Significance
Besides d-glucose, d-xylose is the second most abundant sugar present in lignocellulosic biomass that is regarded as a renewable feedstock for biotechnological production of fuels and chemicals. Simultaneous consumption of both sugars—a prerequisite for economically feasible bioconversions—has primarily been prevented by one impediment: all known d-xylose transporters are competitively inhibited by d-glucose. Using a growth-based screening platform, we could identify two positions in yeast hexose transporters Hxt7 and Gal2 that can be mutated to yield glucose-insensitive xylose transporters. One of the positions is conserved among the hexose transporter family members, which opens the possibility to transfer our findings to other biotechnologically relevant organisms. This work will also significantly contribute to the understanding of sugar-transport mechanisms.
Keywords: xylose transport, major facilitator superfamily, transporter engineering, pentose metabolism, HXT
Abstract
All known d-xylose transporters are competitively inhibited by d-glucose, which is one of the major reasons hampering simultaneous fermentation of d-glucose and d-xylose, two primary sugars present in lignocellulosic biomass. We have set up a yeast growth-based screening system for mutant d-xylose transporters that are insensitive to the presence of d-glucose. All of the identified variants had a mutation at either a conserved asparagine residue in transmembrane helix 8 or a threonine residue in transmembrane helix 5. According to a homology model of the yeast hexose transporter Gal2 deduced from the crystal structure of the d-xylose transporter XylE from Escherichia coli, both residues are found in the same region of the protein and are positioned slightly to the extracellular side of the central sugar-binding pocket. Therefore, it is likely that alterations sterically prevent d-glucose but not d-xylose from entering the pocket. In contrast, changing amino acids that are supposed to directly interact with the C6 hydroxymethyl group of d-glucose negatively affected transport of both d-glucose and d-xylose. Determination of kinetic properties of the mutant transporters revealed that Gal2-N376F had the highest affinity for d-xylose, along with a moderate transport velocity, and had completely lost the ability to transport hexoses. These transporter versions should prove valuable for glucose–xylose cofermentation in lignocellulosic hydrolysates by Saccharomyces cerevisiae and other biotechnologically relevant organisms. Moreover, our data contribute to the mechanistic understanding of sugar transport because the decisive role of the conserved asparagine residue for determining sugar specificity has not been recognized before.
Production processes in biotechnology often rely on starch- or glucose-rich substrates like corn, wheat, or sugarcane. Using these potential foods for production of fuels or chemicals constitutes an ethical conflict and also has economical and ecological drawbacks. Current research is thus trying to exploit alternative substrates such as lignocellulosic biomass for biotechnological conversion. Besides a large amount of d-glucose, mainly from the cellulose fraction, d-xylose is the second most abundant monosaccharide, making up 5–35% of total dry weight (1) (all sugar mentions refer to the d-enantiomer; the descriptor “d-” is omitted from now on).
Saccharomyces cerevisiae, one of the most common organisms in biotechnology, is naturally not able to use xylose as a carbon source, but two degradative pathways could be established by metabolic engineering and overexpression of heterologous enzymes. Conversion of xylose to xylulose is achieved either by a reductive/oxidative pathway using xylose reductase and a xylitol dehydrogenase (2) or by direct isomerization using a xylose isomerase (XI) (3–5). In both cases, xylulose is then phosphorylated by an endogenous or heterologous xylulokinase (XKS) and channeled through the nonoxidative branch of the pentose phosphate pathway (PPP) into glycolysis. The usual fermentation product of S. cerevisiae is ethanol, but yeast research also strives to produce other components, like advanced biofuels and chemicals (6–8).
Despite being no natural substrate for S. cerevisiae, xylose can enter the cell by facilitated diffusion through some yeast hexose transporters (9–11). This intrinsic transport is enough to enable growth on xylose and has not limited xylose consumption in early experiments (12). However, with improvements of intracellular xylose conversion being made (e.g., by overexpression of xylulokinase XKS1 and the PPP enzymes) (13, 14), transport becomes a bottleneck, especially at low xylose concentrations (15–18). In several studies, enhanced transport could be used to improve engineered strains further or could be seen as a result of evolutionary engineering (19–22).
Although consumption rates and ethanol yields from xylose alone have been increased, a major obstacle for efficient and fast fermentation of lignocellulosic hydrolysates still is the lack of cofermentation: i.e., the simultaneous consumption of glucose and pentoses (23). Although intracellular xylose conversion is only slightly affected by the presence or catabolism of intracellular glucose, xylose transport is strongly inhibited by glucose (24). Avoiding transport inhibition in a cofermentation of cellobiose and xylose showed no impediments but even synergistic effects for a parallel catabolism (25). Inhibition of transport has also been directly verified by uptake assays for single transporters (11, 26), as well as for S. cerevisiae (9) and different xylose-using yeast species (e.g., Candida shehatae, which also shows a sequential consumption of glucose and xylose) (27–30). All known xylose transporters that can functionally be expressed in S. cerevisiae are neither selective for xylose, nor do they have a higher affinity for xylose, leading to competitive inhibition by glucose (31–33). Transport affinity for glucose is often two orders of magnitude higher than for xylose. XylE of Escherichia coli, a xylose/H+ symporter, is exceptionally selective for xylose and, like many symporters, has a very high affinity (0.47 mM). However, despite the fact that glucose is not transported by XylE, glucose still is a competitive inhibitor of xylose transport (“dead-end” inhibition). Isothermal titration calorimetry measurements have revealed that xylose and glucose bind with similar high affinities to XylE (Kd Xyl = 0.35 mM vs. Kd Glc = 0.77 mM) (34–36).
Taken together, a xylose transporter that is not inhibited by glucose is a vital prerequisite for simultaneous fermentation of glucose and xylose, but it seems to be a rare feature in nature. As seen with XylE and the recent results of Young et al. (37), selectivity for xylose does not necessarily result in resistance to inhibition by glucose. To address this problem, we created a precise screening system for uninhibited xylose transporters. With this system, we carried out evolutionary engineering/mutagenesis and screening approaches with individual hexose transporters. These experiments led to different mutants of yeast hexose transporters Hxt7 and Gal2 that can still transport xylose but are no longer inhibited by glucose. Detailed kinetic assessment of xylose and glucose transport is shown for Gal2, Hxt7, and some of the identified mutants. The discussed amino acid positions are in good accordance with the recently resolved structure of XylE and provide valuable information about sugar recognition and discrimination between xylose and glucose.
Results
Construction of a Screening Strain for Xylose Transporters Not Inhibited by Glucose.
The hxt0 yeast strain EBY.VW4000 is widely used for characterization and screening of sugar transporters. Deletion of all hexose transporter genes in this strain prevents uptake of glucose and xylose and allows manipulation and assessment of distinct, reintroduced transporters. This strain was the parental strain to our screening strain. The basic principle of the screening system was to turn glucose into a mere inhibitor of xylose-based growth. We therefore (i) disrupted glycolysis and thereby uncoupled the presence of glucose from its utilization as a carbon source, (ii) introduced a xylose utilization pathway, and (iii) expressed individual sugar transporters that support uptake of both glucose and xylose. Under obstructive conditions of a xylose–glucose mixture, growth would only be possible if the transport of xylose is no longer inhibited by glucose. To create this strain, we sequentially deleted the genes of hexokinases HXK1/2, glucokinase GLK1, and the putative hexokinase YLR446W in strain EBY.VW4000. We then integrated a multigene cassette for overexpression of the enzymes of the nonoxidative pentose phosphate pathway (TAL1, TKL1, RPI1, RKI1, and opt. XKS1) into the PYK2 locus (strain AFY10) and overexpressed the xylose isomerase xylA of Clostridium phytofermentans from a multicopy plasmid (AFY10X) to enable efficient utilization of xylose without any limitations downstream of the transport. When overexpressing hexose transporter HXT7 or GAL2 from a multicopy plasmid, this strain did grow on xylose, but only in the absence of glucose. Growth on glucose or maltose as a sole carbon source was not possible (Fig. S1A). Therefore, this strain is suited for a growth-based screening of xylose transporters that are not inhibited by glucose.
Evolutionary Engineering Generates Single Amino Acid Mutants.
We first used AFY10X in an evolutionary engineering approach to release Gal2, the galactose/glucose transporter of S. cerevisiae that also transports the pentoses xylose and l-arabinose, from steric inhibition by glucose. AFY10X was transformed with plasmid p426-GAL2 for strong and constitutive expression of GAL2 and grown in serial aerobic batch fermentations. Starting out in synthetic medium with 10 g/L xylose to adapt the strain to xylose utilization, we switched to medium with 10 g/L xylose and increasing concentrations of glucose. More than 5 g/L glucose in the first step completely inhibited growth on xylose. In seven batches over a total of 50 d, the glucose concentration was increased from 2 g/L to 20 g/L, and the culture concomitantly adapted to the intensified conditions. After reaching 10 g/L xylose plus 20 g/L glucose, best growing single clones were isolated on glucose–xylose plates (Materials and Methods, Clone Selection and Analysis). In four of five cases, sequencing of the GAL2 gene from the isolated plasmids showed identical mutations at position 1,127 (aac to atc), which causes an N376I mutation in the Gal2 protein (Table 1). The ORF of the fifth single clone and the promoter regions in all plasmids were unchanged. In a parallel setup, Hxt5 and Hxt7 were submitted to evolutionary engineering. Cultures expressing HXT7 failed to show significant improvements beyond 10 g/L xylose and 5 g/L glucose whereas HXT5 cultures could be improved to overcome addition of 50 g/L glucose. Analysis revealed an N391T mutation (aac to act), the residue corresponding to asparagine 376 in Gal2, in three of four single clones. One Hxt5 clone showed a T234S mutation.
Table 1.
Overview of mutations at the conserved threonine and asparagine positions identified in the different experimental approaches
| Transporter | Position | Experimental approach | ||
| Evolutionary engineering | epPCR | Site-directed mutagenesis | ||
| Gal2 | T219 | n.m. | S | N/S/G |
| N376 | I | Y | V/F/A | |
| Hxt7 | T213 | — | N/S/A | N/S/G |
| N370 | — | Y | S/L/T/V/A | |
| Hxt5 | T234 | S | — | — |
| N391 | T | — | — | |
Replacing amino acids are given in single-letter code. —, experiment has not been carried out; n.m., no mutation found in ORF.
epPCR Mutagenesis and Screening Yields Additional Asparagine and Threonine Mutants.
To increase the number of mutations, we specifically mutagenized GAL2 and HXT7 by error-prone PCR (epPCR) and screened for improvements. The ORFs were amplified, applying various mutation rates, and prepared for recombinational cloning in a second PCR. Functional plasmids were in vivo assembled directly in the screening strain AFY10X and subsequently screened. Clones able to grow on media with different xylose–glucose mixtures were selected, separated, and analyzed as described. Four clones of GAL2 and HXT7 could be isolated, and ORFs were sequenced. Three Gal2 clones had mutation N376Y, and one Hxt7 clone showed the same mutation at the corresponding asparagine 370. The other four clones all had mutations at threonine 219/213 (Table 1), which corresponds to T234 of Hxt5.
Site-Directed Mutagenesis of the Identified Residues.
The cumulated occurrence of mutations at one of two conserved amino acid positions in all of the improved transporter clones suggested that these mutations are essential and sufficient to decrease inhibition of xylose transport by glucose. To confirm this hypothesis and to identify other possible substituents at these positions, we performed two independent site-directed mutageneses. Threonine 213 in Hxt7 has already been described by Kasahara et al. as a key residue for determination of glucose affinity (38). We chose to replace the threonine 219/213 of Gal2/Hxt7 by the seven amino acids that did not completely eliminate sugar uptake in their study. Mutant constructs were assembled by homologous recombination in AFY10X and subsequently screened as described. Similarly, asparagine 376/370 was randomly changed to the 20 proteinogenic amino acids. Multiple colonies grew on xylose–glucose plates after the threonine mutagenesis of Hxt7 whereas, in the case of Gal2, only a few colonies could be seen. For both transporters, threonine 219/213 to glycine, serine, or asparagine were the only beneficial exchanges. In the case of the random asparagine 376/370 mutagenesis, multiple amino acids were found to attenuate steric inhibition by glucose (Table 1). The presence of various clones that were able to grow on xylose–glucose media proved that single mutations at one of the two identified positions can render the transporters resistant to inhibition by glucose.
Gal2 and Hxt7 Mutants Show Various Growth Characteristics.
We selected five mutations for Gal2 and Hxt7, two at the threonine and three at the asparagine, for further characterization. The mutations were selected based on their superior performance observed in the screenings and the number of clones found within one experiment and between different experiments. The N370F mutation was not found in any Hxt7 mutant but was chosen because of the good performance of Gal2-N376F. EBY.VW4000 and AFY10X were transformed with the respective plasmids, and a serial dilution drop test was performed with ethanol-grown cells. EBY.VW4000 (hxt0) transformants were assessed on various hexoses (glucose, galactose, fructose, mannose) and AFY10X (hxt0 hxk0 +xylA) transformants at various xylose concentrations, with and without glucose addition, to analyze uptake of hexoses and xylose, respectively. Low and high concentrations of sugars were used in the growth tests to evaluate the affinity of the transporters. The growth tests showed that, opposed to the respective wild-type transporter, all mutant transporters enable the cells to grow on media with xylose–glucose mixtures (Fig. 1 and Fig. S1B). Three different phenotypes can be seen with the N376 mutants of Gal2. Tyrosine at this position completely abolishes growth on hexoses, and growth on xylose is glucose-resistant albeit very ineffective (Fig. S1C). Gal2-N376F is also unable to transport hexoses but shows strong and uninhibited transport of xylose. Gal2-N376V likewise confers good growth on xylose regardless of the concentration or the presence of glucose, but without the major impairments concerning hexose transport seen with Gal2-N376F. Mutation of threonine 219 to asparagine, but not to serine, decreases overall sugar affinity. All Hxt7 mutants show similar growth on the respective sugars, with reduced affinities for hexoses. Hxt7-N370F, just like Gal2-N376F, transports only xylose (Fig. S1 B and C). These results show that, by changing single amino acid positions, a broad range of transport characteristics can be achieved. Also, the comparison between Gal2-N376F (xylose-specific) and Gal2-N376V (unaffected hexose transport) demonstrates that xylose specificity is not necessarily needed to lessen steric inhibition by glucose.
Fig. 1.
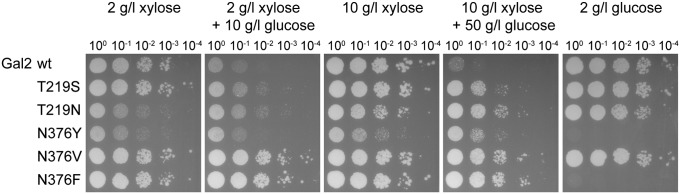
Functional characterization of Gal2 wild type and mutants. Growth assay of AFY10X (xylose and xylose–glucose mixtures) and EBY.VW4000 (glucose) overexpressing the indicated transporters. Cells were pregrown in liquid selective SCE or YEPE medium, respectively. Serial dilutions of washed cells were dropped on solid SC media with the indicated carbon sources. Cells were grown at 30 °C for 3 (glucose) or 6 d (xylose and mixtures).
Kinetic Measurements of Gal2, Hxt7, and N376/370 Mutants.
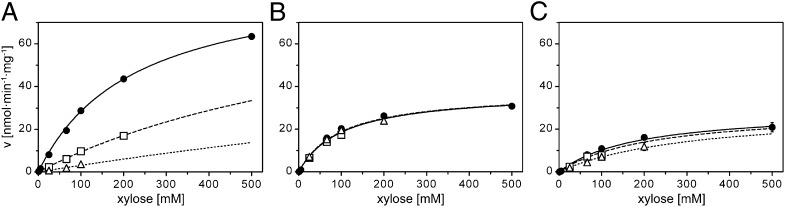
The kinetic properties of three mutants and the corresponding wild-type transporters were assayed (Table 2 and Fig. S2). Hxt7 and Gal2 were confirmed to be high-affinity glucose transporters with similar Km (0.5 and 1.5 mM) and Vmax (26.0 and 27.2 nmol⋅min−1⋅mgwetweight−1) values. Affinity for xylose is also similar between both transporters, but substantially lower than for glucose (Km 200.3 and 225.6 mM). Vmax for xylose is higher than for glucose, especially for Gal2. All mutant transporters that were tested showed an increased affinity for xylose, accompanied by a decrease in transport velocity. Gal2-N376F did not transport glucose at all, as already suggested by the growth assay (Fig. 1). Additionally, this mutant shows the highest affinity for xylose (Km 91.4 mM) while being less impaired in transport velocity than the other two mutants. Mutants Hxt7-N370S and Gal2-N376V both have nearly equal kinetic properties for xylose uptake, and both have decreased affinity for glucose. However, affinity for glucose is still about one order of magnitude higher than for xylose. This apparent discrepancy prompted us to perform xylose-uptake assays at different glucose concentrations (25 and 100 mM) to directly measure the inhibitory effect of glucose. As can be seen in Fig. 2 and Fig. S2, both wild types show strong inhibition of xylose transport even with 25 mM glucose. Despite higher affinity for glucose, Hxt7-N370S shows no inhibition at all, just like the xylose-specific mutant Gal2-N376F. Gal2-N376V is still, but relatively weakly, inhibited. Ki values were calculated assuming that inhibition is competitive. Ki values for glucose inhibition of xylose uptake for wild-type Gal2 and Hxt7 are very low (16.9 and 8.6 mM) but strongly increased for the mutants (Table 2).
Table 2.
Kinetic properties of different Gal2 and Hxt7 mutants and their respective wild type
| Transporter | Glucose | Xylose | |||
| Km (mM) | Vmax (nmol·min−1·mg−1) | Km (mM) | Vmax (nmol·min−1·mg−1) | Ki (mM) | |
| Gal2 | |||||
| WT | 1.5 ± 0.2 | 27.2 ± 0.9 | 225.6 ± 15.8 | 91.3 ± 3.2 | 8.6 ± 0.6 |
| N376F | n.d. | b.d. | 91.4 ± 8.9 | 37.3 ± 1.3 | n.d. |
| N376V | 22.1 ± 1.8 | 50.5 ± 1.4 | 168.3 ± 31.6 | 28.4 ± 2.3 | 137.2 ± 45.4 |
| Hxt7 | |||||
| WT | 0.5 ± 0.1 | 26.0 ± 1.1 | 200.3 ± 13.2 | 67.0 ± 2.0 | 16.9 ± 1.3 |
| N370S | 10.8 ± 1.0 | 47.3 ± 1.2 | 169.9 ± 26.3 | 24.1 ± 1.6 | n.d. |
Determined by zero-trans influx measurements with transporter-overexpressing EBY.VW4000 and calculated with cell wet weight. SEM is indicated. n.d., not determinable; b.d., below detection limit.
Fig. 2.
Inhibitory effect of glucose on xylose transport of wild-type and mutant Gal2. Zero-trans influx of xylose was measured in absence of glucose (solid line, filled circle) and in the presence of 25 mM (dashed line, open square) or 100 mM glucose (dotted line, open triangle). EBY.VW4000 overexpressing (A) GAL2 wild type, (B) GAL2-N376F, or (C) GAL2-N376V was grown in selective YEPE. Global curve fitting for Michaelis–Menten kinetics with competitive inhibition was applied to data of three independent measurements at each concentration. Error bars (SEM) may be smaller than the symbols. v, initial rate of uptake.
Amino Acid Residues Are Conveniently Located for Glucose–Xylose Discrimination.
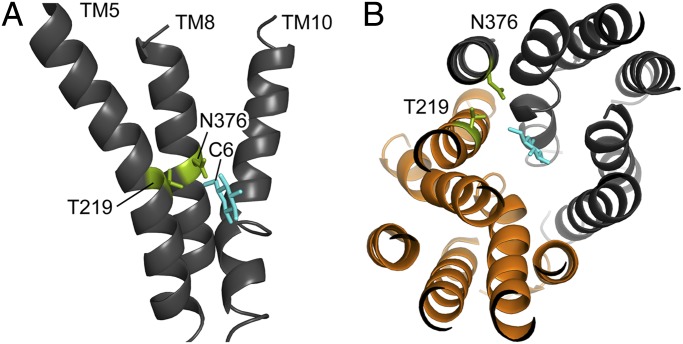
The crystal structure of xylose/H+ symporter XylE of E. coli has recently been resolved in the outward-facing partially occluded conformation. In contrast to the structure of GlcPSE, a specific glucose transporter from Staphylococcus epidermidis (39), the XylE structure contains complexed xylose or glucose, giving important information about substrate binding. XylE is a member of the major facilitator superfamily (MFS) and exhibits high similarities to the human glucose transporters GLUT1 to 4, as well as Gal2. To gain insights into the spatial position of N376 and T219 and their orientation toward the bound sugar, we constructed a homology model of Gal2 (Fig. 3 and Fig. S3) using SWISS-MODEL and PyMOL (40, 41). MFS members usually consist of 12 transmembrane helices (TM) that are organized in two distinct domains of 6 TMs (N and C domain). The two residues are found in the central part of neighboring TMs 5 (T219) and 8 (N376), and their side chains are facing the translocation pore. Glucose is complexed in its pyranose form, with the C6 (which is absent in xylose) oriented toward N376 and T219. Even though both residues do not participate in binding of glucose or xylose (in XylE), changes at these positions could result in alteration of size and form of the binding pocket. Especially, the N376F mutation would drastically reduce the space in the central cavity otherwise occupied by the hydroxymethyl group distinctive for hexoses and may offer an explanation for the xylose specificity of this mutant.
Fig. 3.
Homology model of the Gal2 structure. The model is based on the outward-facing partly occluded structure of E. coli XylE with bound glucose (PDB ID code 4GBZ). (A) Side view of Gal2; for reasons of clarity, only TMs 5, 8, and 10 are shown. The two amino acid residues T219 and N376 (green) are located at the center of their respective helix, with their side chains protruding toward the C6 of glucose (cyan). (B) Top view of Gal2 from the extracellular side, with a cross-sectional plane for better view; glucose (cyan) is found in between subdomains N (orange) and C (dark gray). The 3D images were created with PyMOL (41).
Alteration of Residues Participating in Glucose Binding Is Not Advantageous.
The XylE structure identified the residues that are directly involved in binding of glucose and xylose. Strikingly, most residues involved in the coordination of the pyranose ring (which is invariant in both sugars) are conserved between XylE and Gal2 (Figs. S4 and S5). On the contrary, significant differences between XylE and Gal2 are found in the proximity of the hydroxymethyl group of glucose. Among the three residues undergoing a hydrophobic contact with C6 of the sugar, only I171/I218 is conserved whereas L297 and F383 correspond to F350 and Y446, respectively. The polar residue Q175, which forms a hydrogen bond with the 6-hydroxyl group of glucose, is substituted by the hydrophobic amino acid I222 in Gal2. We investigated whether alterations at these four positions could selectively abolish binding of glucose to Gal2, which possibly would lead to decreased inhibition of xylose transport. For this purpose, a series of single amino acid mutants, introducing different types of side chains, were generated (Table S1). The transport capacity of the mutants was assessed in strains EBY.VW4000 and AFY10X by growth on agar plates containing different carbon sources (xylose, glucose, and mixtures thereof). The results are summarized in Table S1. Interestingly, the substitutions of I222 and F350 by the corresponding residues from XylE (I222Q, F350L) were the only ones at these positions that did not show a significant change of the transport capability. Most of all other mutations negatively affected the transport of either all three sugars tested or of just xylose. None of the changes resulted in uninhibited xylose transport on mixed media, not even in the I218G variant that confers weak growth on xylose but not on glucose.
Discussion
The simultaneous fermentation of glucose and xylose, the two most abundant sugars in lignocellulosic biomass, is a much sought-after trait in S. cerevisiae that could not be realized so far. The main hurdle is the competitive inhibition of xylose transport into the cell by glucose (24). To our knowledge, this work is the first to explicitly target transport to work toward cofermentation. The central key to our success in generating uninhibited xylose transporters was a screening strain that tightly restrains all effects to the desired feature and allows easy, growth-based selection. Different mutations at two conserved amino acid positions, asparagine 376/370 and threonine 219/213 in Gal2 and Hxt7, respectively, were found to diminish the inhibition by glucose. Among the mutants, Gal2-N376F is completely uninhibited, has lost the ability to transport hexoses, and has the highest affinity for xylose along with a moderate transport velocity.
So far, research has been focused on xylose transport per se. Transport is a bottleneck in xylose fermentation, at least at low concentrations, and certainly is exacerbated with improvements of intracellular xylose metabolism (15, 16, 21). Some heterologous xylose transporters have been identified (11, 31–33), and increased transporter expression has proven to be beneficial for xylose-fermentation performance (17, 19, 20). The drawback of all these transporters is that they are likewise inhibited by glucose. Two xylose transporters, the glucose/xylose symporter 1 [Gxs1 (Candida intermedia)] and Xut3 (Scheffersomyces stipitis), were successfully improved concerning maximal growth rate on xylose using directed evolution in an hxt0 strain (42), and, based on these results, Gxs1 was recently modified to eliminate glucose transport while retaining xylose transport (37). However, all mutants still showed inhibition. Kuyper et al. subjected an engineered, xylose-fermenting strain to evolutionary engineering to improve xylose–glucose fermentation (13, 19). Although the evolved strain showed superior xylose transport kinetics, which led to faster diauxic shift and xylose consumption, both sugars were still consumed sequentially. So, although simultaneous fermentation is highly desirable in industry, uninhibited xylose transporters, which are an essential requirement for this, have not become prevalent in either natural or artificial evolution. Our screening system directs the selective pressure toward an uninhibited xylose transport and led to discovery of the desired transporter mutants by different experimental approaches. Mutation of single amino acid positions was sufficient to ease the pressure substantially enough. The influence of single amino acid mutations on transport characteristics has been shown before (42, 43).
All of the mutated transporters that were no longer inhibited by glucose had a mutation at either asparagine 376/370 or threonine 219/213. The model of Gal2 that was deduced from the XylE structure (35) shows that both residues are found in the same region of the transport protein. Referring to the outward-facing, partly occluded conformation, they are positioned slightly to the extracellular side of the central binding pocket so that alterations of the amino acids side chain could sterically prevent glucose from entering the pocket and relieve competitive inhibition. The proposed transport mechanism is a tilting motion of the N and C domain in respect to each other, without extensive conformational changes within the domains themselves (44, 45). T219/213 and N376/370 are found at different sides of the junction between the two domains and are close to the axis of movement where positional changes are minimal. It is conceivable that mutations at this position barely interfere with the mechanisms relevant for the transport process. Another region of a sugar-transport protein, a sequence motif in TM1, was altered in Gxs1 by Young et al. and could increase specificity for xylose but did not eliminate inhibition by glucose (37). This observation indicates that glucose might still be bound but not transported. The motif is close to the C2/C3 position of the sugar and includes residue F40, which is important for sugar binding in XylE. Hxt7 mutants of this motif that could transport xylose but not glucose could be generated only in combination with a D340M mutation (37, 46).
Several amino acids were found to be functional as replacements for asparagines 376/370. They had either hydrophobic (A/V/I/L/Y/F) or smaller and hydrophilic (S/T) side chains. The asparagine-to-phenylalanine mutation abolishes hexose transport completely, most likely due to the larger side chain that protrudes into the substrate translocation pathway. Most of the other asparagine mutants retain hexose transport ability but are less inhibited. In both, the crystal structure of XylE and the derived homology model of Gal2, neither N325/N376 nor I172/T219 directly participates in binding of xylose or glucose. Substitution of asparagine or threonine with smaller-sized amino acids (A/S/T/V/G or N/S/A/G, respectively) is also unlikely to narrow the translocation pore. Instead, changes of van der Waals interactions between neighboring amino acids could alter the structure of the substrate binding site, leading to reduced glucose affinity (38). Threonine 219/213 has already been found to be crucial for high-affinity glucose transport (38) and is located in close proximity to Q215, I218, and I222 in TM5 that contribute to binding of the C6-hydroxymethyl of glucose. The Hxt7 mutants that emerged from the screening process (T213N/S/A/G) are those that are characterized by an increased Km combined with an increased Vmax for glucose (38). The same tendencies can be seen with the transport kinetics of all measured Gal2 and Hxt7 mutants. Along with weakened affinity for glucose, kinetic measurements also show an increased affinity for xylose in all mutants, especially in the Gal2-N376F mutant. This increase suggests that further contacts to xylose are formed, presumably to the C5, which is normally bound neither in glucose nor in xylose. Phenylalanine as an aromatic residue could contribute to coordination of xylose, just like some other aromatic residues in the binding pocket (F85, Y351, W455, W479). Despite strongly diminished inhibition of xylose transport by glucose, affinity for glucose is still higher than for xylose (except for Gal2-N376F).
All our results show that changes in a confined region result in increased affinity for xylose and decreased or even abolished affinity for glucose. This region is in close vicinity to the binding region of the C6 of glucose that is predestined for discrimination between these two very similar sugars. Surprisingly, changing the amino acids that are supposed to directly interact with the C6 of glucose did not prove to be valuable. Most transporters lost their transport function completely, which implies that residues directly involved in substrate binding are very sensitive to changes and affect the function of the protein too severely.
The generated transporters should contribute to the realization of cofermentation of glucose and xylose in S. cerevisiae and possibly other organisms. Aside from their application related to biotechnology, the effects of the different mutants on transport of glucose and xylose will increase our understanding of the structural mechanisms and detailed requirements for sugar binding and transport.
Materials and Methods
Strains and media, transformation and DNA preparation, plasmid construction, epPCR mutagenesis, and site-directed mutagenesis are described in SI Materials and Methods. Media are abbreviated SC (synthetic complete), SM (synthetic minimal), or YEP (yeast extract peptone) together with the respective carbon source code M (maltose), E (ethanol), or X (xylose). Strains, plasmids, and primers are listed in Table S2, Table S3, and Table S4, respectively.
Strain Construction.
Gene deletions in EBY.VW4000 were performed using the loxP/cre recombinase system with kanMX and hphNT1 markers (47, 48). Further details are found in SI Materials and Methods. GLK1 and HXK1 were deleted first, growing cells on selective YEPM. Marker cassettes were then removed using the cre recombinase expressed from plasmid pSH47, which was later removed by 5-fluoroorotic acid (FOA) counterselection (49). Before deletion of HXK2 and YLR446W, the strain was transformed with GAL2-coding plasmid pHL125re (50) to enable growth on galactose and thus easier handling. The hxk0-phenotype in the hxt0 background was verified on maltose media. For improvement of xylose consumption, a multigene cassette for overexpression of TAL1, TKL1, RPE1, RKI1, and codon-optimized XKS1 was integrated into the genome locus of the hxk0 hxt0 strain. To this end, it was transformed with linearized plasmid pAF-HD8.3, which contains the multigene cassette and a kanMX marker flanked by sequences homologous to the PYK2 locus. Transformants were selected by G418 resistance. The resulting strain was named AFY10. To complete the xylose-utilization pathway, AFY10 was transformed with plasmid YEp181_pHXT7-optXI_Clos (bearing the codon-optimized xylA from C. phytofermentans), resulting in strain AFY10X.
Evolutionary Engineering.
Strain AFY10X was transformed with plasmid p426_GAL2, p426_HXT7, or p426_HXT5 and grown on selective SCE plates. The evolutionary engineering was performed as sequential aerobic batch fermentations in shake flasks on rotary shakers (Infors Orbitron, 180 rpm) at 30 °C. Cultures were inoculated to an OD600 of 0.2, grown until reaching late exponential to early stationary phase, and then harvested by centrifugation (2,000 × g, 2 min) for fresh inoculation. For selection of plasmids, media lacked l-leucine and uracil. G418 and 0.5 g/L 2-deoxy-d-glucose (2-DOG) were added to select for the AFY10 strain background and prevent growth of suppressor mutants of the hxk0 phenotype. hxk0 strains, but not wild-type strains, are resistant to 2-DOG (24). For generation of biomass and for adaptation to growth on xylose, the first three batches were performed in SCE, SCX, and SMX, respectively. From the latter culture, one backup culture was inoculated in SMX whereas another was set up in SMX plus 5 or 10 g/L glucose. In case of Gal2, the latter cultures failed to grow so the backup culture was transferred to a culture in SMX plus 2 g/L glucose. The approach with Hxt7 was conducted on SC medium due to failed growth in SM medium. The experiment was aborted because growth ceased at 10 g/L glucose. Concentrations were increased in the following order [g/L]: for Gal2, 2/3/5/5/10/10/20/20; for Hxt5, 10/20/20/20/30/30/40/50/50/50; for Hxt7, 5/5/5/10. Samples from the last batch were plated out on selective SCE for clone selection (see Clone Selection and Analysis).
Clone Selection and Analysis.
Screening of the evolutionary engineering culture samples and the AFY10X transformants of epPCR and targeted mutagenesis was performed as follows. Colonies were transferred from selective SCE plates to plates with various xylose–glucose concentrations (2 + 10, 2 + 20, 10 + 30, and 10 + 50 g/L) by replica plating. Single colonies were selected based on best growth and streaked out on fresh plates for better comparison and to obtain single clones. Single colonies were grown in liquid selective SCE medium for DNA preparation. Plasmids were isolated as described for plasmid construction. In some cases, ORFs were amplified from the DNA preparations by PCR. Both were sequenced to identify mutations.
Growth Assays.
EBY.VW4000 and AFY10X were transformed with the pRS62N-transporter plasmids and inoculated in 5 mL YEPE supplemented with ClonNAT or SCE leu− with G418, ClonNAT, and 2-DOG, respectively. After reaching late exponential phase, the cells were collected by centrifugation (2,000 × g, 2 min), washed with sterile water, and then resuspended to an OD600 of 1.0 in selective SC medium without carbon source. From this cell suspension, a 10-fold serial dilution was prepared in selective SC medium (four dilution steps). Then, 6 µL of each cell suspension were spotted on selective SC plates and grown at 30 °C for up to 7 d. Carbon sources tested with EBY.VW4000 were glucose (2 g/L), galactose, fructose, and mannose (each at 2 and 20 g/L). Xylose (2 and 10 g/L) and xylose–glucose mixtures (2 + 10 and 10 + 50 g/L) were used for the AFY10X growth test. AFY10X, CEN.PK2-1c, and EBY.VW4000 with empty vector (pRS62N) were included as controls. All strains were also checked on ethanol and maltose (10 g/L) plates and showed growth. Controls behaved as expected (Fig. S1 A and C). The growth tests of the Gal2-I218/I222/F350/Y446 mutants were done accordingly, using p426H7-transporter plasmids and selective SC medium for cultivation. EBY.VW4000 transformants were tested for glucose (20 g/L), AFY10X transformants for xylose (20 g/L), and xylose–glucose mixtures (2 + 20 and 10 + 40 g/L). Maltose or ethanol was used as a control, respectively.
Uptake Assays.
Determination of initial uptake rates of sugars was performed as described in refs. 51 and 52, with further modifications. For the assay, EBY.VW4000 expressing the respective transporter was grown in selective YEPE to an OD600 of 1.1–1.6. Details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Heiko Dietz (Goethe University Frankfurt) for providing plasmid pHD8, and Thorsten Subtil, Feline Benisch, and Dawid Brat for helpful suggestions and discussion. We thank Alexander Jung and Martin Brinek for their contributions to the experimental work. This project has been supported by the EC 7th Framework program [NEMO project (novel high-performance enzymes and microorganisms for conversion of lignocellulosic biomass to bioethanol)], Butalco GmbH, and the German Federal Ministry of Food, Agriculture and Consumer Protection following a decision of the German Bundestag [Project ECO-FERM (Effiziente Co-Fermentation von Pentosen und Hexosen durch Hefen), Förderkennzeichen: 22031811].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.J. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323464111/-/DCSupplemental.
References
- 1.Gírio FM, et al. Hemicelluloses for fuel ethanol: A review. Bioresour Technol. 2010;101(13):4775–4800. doi: 10.1016/j.biortech.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 2.Kötter P, Amore R, Hollenberg CP, Ciriacy M. Isolation and characterization of the Pichia stipitis xylitol dehydrogenase gene, XYL2, and construction of a xylose-utilizing Saccharomyces cerevisiae transformant. Curr Genet. 1990;18(6):493–500. doi: 10.1007/BF00327019. [DOI] [PubMed] [Google Scholar]
- 3.Madhavan A, et al. Xylose isomerase from polycentric fungus Orpinomyces: Gene sequencing, cloning, and expression in Saccharomyces cerevisiae for bioconversion of xylose to ethanol. Appl Microbiol Biotechnol. 2009;82(6):1067–1078. doi: 10.1007/s00253-008-1794-6. [DOI] [PubMed] [Google Scholar]
- 4.Brat D, Boles E, Wiedemann B. Functional expression of a bacterial xylose isomerase in Saccharomyces cerevisiae. Appl Environ Microbiol. 2009;75(8):2304–2311. doi: 10.1128/AEM.02522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuyper M, et al. High-level functional expression of a fungal xylose isomerase: The key to efficient ethanolic fermentation of xylose by Saccharomyces cerevisiae? FEMS Yeast Res. 2003;4(1):69–78. doi: 10.1016/S1567-1356(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 6.Brat D, Boles E. Isobutanol production from D-xylose by recombinant Saccharomyces cerevisiae. FEMS Yeast Res. 2013;13(2):241–244. doi: 10.1111/1567-1364.12028. [DOI] [PubMed] [Google Scholar]
- 7.Hong KK, Nielsen J. Metabolic engineering of Saccharomyces cerevisiae: A key cell factory platform for future biorefineries. Cell Mol Life Sci. 2012;69(16):2671–2690. doi: 10.1007/s00018-012-0945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen J, Larsson C, van Maris A, Pronk J. Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotechnol. 2013;24(3):398–404. doi: 10.1016/j.copbio.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Kotyk A. Properties of the sugar carrier in baker’s yeast. II. Specificity of transport. Folia Microbiol (Praha) 1967;12(2):121–131. doi: 10.1007/BF02896872. [DOI] [PubMed] [Google Scholar]
- 10.Hamacher T, Becker J, Gárdonyi M, Hahn-Hägerdal B, Boles E. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology. 2002;148(Pt 9):2783–2788. doi: 10.1099/00221287-148-9-2783. [DOI] [PubMed] [Google Scholar]
- 11.Saloheimo A, et al. Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases. Appl Microbiol Biotechnol. 2007;74(5):1041–1052. doi: 10.1007/s00253-006-0747-1. [DOI] [PubMed] [Google Scholar]
- 12.Kötter P, Ciriacy M. Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1993;38(6):776–783. [Google Scholar]
- 13.Kuyper M, et al. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 2005;5(4-5):399–409. doi: 10.1016/j.femsyr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Demeke MM, et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol Biofuels. 2013;6(1):89. doi: 10.1186/1754-6834-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gárdonyi M, Jeppsson M, Lidén G, Gorwa-Grauslund MF, Hahn-Hägerdal B. Control of xylose consumption by xylose transport in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng. 2003;82(7):818–824. doi: 10.1002/bit.10631. [DOI] [PubMed] [Google Scholar]
- 16.Parachin NS, Bergdahl B, van Niel EW, Gorwa-Grauslund MF. Kinetic modelling reveals current limitations in the production of ethanol from xylose by recombinant Saccharomyces cerevisiae. Metab Eng. 2011;13(5):508–517. doi: 10.1016/j.ymben.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Tanino T, et al. Sugar consumption and ethanol fermentation by transporter-overexpressed xylose-metabolizing Saccharomyces cerevisiae harboring a xyloseisomerase pathway. J Biosci Bioeng. 2012;114(2):209–211. doi: 10.1016/j.jbiosc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Runquist D, Hahn-Hägerdal B, Rådström P. Comparison of heterologous xylose transporters in recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2010;3(1):5. doi: 10.1186/1754-6834-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuyper M, et al. Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res. 2005;5(10):925–934. doi: 10.1016/j.femsyr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Runquist D, Fonseca C, Rådström P, Spencer-Martins I, Hahn-Hägerdal B. Expression of the Gxf1 transporter from Candida intermedia improves fermentation performance in recombinant xylose-utilizing Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2009;82(1):123–130. doi: 10.1007/s00253-008-1773-y. [DOI] [PubMed] [Google Scholar]
- 21.Wahlbom CF, Cordero Otero RR, van Zyl WH, Hahn-Hägerdal B, Jönsson LJ. Molecular analysis of a Saccharomyces cerevisiae mutant with improved ability to utilize xylose shows enhanced expression of proteins involved in transport, initial xylose metabolism, and the pentose phosphate pathway. Appl Environ Microbiol. 2003;69(2):740–746. doi: 10.1128/AEM.69.2.740-746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katahira S, et al. Improvement of ethanol productivity during xylose and glucose co-fermentation by xylose-assimilating S. cerevisiae via expression of glucose transporter Sut1. Enzyme Microb Technol. 2008;43(2):115–119. [Google Scholar]
- 23.Kim SR, Ha S-J, Wei N, Oh EJ, Jin Y-S. Simultaneous co-fermentation of mixed sugars: A promising strategy for producing cellulosic ethanol. Trends Biotechnol. 2012;30(5):274–282. doi: 10.1016/j.tibtech.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Subtil T, Boles E. Competition between pentoses and glucose during uptake and catabolism in recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2012;5(1):14. doi: 10.1186/1754-6834-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha S-J, et al. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc Natl Acad Sci USA. 2011;108(2):504–509. doi: 10.1073/pnas.1010456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WJ, Kim MD, Ryu YW, Bisson LF, Seo JH. Kinetic studies on glucose and xylose transport in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2002;60(1-2):186–191. doi: 10.1007/s00253-002-1085-6. [DOI] [PubMed] [Google Scholar]
- 27.Lucas C, van Uden N. Transport of hemicellulose monomers in the xylose-fermenting yeast Candida shehatae. Appl Microbiol Biotechnol. 1986;23(6):491–495. [Google Scholar]
- 28.Delgenes JP, Moletta R, Navarro JM. Fermentation of D-xylose, D-glucose and L-arabinose mixture by Pichia stipitis Y 7124: Sugar tolerance. Appl Microbiol Biotechnol. 1988;29(2-3):155–161. doi: 10.1002/bit.260340314. [DOI] [PubMed] [Google Scholar]
- 29.Gárdonyi M, Österberg M, Rodrigues C, Spencer-Martins I, Hahn-Hägerdal B. High capacity xylose transport in Candida intermedia PYCC 4715. FEMS Yeast Res. 2003;3(1):45–52. doi: 10.1111/j.1567-1364.2003.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 30.Nobre A, Lucas C, Leão C. Transport and utilization of hexoses and pentoses in the halotolerant yeast Debaryomyces hansenii. Appl Environ Microbiol. 1999;65(8):3594–3598. doi: 10.1128/aem.65.8.3594-3598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du J, Li S, Zhao H. Discovery and characterization of novel d-xylose-specific transporters from Neurospora crassa and Pichia stipitis. Mol Biosyst. 2010;6(11):2150–2156. doi: 10.1039/c0mb00007h. [DOI] [PubMed] [Google Scholar]
- 32.Leandro MJ, Gonçalves P, Spencer-Martins I. Two glucose/xylose transporter genes from the yeast Candida intermedia: First molecular characterization of a yeast xylose-H+ symporter. Biochem J. 2006;395(3):543–549. doi: 10.1042/BJ20051465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young E, Poucher A, Comer A, Bailey A, Alper H. Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host. Appl Environ Microbiol. 2011;77(10):3311–3319. doi: 10.1128/AEM.02651-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson PJ, Maiden MC. Homologous sugar transport proteins in Escherichia coli and their relatives in both prokaryotes and eukaryotes. Philos Trans R Soc Lond B Biol Sci. 1990;326(1236):391–410. doi: 10.1098/rstb.1990.0020. [DOI] [PubMed] [Google Scholar]
- 35.Sun L, et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature. 2012;490(7420):361–366. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 36.Lam VM, Daruwalla KR, Henderson PJ, Jones-Mortimer MC. Proton-linked D-xylose transport in Escherichia coli. J Bacteriol. 1980;143(1):396–402. doi: 10.1128/jb.143.1.396-402.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young EM, Tong A, Bui H, Spofford C, Alper HS. Rewiring yeast sugar transporter preference through modifying a conserved protein motif. Proc Natl Acad Sci USA. 2014;111(1):131–136. doi: 10.1073/pnas.1311970111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasahara T, Shimogawara K, Kasahara M. Crucial effects of amino acid side chain length in transmembrane segment 5 on substrate affinity in yeast glucose transporter Hxt7. Biochemistry. 2011;50(40):8674–8681. doi: 10.1021/bi200958s. [DOI] [PubMed] [Google Scholar]
- 39.Iancu CV, Zamoon J, Woo SB, Aleshin A, Choe JY. Crystal structure of a glucose/H+ symporter and its mechanism of action. Proc Natl Acad Sci USA. 2013;110(44):17862–17867. doi: 10.1073/pnas.1311485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 41. Schrödinger (2010) The PyMOL Molecular Graphics System (Schrödinger, Cambridge, MA), Version 1.3r1.
- 42.Young EM, Comer AD, Huang H, Alper HS. A molecular transporter engineering approach to improving xylose catabolism in Saccharomyces cerevisiae. Metab Eng. 2012;14(4):401–411. doi: 10.1016/j.ymben.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Weierstall T, Hollenberg CP, Boles E. Cloning and characterization of three genes (SUT1-3) encoding glucose transporters of the yeast Pichia stipitis. Mol Microbiol. 1999;31(3):871–883. doi: 10.1046/j.1365-2958.1999.01224.x. [DOI] [PubMed] [Google Scholar]
- 44.Quistgaard EM, Löw C, Moberg P, Trésaugues L, Nordlund P. Structural basis for substrate transport in the GLUT-homology family of monosaccharide transporters. Nat Struct Mol Biol. 2013;20(6):766–768. doi: 10.1038/nsmb.2569. [DOI] [PubMed] [Google Scholar]
- 45.Henderson PJF, Baldwin SA. This is about the in and the out. Nat Struct Mol Biol. 2013;20(6):654–655. doi: 10.1038/nsmb.2604. [DOI] [PubMed] [Google Scholar]
- 46.Kasahara T, Kasahara M. Identification of a key residue determining substrate affinity in the yeast glucose transporter Hxt7: A two-dimensional comprehensive study. J Biol Chem. 2010;285(34):26263–26268. doi: 10.1074/jbc.M110.149716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter Z, Delneri D. New generation of loxP-mutated deletion cassettes for the genetic manipulation of yeast natural isolates. Yeast. 2010;27(9):765–775. doi: 10.1002/yea.1774. [DOI] [PubMed] [Google Scholar]
- 48.Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24(13):2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 50.Liang H, Gaber RF. A novel signal transduction pathway in Saccharomyces cerevisiae defined by Snf3-regulated expression of HXT6. Mol Biol Cell. 1996;7(12):1953–1966. doi: 10.1091/mbc.7.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bisson LF, Fraenkel DG. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1983;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh MC, Smits HP, Scholte M, van Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176(4):953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.