Significance
The leading accounts of face recognition are the face-specific view and the expertise view. The face-specific account claims upright faces are processed by brain mechanisms specialized for faces, whereas the expertise hypothesis claims faces are processed by mechanisms engaged by fine-grained discrimination of the object of expertise. The effects of expertise on visual recognition have been studied in the laboratory using greebles (objects designed to place face-like demands on recognition mechanisms), and behavioral and neural markers characteristic of face processing have been reported for greebles after training. Here we present compelling evidence that performance with greebles does not depend on face recognition mechanisms. Two individuals with acquired prosopagnosia displayed normal greeble learning despite severely impaired face performance. These results support the face-specific account.
Abstract
Face recognition is generally thought to rely on different neurocognitive mechanisms than most types of objects, but the specificity of these mechanisms is debated. One account suggests the mechanisms are specific to upright faces, whereas the expertise view proposes the mechanisms operate on objects of high within-class similarity with which an observer has become proficient at rapid individuation. Much of the evidence cited in support of the expertise view comes from laboratory-based training experiments involving computer-generated objects called greebles that are designed to place face-like demands on recognition mechanisms. A fundamental prediction of the expertise hypothesis is that recognition deficits with faces will be accompanied by deficits with objects of expertise. Here we present two cases of acquired prosopagnosia, Herschel and Florence, who violate this prediction: Both show normal performance in a standard greeble training procedure, along with severe deficits on a matched face training procedure. Herschel and Florence also meet several response time criteria that advocates of the expertise view suggest signal successful acquisition of greeble expertise. Furthermore, Herschel’s results show that greeble learning can occur without normal functioning of the right fusiform face area, an area proposed to mediate greeble expertise. The marked dissociation between face and greeble expertise undermines greeble-based claims challenging face-specificity and indicates face recognition mechanisms are not necessary for object recognition after laboratory-based training.
Cognitive neuroscientists generally agree that the visual mechanisms involved in face recognition are different from those involved in most other types of object recognition, but the question of what these mechanisms are specialized for is a long-running debate. Are the mechanisms specific to faces, or do objects sharing certain properties with faces also engage them?
The expertise hypothesis (1, 2) suggests the mechanisms involved in face processing are also engaged by objects with high within-class similarity for which people have become experts at rapid individuation. Evidence cited in support of this view comes from studies with real-world experts and laboratory-trained experts. Behavioral markers characteristic of face perception have been claimed to occur for the perception of dogs (1), fingerprints (3), handwriting (4), and chess configurations (5) in individuals who are long-time experts with these categories (but see refs. 6–8). Furthermore, brain areas involved in face processing were reported to show a stronger response to bird and car stimuli in participants with expertise for these objects, although it is important to note that areas not selective for faces also showed an elevated response (9–11; see also ref. 12). Real-world expertise, however, is difficult to study because experts are difficult to find, expertise level cannot be experimentally controlled, and the acquisition of face-like expertise with objects has been suggested to take many years (1, 2). To overcome these limitations, advocates of the expertise hypothesis developed an alternative approach in which participants are trained in the laboratory to become experts with “greebles,” an artificial class of objects designed to place face-like demands on recognition mechanisms (2, 13). After 7 to 10 sessions of learning to identify individual greebles along with the family or sex of the greebles, most participants become proficient at recognizing greebles and reach the criterion claimed to indicate expertise (2). The greeble training procedure is relatively fast and simple, which makes it an attractive method to investigate the effects of short-term training on object recognition processes. Furthermore, unlike studies of real-world object expertise (1, 7), studies with greebles allow participants to be tested before and after training. Several studies have reported that greeble training elicited perceptual (2, 14) and neural (15, 16) effects similar to the effects characteristic of face processing (but see refs. 17–18 for contrary evidence with greebles and novel nongreeble objects). Greebles were also found to interfere with the N170 signal, a reliable marker of face mechanisms (19, 20), when presented concurrently with faces, suggesting shared processing (21). Importantly, all these effects were reported after, but not before, training, implying that face perception mechanisms may be recruited by other object categories with which people acquire sufficient expertise.
A fundamental prediction of the expertise hypothesis is that individuals with compromised face recognition (prosopagnosia) should also be impaired at acquiring and applying expertise with other objects such as greebles. Previous studies of greeble learning in prosopagnosics have been performed, but several factors complicated their interpretation. Two acquired cases, SM (22) and LR (23), were unable to develop normal greeble expertise, in line with the prediction of the expertise hypothesis. However, their poor performance may be explained by factors other than a disruption of expertise mechanisms, such as general object recognition deficits in the case of SM (24) or difficulty in learning the individual greeble names in the case of LR (23). Consistent with this interpretation, both cases were impaired in greeble recognition from the beginning of training, when neither patients nor controls are greeble experts. Participants with deficits limited to mechanisms necessary for expert processing would be expected to perform normally in early sessions but show deficits in later sessions after control participants have acquired expertise. Edward, a developmental prosopagnosic with normal object recognition, performed as well as controls in greeble training (25), but his performance with greebles was not contrasted with performance on a parallel face training paradigm, so it is not clear that the greeble training procedure would have elicited deficits in Edward if faces were used.
Here we test whether two prosopagnosics, Florence and Herschel, are impaired in a standard greeble training procedure in a manner that avoids previous limitations. First, their normal performance in the first sessions with greebles (when everyone is a greeble novice) ensured the prosopagnosic and control participants started on equal footing and provided assurance that nonexpertise-related factors would not affect the prosopagnosics’ performance in later sessions. Second, to ensure the training procedure elicited impaired performance in Florence and Herschel when faces were used rather than greebles, we also created a similar procedure with computer-generated faces that was matched for difficulty with the greeble training. The training routine in the current study was very similar to that used in prior greeble studies (16, 25–27), including the most recent study, in which a prosopagnosic participant was tested by advocates of the expertise view (23). It involved eight sessions during which participants progressively learned to identify greebles at the individual and family level (or learned to identify faces at the individual level). Normal greeble learning by Florence and/or Herschel would be compelling evidence that the acquisition of expertise in the greeble task (or whatever is needed for successful performance) does not depend on the same mechanisms used for face perception, whereas normal performance in early sessions followed by impaired performance in later sessions would provide support that greeble experts depend on the same mechanisms for faces and greebles.
Results
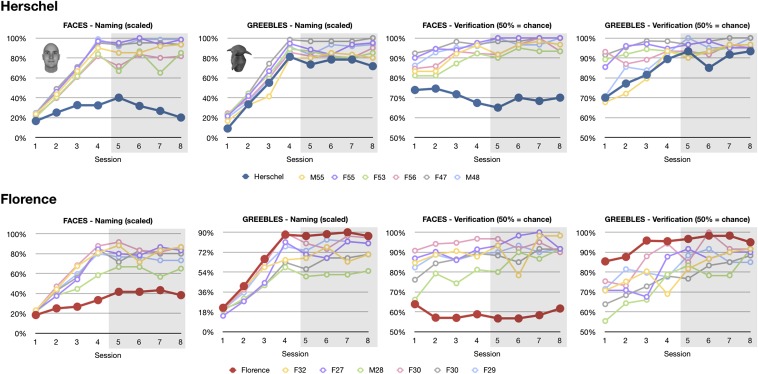
Florence and Herschel showed learning profiles comparable to controls for the greebles, but not for the faces throughout the training procedures (Fig. 1). To compare performance within and between participants, we computed an average percentage correct score for the naming trials and an average percentage correct score for the verification trials in the criterion phase (last four sessions) after all individual greebles (or faces) were introduced. Because participants are expected to become experts during the later sessions, this is also where the expertise hypothesis predicts a dissociation in greeble performance between controls and prosopagnosics. Each of the criterion phase sessions had 60 individual naming trials and 60 family verification and 60 individual verification trials. Naming trials included 40 learned and 20 unlearned stimuli, and participants were informed of these base rates. For verification trials, the chance level was 50% (i.e., labels and greebles matched in half of the trials). Differences between prosopagnosics and controls were evaluated for statistical significance using Crawford’s modified t test for single case studies (28).
Fig. 1.
Individual scores during the greeble and face training procedures (criterion sessions are shaded). Herschel and Florence were severely impaired at learning faces but showed normal learning of greebles. The naming scores were scaled to reflect the varying difficulty of each session, corresponding to the total number of individuals learned up to that point (participants were tested on five individuals in session 1, 10 in session 2, 15 in session 3, and 20 thereafter). The naming conventions for controls reflect sex (M/F) and age. Each prosopagnosic was compared with his/her age-matched control group. Because of technical problems, F53‘s results for session 1 faces and session 2 greebles were not recorded. For illustration purposes, values for the missing sessions were assumed to be the same as the results for the previous session (faces session 1) or the average of the previous and next sessions (greebles session 2).
Controls’ Accuracy.
Paired samples t tests showed that Florence’s control group did better with faces than greebles at both naming (78.0% vs. 70.3%; P = 0.045) and verification (91.5% vs. 87.2%; P = 0.002). No significant differences in performance with faces or greebles were found with Herschel’s control group at either naming (88.5% and 87.8%, respectively; P = 0.784) or verification (96.9% and 95.8%, respectively; P = 0.115). The fact that older controls’ performance with greebles approached that with faces might be a result of the different training order (older controls completed the greeble training last). Note that the increased greeble performance of the older controls raises the threshold for Herschel to be considered normal at greeble learning.
Prosopagnosic Accuracy.
As expected, Florence and Herschel had severe difficulties learning faces (Fig. 1). Florence responded correctly on only 41% of the naming trials and 58% of the verification trials. Herschel scored 30% for naming and 68% for verification. All scores were substantially lower than their respective controls’ average scores (Table 1).
Table 1.
Average accuracy and response times in the criterion phase (last four sessions)
| Accuracy, % | Response time, ms | ||||||||||||
| Young controls | Florence | Old controls | Herschel | Young controls | Florence | Old controls | Herschel | ||||||
| Stimuli | Task | M (SD) | Score | P | M (SD) | Score | P | M (SD) | Score | P | M (SD) | Score | P |
| Faces | Naming | 78.0 (7.8) | 41.3 | 0.007 | 88.5 (9.1) | 29.6 | 0.002 | 1747 (121) | 4441 | <0.001 | 2307 (699) | 6560 | 0.002 |
| Verification | 91.5 (3.1) | 58.3 | <0.001 | 96.9 (2.7) | 68.3 | <0.001 | 938 (61) | 2534 | <0.001 | 1491 (381) | 2978 | 0.015 | |
| Naming | 70.3 (10.9) | 87.9 | 0.195 | 87.8 (6.0) | 75.4 | 0.114 | 1619 (158) | 2128 | 0.030 | 2074 (607) | 3428 | 0.094 | |
| Greebles | Verification | 87.2 (3.6) | 97.1 | 0.052 | 95.8 (2.1) | 90.8 | 0.079 | 1017 (194) | 1241 | 0.334 | 1480 (527) | 1882 | 0.512 |
| Verification family | 90.7 (7.3) | 96.7 | 0.481 | 95.3 (7.9) | 96.7 | 0.876 | 814 (153) | 890 | 0.663 | 1288 (801) | 1690 | 0.661 | |
P values were calculated using Crawford’s t test for single case studies (28). Significant differences are in bold.
In contrast to their performance with faces, both Florence and Herschel showed greeble learning curves comparable to those of controls (Fig. 1). Florence’s average performance in the criterion phase, after all 20 greebles and their families were learned, was 88% for naming, 97% for verification individual, and 97% for verification family. Herschel’s average scores were 75%, 91%, and 97%, respectively. These scores were not significantly different from controls (Table 1).
The dissociation between the impaired face learning and the normal greeble learning for Florence and Herschel was further confirmed by the Bayesian Standardized Difference Test (29), which estimates the percentage of the control population exhibiting a more extreme difference between two tasks than a patient. For naming and verification, for both Florence and Herschel, these estimates were below 0.01%.
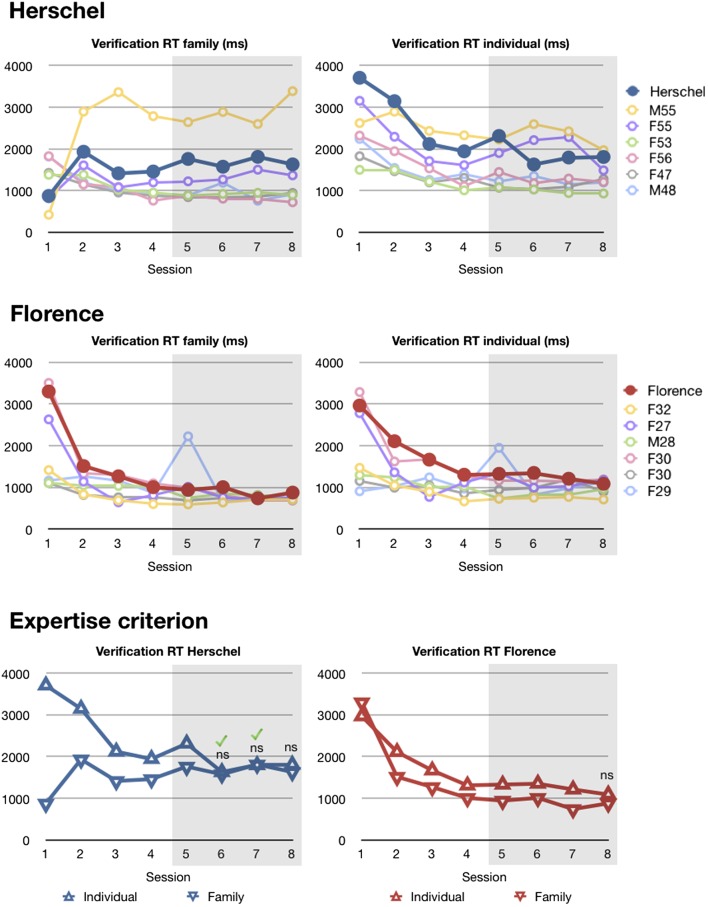
Response Times.
To check whether their normal accuracy with greebles could be explained by speed–accuracy tradeoffs, we compared Florence and Herschel’s response times (RTs) with controls’ RTs. For each participant, session, and trial type (individual naming, individual verification, and family verification), we computed a mean RT for “hit” trials (23). Means were computed after excluding trials with RTs more than two SDs from the mean to remove outliers and prevent statistical equivalence resulting from high variability (between 3% and 8% of trials were excluded per participant). For the criterion phase (sessions 5–8), Herschel’s average RTs were comparable with those of controls in all conditions. Florence was slower than controls in the greeble naming trials but was normal in both types of verification trials (see Table 1 for RT data and statistical results). Note that naming RTs are generally slower than verification RTs and have limited informational value because participants need to find one key among 21 alternatives, and therefore they reflect more than perceptual differences between participants.
In the face-learning task, Florence and Herschel were significantly slower than controls for both verification and naming trials (Table 1). Furthermore, the differences between RTs in face trials and RTs in greeble trials were significantly larger for Florence and Herschel than for controls on both naming and verification (all P < 0.001, using the Bayesian Standardized Difference Test) (29).
Expertise Criterion.
According to advocates of the expertise hypothesis (2), participants undergoing greeble training should be considered greeble experts when average RTs for hit trials at individual-level recognition become comparable (i.e., not statistically different) to average RTs for hit trials at family-level recognition. Although we discuss our strong reservations about the value of this criterion or variations on it in the discussion (see also ref. 30), both prosopagnosics met this criterion. Herschel reached it in sessions 6, 7, and 8 (P = 0.748, P = 0.946, and P = 0.469), whereas Florence reached the criterion in the final session (P = 0.113; Fig. 2). As an additional criterion for expertise, Gauthier and colleagues recently (23) recommended adding the constraint that the difference between individual- and family-level response times should be lower than 95 ms (corresponding to a 95% interval of response times differences from a previous greeble study). Herschel’s RTs satisfied this criterion in sessions 6 and 7 (54 ms and −16 ms, respectively). Florence did not meet this criterion; however, we note that three young controls and four older controls did not meet this criterion either.
Fig. 2.
Response times during the greeble training procedure (criterion sessions are shaded). Herschel and Florence’s displayed comparable RTs to their age-matched control groups, indicating that normal greeble learning cannot be explained by speed–accuracy tradeoffs. In addition, in three (Herschel) and one (Florence) of the final four sessions, there were no significant differences between individual and family verification RTs. According to Gauthier and Tarr (2), the absence of a difference indicates that Herschel and Florence were greeble experts. The green ticks mark sessions where the absolute difference between individual and family level hit trials was below 95 ms, the most stringent expertise criterion (23). Herschel achieved this criterion twice, whereas Florence did not reach it. Three young controls and four old controls did not meet this criterion either. ns, difference not significant.
Discussion
Our results demonstrate a marked dissociation between greeble learning and face learning. It has been suggested that Greeble recognition after training engages the same mechanisms used for face processing (2, 15, 21), which predicts lesions disrupting face recognition will also disrupt greeble recognition after training. However, two acquired prosopagnosics showed normal performance in a standard greeble training procedure while exhibiting severe impairments with a comparable face task. Both Herschel and Florence also showed response time effects that are putative markers of greeble expertise.† Herschel was as fast as controls and fulfilled both expertise criteria. Florence was as fast as controls on greeble verification trials, although she was slightly slower on naming trials (but still significantly faster than on face naming trials) and did not achieve the more stringent expertise criterion. However, Florence’s accuracy for both naming and verification was better than the control average. Her successful learning of greebles is interesting considering she shows deficits with learning exemplars from other object classes (Table S1), raising the possibility that different mechanisms are used to process different nonface object classes at the within-category level. If distinct mechanisms are engaged by nonexpert object classes, expertise acquired with one class (i.e., greebles) may not be informative about expertise acquired with other classes (e.g., faces, cars).
Implications for the Specificity of the Mechanisms Used for Face Recognition.
Substantial evidence suggests faces are processed by mechanisms that are not involved in the recognition of most other objects (19, 30–35), but whether these mechanisms are face-specific or operate on other object classes as well remains a matter of debate. One influential alternative to the face-specific account is that these mechanisms contribute to individuation of objects with which observers have become experts (1). The greeble training program was designed to study the acquisition of expertise in the laboratory (2, 23, 26). Although several reviews have disputed the interpretation of the behavioral and neural results by the expertise advocates (6, 36–38; see also Implications for the Expertise Hypothesis and for the Greeble Training Program), greeble training is said to cause a shift in how participants process greebles. Participants putatively shift from a feature-based approach to a more holistic process, similar to the holistic processes applied to upright faces (33, 34), which in turn leads to face-like perceptual effects (2, 14). Also consistent with this expertise view, training has been reported to lead to increased activation to greebles alone (15) and to a reduced response to faces presented simultaneously with greebles (21) in face-linked neural markers. However, our findings challenge the main claim of the expertise hypothesis. Together, Herschel’s and Florence’s results indicate that intact face mechanisms are not required for building greeble expertise and bolster the evidence supporting the face-specific account.
Herschel’s normal performance with greebles is also theoretically interesting, given claims that the fusiform face area (FFA) is the locus of mechanisms mediating general visual expertise (9, 10, 15; but see refs. 17–18). Although Herschel still shows a right and a left FFA, these areas exhibit reduced face-selectivity relative to healthy controls, and his right FFA size is severely reduced (39). Previous discussions of the role of FFA for expertise would predict that this would interfere with the acquisition of greeble expertise. Contrary to this prediction, Herschel’s normal greeble learning suggests greeble expertise is not dependent on a typically functioning FFA. It is possible that reduced functioning of the right FFA may suffice for greeble learning, but several previous studies failed to find a correlation between activation in the FFA and expertise level with novel objects (17, 18, 40, 41) or real objects (12), suggesting that acquisition of expertise does not depend on the FFA. In two of these studies (18, 41), changes associated with the acquisition of expertise occurred in the lateral occipital complex, a region implicated in object processing that appears to be normal in Herschel (39).
We believe the previous findings linking expert greeble processing to neural areas implicated in face processing can be explained by other factors. Thus, in one study (15) the selected region of interest most likely extended beyond standardly defined FFA into adjacent areas involved in object processing, and these object areas have been found to be more strongly activated by objects of expertise (18, 41). In another study (16), the reported face-like delayed and enhanced N170 to inverted greebles in greeble experts was much smaller than for faces and left-lateralized (in contrast with face effects that occurred in both hemispheres). A second study from the same group (21) reported a selective decrease in the N170 signal to faces when processed concurrently with greebles in greeble experts. However, a general problem with event-related potentials recorded on the human scalp is source localization, which makes it difficult to determine whether the reported effects involve face-selective regions or other regions.
Implications for the Expertise Hypothesis and for the Greeble Training Program.
In interpreting our results, it is important to distinguish between two separate claims made by advocates of the greeble framework. First is a “methods claim,” arguing that greeble training can lead to expertise with a novel object class. Second is an “expertise claim,” suggesting that expertise with a novel object class depends on the same processes as expertise with faces. The dissociations we find indicate that at least one of these hypotheses must be false.
If the methods claim is true, our results imply that greeble expertise and face expertise rely on separate mechanisms; that is, the expertise claim is false. If the expertise claim is true, that is, the same mechanisms mediate object and face expertise, the methods claim must be false because our results show that the presumed common expertise mechanisms (damaged in our prosopagnosics) are not needed for successful completion of the greeble training.
In the case that the methods claim is false (i.e., the greeble method is not a suitable probe of object expertise at a level that parallels face expertise), our findings leave open the possibility that category-general expertise mechanisms are important for the recognition of both faces and real-world objects of expertise. One potentially important point of difference between expertise generated in the laboratory and real-world expertise is the time scale of acquisition. The 5–10 h of practice with greebles is quite different from the amount of experience that has been suggested to lead to real-world expertise (1, 42), and this limited practice may explain why, despite many studies, very few results suggest training leads greebles to be processed in a face-like manner (see ref. 43 for experiments with another novel stimulus class).
The evidence that extensive real-world experience with particular object classes leads to face-like object recognition is stronger, but is also weak (see refs. 6 and 36 for reviews). The finding that dog show judges show face-sized inversion effects provided important impetus for the expertise view, but an attempted replication and extension of it failed to find any behavioral effects suggesting dog experts process dogs in a face-like manner (7). Experts with a number of other stimulus classes (cars, biological cells) have also not shown behavioral indicators of face-like processing (8). Neural evidence also provides mixed support. Rossion and colleagues replicated the N170 interference effect initially found for greebles (21) in a subsequent study with car experts (44). Furthermore, Gauthier and colleagues reported increased activation to cars and birds in the right FFA in line with behavioral expertise (9), and the effect was replicated by Xu (11); however, the effects were also present outside face-selective areas in both studies, raising the possibility that the increase resulted from enhanced attention to objects of expertise. More recently, a correlation of FFA activation with behavioral expertise was shown for cars in a high-resolution functional MRI study (10). However, there is also considerable neural evidence against a common mechanism for faces and other real-world objects of expertise. A recent study investigating the effects of long-term expertise on gray matter structure found significant and selective changes restricted to the prefrontal cortex (45). Results from neuropsychological studies suggest face processing may dissociate from long-term object expertise. After brain lesions causing prosopagnosia, WJ acquired a flock of sheep and became capable of recognizing sheep faces better than normal controls and scored best among a group of sheep farmers (46), whereas RM retained his superior ability to recognize car makes and models (47). Florence and another acquired prosopagnosic could successfully discriminate bodies, a category that generates substantial inversion effects and is relatively well-matched to faces in terms of visual exposure and perceptual experience (48). Conversely, several brain-damaged individuals lost the ability to recognize classes of nonface objects that they had an intense interest in, but their face recognition remained normal (e.g., CK in ref. 49; MX in ref. 50).
Our results are also inconsistent with a criticism of greeble studies. It has been suggested that the greeble part configuration (two features above one feature above another feature) is so similar to the face configuration that face perception mechanisms are activated by them (27, 37). This activation of the face system then causes greeble processing to show the cognitive and neural hallmarks seen for face processing. Normal greeble performance in the two acquired prosopagnosics, however, indicates that their visual systems did not treat the greebles as faces. This finding complements results from CK, an object agnosic who can recognize faces; CK was severely impaired with greebles despite his intact face recognition (51). Herschel, Florence, and CK’s dissociation, however, does not rule out the possibility that some participants treat the greebles as faces because of their similarity (27).
Conclusion
Our study shows normal greeble learning by two acquired prosopagnosics, providing straightforward evidence that face and greeble recognition rely on different mechanisms. This finding is consistent with the view that faces are processed by mechanisms specialized for faces rather than objects of expertise. Although the current study does not directly address the possibility that real objects of expertise and faces depend on common expertise mechanisms, it compellingly demonstrates that the mechanisms used for face processing are not necessary for normal performance in greeble training, and it undermines interpretations of previous greeble-based results used to support the expertise hypothesis.
Materials and Methods
Prosopagnosic Participants.
Herschel is a right-handed British male with a degree in astronomy who runs a research laboratory. He was 55 y old at the time of testing (born 1956). In 2008, after two strokes that lesioned his occipitotemporal cortex (primarily in the right hemisphere) and right hippocampus (Fig. S1), he lost the upper left visual field and a large part of his upper right visual field and became severely prosopagnosic. All core face areas (the FFA, occipital face area, and face area in posterior superior temporal sulcus) were found bilaterally (except left occipital face area, which could not be identified), using a contrast of dynamic faces greater than dynamic objects (52). These face-selective areas, however, showed lower percentage signal changes to faces in Herschel than in controls (39). In addition, Herschel’s right FFA consisted of only nine voxels, whereas controls averaged 69.3 voxels and ranged from 32 to 117 voxels (statistical threshold, P = 10−4 uncorrected). Herschel’s activation to objects in the lateral occipital cortex bilaterally was normal (see ref. 39 for functional MRI details and neuropsychological assessment).
Florence is a right-handed female nurse from Canada who was 29 y old at the time of testing (born 1982). She was described in previous studies as R-AT1 (53–54). In 2006, she became prosopagnosic after a resection of her right amygdala and right hippocampus to control epilepsy. Neuropsychological assessment and functional MRI were conducted in 2007 (see ref. 54 for details). Florence performed normally on a battery of cognitive and low-level visual tests. Despite her face impairments, a static face localizer (activation to faces minus activation to objects) revealed face activation bilaterally in all core face-selective areas: FFA, occipital face area, and face area in posterior superior temporal sulcus. In 2008, she underwent a second operation that removed most of her right anterior temporal lobe, sparing the areas previously found to be face-selective (Fig. S1). Florence has noted no visual changes since her second surgery, and her normal performance on tasks described here suggests her early visual processes were unaffected by the second procedure.
Test results confirming Florence and Herschel’s prosopagnosia are summarized in Table S1. In addition to deficits in face perception and face memory, Florence shows mixed performance with within-class object matching and recognition. Herschel’s impairments with within-class discrimination, however, are restricted to faces. In addition, in a previous paper (39), we reported his normal performance with basic-level recognition of objects presented for 50 ms (with the exception of mammals). He had difficulties recognizing severely degraded objects and words in visual closure tests, but the origin of this deficit may involve mechanisms such as object segmentation that are not taxed by the greeble and face tasks.
Control Participants.
Two control groups consisted of six participants each. The first group was age-matched to Florence (mean age, 29.3 y; age range, 27–32 y; five women), and the second group was age-matched to Herschel (mean age, 52.3 y; age range, 47–56 y; four women).
Stimuli.
Greeble stimuli were selected from those used in the original study (2). Similar to faces, greebles have a common first-order configuration (Fig. 3). They can be identified at the family level on the basis of their overall shape and at the individual level on the basis of the sizes and shapes of their parts. The face stimuli (Fig. 3) were selected from a large set of computer-generated male faces produced by Facegen (Singular Inversions), according to a pixel-based similarity matrix (i.e., highly similar faces were preferred).
Fig. 3.
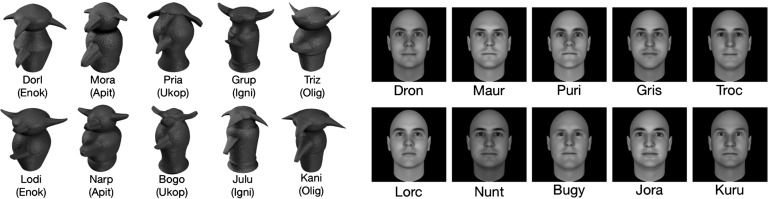
Ten of the 20 greebles and faces learned during the experiments. Individual greebles belong to one of five families (in parentheses) and could be identified at the individual level and at the family level. Faces were identified only at the individual level. Faces and greebles had abstract four-letter names starting with a consonant (family names started with a vowel).
Procedure.
The greeble training procedure closely followed procedures used in previous studies (23, 25, 26). We asked participants to complete eight training sessions, one per day, on consecutive days (one control participant had a 1-d break between two sessions), with the goal of learning to identify greebles at the individual and family level. Consistent with previous terminology (23), the training included a “learning phase” (first four sessions) and a “criterion phase” (last four sessions). The five greeble families were introduced in the first session and probed for learning/recognition throughout the procedure (six exemplars per family). Twenty greebles identified at the individual level (four from each family) were introduced gradually during the learning phase (five new greebles per session). Families had four-letter names each starting with a different vowel, and individual greebles had four-letter names each starting with a different consonant. Participants learned the greebles in several different types of trials. In inspect trials, participants viewed greebles and their corresponding family or individual name one at a time. In naming with feedback trials, participants were asked to identify individual greebles by pressing the key corresponding to the first letter of their name; after each trial, the correct name of the greeble was presented. In naming trials, participants were asked to identify greebles at the individual or family level but were not given the correct response. In verification trials, participants were presented with a name, which could be an individual or a family name, followed by a greeble, and asked to indicate whether the name and the greeble matched. The naming and verification trials included auditory feedback (a beep) for incorrect answers. During the criterion phase, participants were tested with the naming and verification trials for successful learning of 30 greebles at the family level and 20 greebles at the individual level. Learning sessions lasted about 60 min, whereas criterion sessions lasted about 15 min.
A similar training procedure was created with computer-generated faces instead of greebles, with the difference that faces were not grouped into families (thus there were no family trials). Note that although participants had extensive exposure to faces and none to greebles before the experiment, all individual stimuli (faces and greebles) were unfamiliar, and pilot testing and control data presented in the results section indicated that the difficulty of the face training procedure was comparable to that of the greeble procedure. Sessions 1–4 lasted ∼45 min, whereas sessions 5–8 lasted about 12 min.
All participants underwent both training procedures (greebles and faces), with a break of at least 4 wk in between. Florence, Herschel, and Florence’s control group completed the greeble training first, and Herschel’s control group completed the face training first.
Supplementary Material
Acknowledgments
We thank Florence and Herschel for the generous contribution of their time and effort. This research was partly supported by AXA Research Fund, a Bogue Research Fellowship (to C.R.), and a Hitchcock Foundation grant (to B.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.M. is a guest editor invited by the Editorial Board.
†Although it has been suggested that the response time comparison in the verification trials is the critical measure of expertise, we have empirical and theoretical concerns about it. In one of the first papers involving greebles (13), of 12 subjects, two met the expertise criterion in the fourth session, two in the third session, one in the second session, and one in the first session. In another influential paper (26), of the five controls, two met the expertise criterion in the first session and another two in the second session. Thus, many subjects achieve the criterion after very little training. In addition, regardless of the object classes used, response time with the two types of verification will depend on the amount of practice with each type and on the similarity between individuals and between families. Thus, because this criterion is strongly influenced by the parameters of the experiment, it is a questionable measure of a qualitative shift in recognition processes.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317125111/-/DCSupplemental.
References
- 1.Diamond R, Carey S. Why faces are and are not special: An effect of expertise. J Exp Psychol Gen. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier I, Tarr MJ. Becoming a “Greeble” expert: Exploring mechanisms for face recognition. Vision Res. 1997;37(12):1673–1682. doi: 10.1016/s0042-6989(96)00286-6. [DOI] [PubMed] [Google Scholar]
- 3.Busey TA, Vanderkolk JR. Behavioral and electrophysiological evidence for configural processing in fingerprint experts. Vision Res. 2005;45(4):431–448. doi: 10.1016/j.visres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Bruyer R, Crispeels G. Expertise in person recognition. Bull Psychon Soc. 1992;30(6):501–504. [Google Scholar]
- 5.Boggan AL, Bartlett JC, Krawczyk DC. Chess masters show a hallmark of face processing with chess. J Exp Psychol Gen. 2012;141(1):37–42. doi: 10.1037/a0024236. [DOI] [PubMed] [Google Scholar]
- 6.McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends Cogn Sci. 2007;11(1):8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Robbins R, McKone E. No face-like processing for objects-of-expertise in three behavioural tasks. Cognition. 2007;103(1):34–79. doi: 10.1016/j.cognition.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka JW, Gauthier I. Expertise in object and face recognition. Psychol Learn Motiv. 1997;36:83–125. [Google Scholar]
- 9.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3(2):191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 10.McGugin RW, Gatenby JC, Gore JC, Gauthier I. High-resolution imaging of expertise reveals reliable object selectivity in the fusiform face area related to perceptual performance. Proc Natl Acad Sci USA. 2012;109(42):17063–17068. doi: 10.1073/pnas.1116333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y. Revisiting the role of the fusiform face area in visual expertise. Cereb Cortex. 2005;15(8):1234–1242. doi: 10.1093/cercor/bhi006. [DOI] [PubMed] [Google Scholar]
- 12.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7(5):555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier I, Williams P, Tarr MJ, Tanaka J. Training ‘greeble’ experts: A framework for studying expert object recognition processes. Vision Res. 1998;38(15-16):2401–2428. doi: 10.1016/s0042-6989(97)00442-2. [DOI] [PubMed] [Google Scholar]
- 14.Tarr MJ. Visual object recognition: Can a single mechanism suffice? In: Peterson M, Rhodes G, editors. Perception of Faces, Objects and Scenes: Analytic and Holistic Processes. New York: Oxford UP; 2003. pp. 172–211. [Google Scholar]
- 15.Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2(6):568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 16.Rossion B, Gauthier I, Goffaux V, Tarr MJ, Crommelinck M. Expertise training with novel objects leads to left-lateralized facelike electrophysiological responses. Psychol Sci. 2002;13(3):250–257. doi: 10.1111/1467-9280.00446. [DOI] [PubMed] [Google Scholar]
- 17.Kung C-C, Peissig JJ, Tarr MJ. Is region-of-interest overlap comparison a reliable measure of category specificity? J Cogn Neurosci. 2007;19(12):2019–2034. doi: 10.1162/jocn.2007.19.12.2019. [DOI] [PubMed] [Google Scholar]
- 18.Op de Beeck HP, Baker CI, DiCarlo JJ, Kanwisher NG. Discrimination training alters object representations in human extrastriate cortex. J Neurosci. 2006;26(50):13025–13036. doi: 10.1523/JNEUROSCI.2481-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eimer M. The face-sensitive N170 component of the event-related brain potential. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford UP; 2011. pp. 329–344. [Google Scholar]
- 21.Rossion B, Kung C-C, Tarr MJ. Visual expertise with nonface objects leads to competition with the early perceptual processing of faces in the human occipitotemporal cortex. Proc Natl Acad Sci USA. 2004;101(40):14521–14526. doi: 10.1073/pnas.0405613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrmann M, Marotta J, Gauthier I, Tarr MJ, McKeeff TJ. Behavioral change and its neural correlates in visual agnosia after expertise training. J Cogn Neurosci. 2005;17(4):554–568. doi: 10.1162/0898929053467613. [DOI] [PubMed] [Google Scholar]
- 23.Bukach CM, et al. Does acquisition of Greeble expertise in prosopagnosia rule out a domain-general deficit? Neuropsychologia. 2012;50(2):289–304. doi: 10.1016/j.neuropsychologia.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier I, Behrmann M, Tarr MJ. Can face recognition really be dissociated from object recognition? J Cogn Neurosci. 1999;11(4):349–370. doi: 10.1162/089892999563472. [DOI] [PubMed] [Google Scholar]
- 25.Duchaine BC, Dingle K, Butterworth E, Nakayama K. Normal greeble learning in a severe case of developmental prosopagnosia. Neuron. 2004;43(4):469–473. doi: 10.1016/j.neuron.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier I, Tarr MJ. Unraveling mechanisms for expert object recognition: Bridging brain activity and behavior. J Exp Psychol Hum Percept Perform. 2002;28(2):431–446. doi: 10.1037//0096-1523.28.2.431. [DOI] [PubMed] [Google Scholar]
- 27.Brants M, Wagemans J, Op de Beeck HP. Activation of fusiform face area by Greebles is related to face similarity but not expertise. J Cogn Neurosci. 2011;23(12):3949–3958. doi: 10.1162/jocn_a_00072. [DOI] [PubMed] [Google Scholar]
- 28.Crawford JR, Garthwaite PH. Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40(8):1196–1208. doi: 10.1016/s0028-3932(01)00224-x. [DOI] [PubMed] [Google Scholar]
- 29.Crawford JR, Garthwaite PH. Comparison of a single case to a control or normative sample in neuropsychology: Development of a Bayesian approach. Cogn Neuropsychol. 2007;24(4):343–372. doi: 10.1080/02643290701290146. [DOI] [PubMed] [Google Scholar]
- 30.Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9(5):415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- 31.Busigny T, Joubert S, Felician O, Ceccaldi M, Rossion B. Holistic perception of the individual face is specific and necessary: Evidence from an extensive case study of acquired prosopagnosia. Neuropsychologia. 2010;48(14):4057–4092. doi: 10.1016/j.neuropsychologia.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Duchaine BC, Yovel G, Butterworth EJ, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cogn Neuropsychol. 2006;23(5):714–747. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka JW, Farah MJ. Parts and wholes in face recognition. Q J Exp Psychol A. 1993;46(2):225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- 34.Yin RK. Looking at upside-down faces. J Exp Psychol. 1969;81:141–145. [Google Scholar]
- 35.Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage. 2010;52(4):1559–1573. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKone E, Robbins R. Are faces special? In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford UP; 2011. pp. 149–176. [Google Scholar]
- 37.Kanwisher N. Domain specificity in face perception. Nat Neurosci. 2000;3(8):759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- 38.Rossion B. The composite face illusion : A whole window into our understanding of holistic face perception. Vis Cogn. 2013;21:139–253. [Google Scholar]
- 39.Rezlescu C, Pitcher D, Duchaine B. Acquired prosopagnosia with spared within-class object recognition but impaired recognition of degraded basic-level objects. Cogn Neuropsychol. 2012;29(4):325–347. doi: 10.1080/02643294.2012.749223. [DOI] [PubMed] [Google Scholar]
- 40.Moore CD, Cohen MX, Ranganath C. Neural mechanisms of expert skills in visual working memory. J Neurosci. 2006;26(43):11187–11196. doi: 10.1523/JNEUROSCI.1873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue X, Tjan BS, Biederman I. What makes faces special? Vision Res. 2006;46(22):3802–3811. doi: 10.1016/j.visres.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195(4275):312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- 43.Wong ACN, Palmeri TJ, Gauthier I. Conditions for facelike expertise with objects: Becoming a Ziggerin expert—but which type? Psychol Sci. 2009;20(9):1108–1117. doi: 10.1111/j.1467-9280.2009.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossion B, Collins D, Goffaux V, Curran T. Long-term expertise with artificial objects increases visual competition with early face categorization processes. J Cogn Neurosci. 2007;19(3):543–555. doi: 10.1162/jocn.2007.19.3.543. [DOI] [PubMed] [Google Scholar]
- 45.Gilaie-Dotan S, Harel A, Bentin S, Kanai R, Rees G. Neuroanatomical correlates of visual car expertise. Neuroimage. 2012;62(1):147–153. doi: 10.1016/j.neuroimage.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeil JE, Warrington EK. Prosopagnosia: A face-specific disorder. Q J Exp Psychol A. 1993;46(1):1–10. doi: 10.1080/14640749308401064. [DOI] [PubMed] [Google Scholar]
- 47.Sergent J, Signoret JL. Varieties of functional deficits in prosopagnosia. Cereb Cortex. 1992;2(5):375–388. doi: 10.1093/cercor/2.5.375. [DOI] [PubMed] [Google Scholar]
- 48.Susilo T, Yovel G, Barton JJS, Duchaine B. Face perception is category-specific: Evidence from normal body perception in acquired prosopagnosia. Cognition. 2013;129(1):88–94. doi: 10.1016/j.cognition.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Moscovitch M, Winocur G, Behrmann M. What is special about face recognition? Nineteen experiments on a person with visual object agnosia and dyslexia but normal face recognition. J Cogn Neurosci. 1997;9(5):555–604. doi: 10.1162/jocn.1997.9.5.555. [DOI] [PubMed] [Google Scholar]
- 50.Assal G, Favre C, Anderes JP. [Nonrecognition of familiar animals by a farmer. Zooagnosia or prosopagnosia for animals] Rev Neurol (Paris) 1984;140(10):580–584. French. [PubMed] [Google Scholar]
- 51.Gauthier I, Behrmann M, Tarr MJ. Are Greebles like faces? Using the neuropsychological exception to test the rule. Neuropsychologia. 2004;42(14):1961–1970. doi: 10.1016/j.neuropsychologia.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 52.Pitcher D, Dilks DD, Saxe RR, Triantafyllou C, Kanwisher N. Differential selectivity for dynamic versus static information in face-selective cortical regions. Neuroimage. 2011;56(4):2356–2363. doi: 10.1016/j.neuroimage.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 53.Barton JJ, Hanif H, Ashraf S. Relating visual to verbal semantic knowledge: The evaluation of object recognition in prosopagnosia. Brain. 2009;132(Pt 12):3456–3466. doi: 10.1093/brain/awp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox CJ, Hanif HM, Iaria G, Duchaine BC, Barton JJS. Perceptual and anatomic patterns of selective deficits in facial identity and expression processing. Neuropsychologia. 2011;49(12):3188–3200. doi: 10.1016/j.neuropsychologia.2011.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.