4-3 HIV-1 virus (5) and infection measured by GFP+ cells 2 d postinfection. (D) Lysates of monocytes and differentiated MDM were run in a polyacrylamide gel, transferred, and blotted with an antibody recognizing the phosphorylated form of SAMHD1 at position T592 (3), anti-SAMHD1 (Abcam), and an anti–β-actin antibody (Sigma-Aldrich). One representative blot is shown. (E) SAMHD1 expression in MDM treated (+) or not (−) with viral-like particles carrying Vpx (VLPVpx) was analyzed by Western blot. Hsp90 antibody (BD Biosciences) was used as a loading control. (F) HIV-1 infection measured as percentage of GFP+ cells of macrophages in the absence (−) or presence (+) of viral-like particles carrying Vpx (VLPVpx). For the production of VLPVpx, HEK293T cells were cotransfected with pSIV3+ and a VSV-G–expressing plasmid. One representative experiment of three is shown.
Fig. 2.
RNA interference of p21-induced SAMHD1 phosphorylation and increased HIV-1 replication. (A) MDM were transfected with a siRNA targeting p21 (sip21) or a nontargeting siRNA control (siNT), and mRNA was quantified by quantitative PCR (qPCR). Mean ± SD of four independent experiments are shown. (B) Lysates of cells treated with the indicated siRNA were immunoblotted with antibodies recognizing the phosphorylated form of SAMHD1 (pSAMHD1) at position T592, anti-SAMHD1, anti-p21 (Cell Signaling Technologies) and anti–β-actin. One representative blot of three is shown. (C) Alternatively, cell lysates were run in 6% polyacrylamide gels and SAMHD1 phosphorylation (pSAMHD1) compared between samples as the appearance of slow-migrating forms of SAMHD1. (D) MDM treated with the indicated siRNA were infected with a VSV-pseudotyped NL4-3 virus and proviral DNA was quantified by qPCR after 16 h and normalized to the proviral DNA detected in macrophages transfected in the absence of siRNA (Mock). Next, 3 μM of the reverse-transcriptase inhibitor AZT and 2 μM of the HIV integrase inhibitor raltegravir (RALT) were used as control. Ct values for proviral DNA were normalized using RNaseP as house-keeping gene using the ΔΔCt method. Mean ± SD of three independent experiments are shown. (E) As in D, MDM were infected with the R5-tropic strain BaL. Proviral DNA was normalized to the proviral DNA detected in macrophages transfected with a nontargeting siRNA control (siNT). Mean ± SD of two independent experiments are shown. (F and G) Virus replication as measured by GFP expression at 48 h postinfection with a VSV-pseudotyped NL4-3 GFP-expressing virus. Evaluated by (F) flow cytometry or (G) immunofluorescence microscopy. (Magnification: 100×.) For F, mean ± SD of six independent experiments are shown. (H) Infection of siRNA-treated macrophages in the absence (black bars) or presence (white bars) of viral-like particles carrying Vpx (VLPVpx). Mean and SD of three independent infections of the same donor are shown. Three independent donors showed similar results. (I) p21 is a known inhibitor of cyclin-dependent kinases shown to regulate CDK1/2 function in cell culture and in vivo. In turn, it has been shown that SAMHD1 activity is controlled by CDK through phosphorylation leading to SAMHD1 deactivation and increased dNTP levels required for infection. Conversely, RNR2 increases dNTP. As shown in ref. 1, p21 may directly affect RNR2 function. We envision that RNR2 may be controlled by p21 upstream of CDK.
p21 is a cyclin-dependent kinase inhibitor (1) controlling cell-cycle progression through activation of cyclin-CDK1/2 complexes. Recently, it has been shown that phosphorylation of SAMHD1 by CDK1 leads to SAMHD1 inactivation (3). Thus, cell-cycle control must include a coordinated regulation of RNR2 and SAMHD1 function and dNTP availability. In this scenario, CDK activity may be the underlying mechanism explaining p21-mediated control of RNR2 and SAMHD1 (Fig. 2I).
Acknowledgments
This work was funded through the Ministerio de Economía y Comercio BFU2012-31569, SAF2010-21617-C02, and PI13/01083.
Footnotes
The authors declare no conflict of interest.
References
- 1.Allouch A, et al. p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci USA. 2013;110(42):E3997–E4006. doi: 10.1073/pnas.1306719110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauls E, Ballana E, Esté JA. Nucleotide embargo by SAMHD1: A strategy to block retroviral infection. Antiviral Res. 2013;97(2):180–182. doi: 10.1016/j.antiviral.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 3.White TE, et al. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe. 2013;13(4):441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldauf HM, et al. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med. 2012;18(11):1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauls E, et al. Restriction of HIV-1 replication in primary macrophages by interleukin-12 and interleukin-18 through the upregulation of SAMHD1. J Immunol. 2013;(190):4736–4741. doi: 10.4049/jimmunol.1203226. [DOI] [PubMed] [Google Scholar]