Significance
Loss of megafauna, termed trophic downgrading, has been found to affect biotic interactions, disturbance regimes, species invasions, and nutrient cycling. One recognized cause in air-breathing marine megafauna is incidental capture or bycatch by fisheries. Characterizing megafauna bycatch patterns across large ocean regions is limited by data availability but essential to direct conservation and management resources. We use empirical data to identify the global distribution and magnitude of seabird, marine mammal, and sea turtle bycatch in three widely used fishing gears. We identify taxa-specific hotspots and find evidence of cumulative impacts. This analysis provides an unprecedented global assessment of the distribution and magnitude of air-breathing megafauna bycatch, highlighting its cumulative nature and the urgent need to build on existing mitigation successes.
Keywords: fisheries bycatch, trophic downgrading, longlines, gillnets, trawls
Abstract
Recent research on ocean health has found large predator abundance to be a key element of ocean condition. Fisheries can impact large predator abundance directly through targeted capture and indirectly through incidental capture of nontarget species or bycatch. However, measures of the global nature of bycatch are lacking for air-breathing megafauna. We fill this knowledge gap and present a synoptic global assessment of the distribution and intensity of bycatch of seabirds, marine mammals, and sea turtles based on empirical data from the three most commonly used types of fishing gears worldwide. We identify taxa-specific hotspots of bycatch intensity and find evidence of cumulative impacts across fishing fleets and gears. This global map of bycatch illustrates where data are particularly scarce—in coastal and small-scale fisheries and ocean regions that support developed industrial fisheries and millions of small-scale fishers—and identifies fishing areas where, given the evidence of cumulative hotspots across gear and taxa, traditional species or gear-specific bycatch management and mitigation efforts may be necessary but not sufficient. Given the global distribution of bycatch and the mitigation success achieved by some fleets, the reduction of air-breathing megafauna bycatch is both an urgent and achievable conservation priority.
Ocean health, a measure of the overall condition of marine ecosystems, has been the focus of a number of recent studies (1) that have shown that the impact fisheries can have on ocean health. Over the past 50 y, total world fisheries production has increased from 19.3 million tons in 1950 to more than 154 million tons today (2), and although fisheries management initiatives have reduced exploitation rates in some regions, a large fraction of stocks (approximately 63%) is still classified as overfished or collapsed (2, 3). Beyond the direct effects of fish removal, fishing exerts indirect effects through incidental capture of nontarget species or bycatch (4, 5). [The term bycatch is also defined as all unwanted, unmanaged, or discarded catch (4). Megafauna species are targets of fisheries in some countries, although targeted fisheries are a less common fishery interaction than incidental capture at the global scale.] Also, it is one of the primary causes of observed declines of seabirds, marine mammals, and sea turtles, collectively termed air-breathing marine megafauna (6–9). Fisheries bycatch is a product of susceptibility (driven by the distribution, type, and magnitude of fisheries effort) and vulnerability (based on ecological characteristics of the bycaught species; e.g., life history and species distribution) (10). For some depleted species, such as Pacific leatherback turtle (Dermochelys coriacea), Amsterdam Albatross (Diomedea amsterdamensis), vaquita (Phocoena sinus), Atlantic humpbacked dolphin (Sousa teuszii), and Australian and New Zealand sea lion (Neophoca cinerea and Phocarctos hookeri), fisheries bycatch has been identified as the single largest threat to extant populations (7, 11–15).
Beyond issues of species viability, declines in marine megafauna lead to major changes in ecosystem function and process (16, 17). This loss of megafauna, referred to as trophic downgrading, has reverberating effects on biotic interactions, disturbance regimes, species invasions, and nutrient cycling (17). Fisheries bycatch, a major driver of trophic downgrading, can be difficult to assess; accurate bycatch data collection requires dedicated and trained observers and considerable resources across fleets and vast oceans (18). Nevertheless, a comprehensive understanding of the extent and magnitude of bycatch is a necessary first step to direct conservation actions that may ameliorate this threat.
We conducted a direct global assessment of fisheries bycatch using empirical data from peer-reviewed publications, agency and technical reports, and symposia proceedings published between 1990 and 2008 to characterize bycatch in three taxonomic groups (seabirds, marine mammals, and turtles) and three general categories of widely used fishing gear (gillnets, longlines, and trawls). This direct and comprehensive assessment differs from most previous studies that have focused on a proxy of bycatch (e.g., fisheries yield) rather than empirical bycatch data (19), a single species or taxon (20–22), or a single type of fishing gear in one region (23, 24), often without consideration of the spatial distribution of bycatch. A direct, spatially explicit cross-taxa and cross-gear bycatch assessment is necessary to analyze the complex patterns of global bycatch. Without such information, well-intentioned fisheries management schemes, such as modifications to gear or the location or timing of fishing effort, may reduce bycatch of one taxon but exert collateral damage on other species (e.g., a switch from demersal longlines that take seabirds to bottom set gillnets may reduce the bycatch of seabirds but increase bycatch of small cetaceans) (25). The concern regarding collateral damage stems from the one-to-many relationship among gear and bycatch species—one species may be caught by multiple gear types and one gear type may catch multiple species within a single fishing region. The one-to-many relationship among gear and bycatch species can lead to cumulative population or assemblage effects that may be greater than the sum of individual effects.
We used a multitaxa, multigear bycatch database to answer three questions. (i) Where is bycatch highest by taxon and gear type? (ii) Are there hotspots of cumulative bycatch across taxa and gear? (iii) Where are the gaps in existing bycatch data? Because there are no standard metrics for reporting bycatch within or among ocean regions, substantial nonuniformity exists among available bycatch data. For example, in a single taxa (sea turtles) and a single gear type (gillnets), 17 different metrics were used to report bycatch (table 1 in ref. 9). The lack of standard metrics for bycatch reporting presents a formidable impediment to direct comparison or integrated analyses of existing bycatch data. To address this lack of conformity, we used expert knowledge to allow for synthesis among bycatch records. Expert knowledge has been shown to be a necessary approach to account for data gaps and synthesize existing knowledge and disparate data (26–29). Our expert panel consisted of 17 independent experts (5, 6, and 6 experts with expertise in seabirds, marine mammals, and sea turtles, respectively) with at least 5 y of experience in fisheries bycatch evaluating the intensity of each bycatch record from high (five) to low (one) within each gear category type. Bycatch intensity represents a common metric of bycatch level or severity that integrates bycatch rate, species affected, spatial location, and fishing effort information (Materials and Methods). Working independently, at least three experts reviewed each record, and we averaged intensity scores to account for differences among respondents. Using these expert-based bycatch intensity scores, we then mapped patterns of bycatch intensity of all documented bycatch reports by taxa and gear and generated a cumulative map integrated across taxa and gear.
Results
Bycatch Intensity by Taxa and Gear Type.
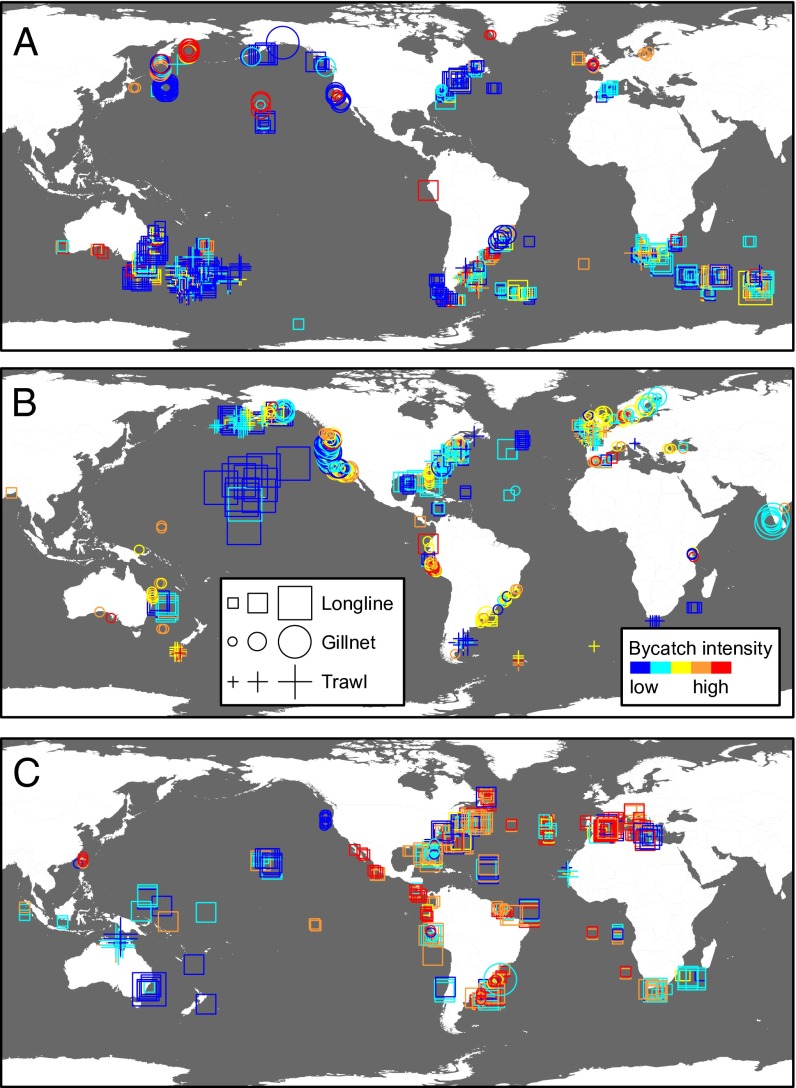
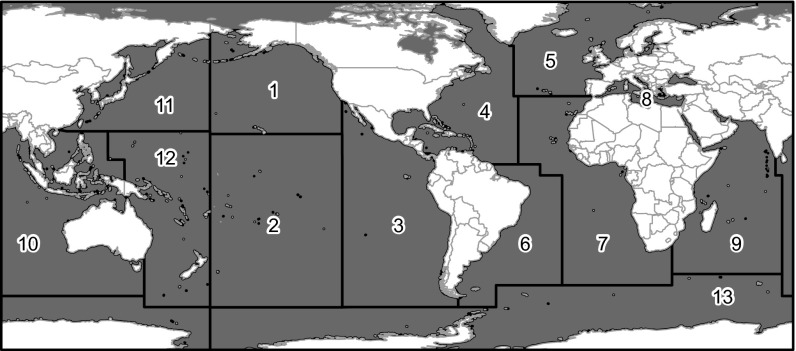
Because the traditional approach to characterizing bycatch has been taxa- or gear-specific, we first mapped bycatch intensity to illustrate patterns in megafauna bycatch by taxonomic group (Fig. 1) and gear type (Fig. S1). Among the three taxa, sea turtles had statistically higher bycatch intensity followed by marine mammals and then seabirds [generalized linear model; F(2,2,117) = 98.65, P < 0.001]. This difference among taxa likely reflects the tenuous conservation status of sea turtles: six of seven sea turtle species are classified as threatened with extinction by the International Union for Conservation of Nature (redlist.org). We also found significant differences within taxa among regions with available data (regions shown in Fig. 2). High-intensity sea turtle bycatch was most prevalent in three fishing areas—the southwest Atlantic Ocean, eastern Pacific Ocean, and Mediterranean Sea. Marine mammal bycatch intensity was highest in the eastern Pacific Ocean and Mediterranean Sea, whereas seabird bycatch was highest in the southwest Atlantic Ocean and the southern Indian Ocean, where several petrel and albatross breeding colonies are located (23).
Fig. 1.
Bycatch intensity for (A) seabirds, (B) marine mammals, and (C) sea turtles for records from 1990 to 2008. Three gear categories (gillnet, longline, and trawl) are represented by separate symbols, and symbol size is scaled to reflect the proportional amount of observed fishing effort for each record. Bycatch data records that did not have reported observed effort are shown in Fig. S2.
Fig. 2.
Geographic regions used for analysis: 1, northeast Pacific; 2, southeast Pacific; 3, eastern Tropical Pacific; 4, northwest Atlantic/Caribbean; 5, northeast Atlantic; 6, southwest Atlantic; 7, eastern Atlantic; 8, Mediterranean; 9, western Indian Ocean; 10, eastern Indian Ocean; 11, northwest Pacific; 12, southwest Pacific; and 13, southern Ocean/Antarctica.
Considering bycatch intensity by gear categories worldwide (Fig. S1), we found that gillnets had the highest bycatch intensity scores followed by longlines and then trawls [F(2,2,052) = 10.927, P < 0.001]. Within a gear type, we found differing patterns of bycatch intensity among regions, with some gears associated with both high- and low-intensity bycatch. For gillnets, however, bycatch intensity was similar among fishing regions (mean SE = 0.35). Longline and trawl bycatch intensity did suggest that there were differences in intensity among regions, because intensity values were significantly higher in the eastern Pacific Ocean than other regions where data are available [F(9,1,844) = 5.927, P < 0.01].
Despite these broad patterns, gear-specific bycatch intensity was found to be variable within a region, with a single gear type associated with both very high- and very low-intensity bycatch. This intraregional variability could reflect bias in bycatch data availability or data accuracy, because the level of fishing effort influences the precision of the reported bycatch rate (9, 30) and differences in fishing effort practice or distribution, or it could reflect the highly variable nature of spatiotemporal overlap between fishing effort and megafauna (31).
Cumulative Bycatch Intensity.
To consider potential impacts to wide-ranging megafauna species as an assemblage, we calculated cumulative bycatch patterns across large ocean regions. To consider the impact of bycatch from different fleets and gears, we calculated a cumulative bycatch intensity index for all bycatch records in each region. Cumulative intensity (IC) was calculated as
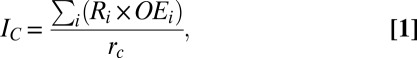 |
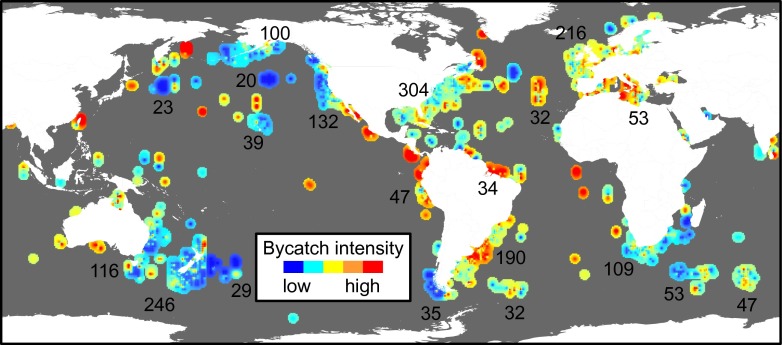
where the mean product of Ri, the bycatch intensity score for each record within a region, and OEi, the scaled observed fishing effort for each record within a region, is divided by rc, the total number of data records per region. Weighting the bycatch scores by the relative amount of fishing effort observed resulted in greater weight being given to the more precise bycatch estimates, because estimate precision is highly correlated with the level of observed effort (9, 30). Cumulative megafauna bycatch intensities varied among regions, with the highest cumulative scores (>1 SD) occurring in the Mediterranean and the southwest Atlantic (Fig. 3 and Table 1), although these values could not be calculated in data-poor regions.
Fig. 3.
Calculated cumulative bycatch intensity (Eq. 1) for all taxonomic groups and gear types. Bycatch intensity values were used to generate a raster surface from an inverse weighted distance function for polygons with available data. Interpolated values are displayed using the same color ramp as in Fig. 1. Numbers represent the number of data records represented within each polygon.
Table 1.
Calculated cumulative bycatch intensity and associated bycatch records for 10 ocean regions
| Ocean region | Cumulative bycatch intensity | Number of bycatch records included |
| Northwest Pacific | 2.53 | 224 |
| Southeast Pacific | 3.59 | 120 |
| Northwest Atlantic/Caribbean | 2.84 | 215 |
| Northeast Atlantic | 3.37 | 124 |
| Southwest Atlantic | 3.84 | 155 |
| Southeast Atlantic | 3.0 | 81 |
| Mediterranean | 4.77 | 90 |
| Eastern Indian Ocean | 3.23 | 69 |
| Southwest Pacific | 2.73 | 217 |
| Southern Ocean/Antarctica | 3.28 | 188 |
Cumulative scores were only calculated for regions with more than 50 bycatch records. Ocean regions are shown in Fig. 2.
Discussion
Widespread Nature of Megafaunal Bycatch.
These results represent the most complete representation of the global distribution of megafauna bycatch. Our synthesis reveals bycatch hotspots that may be driven by megafauna density (32–36), fishing intensity (37), or a combination of both density and intensity. We found evidence of taxon-specific hotspots of bycatch intensity (e.g., southwest Atlantic and Mediterranean for turtles, eastern Pacific Ocean for marine mammals, and southwest Atlantic for seabirds) as well as some significant differences in bycatch intensity among gears and regions (e.g., gillnets in all regions and longlines and trawls in the eastern Pacific Ocean).
These spatial patterns are most directly influenced by data availability but also likely influenced by (i) the global distribution of air-breathing megafauna, (ii) the distribution of fishing effort, and (iii) the success of bycatch mitigation for some gear types, taxa, and regions. The distribution of megafauna and subsequent bycatch is likely influenced by both oceanographic (32–36) and physiological forces [i.e., this group of (primarily) endothermic megafauna is more likely to occur in cooler waters] (38). Additionally, the distribution of fishing effort is known to be nonrandom, with greater effort concentrated in productive Eastern Boundary Currents and shallow coastal zones (39). The patterns of species and vessel distribution and biophysical features may account for the documented hotspots in the eastern Pacific and southwestern Atlantic, respectively. Finally, regional bycatch mitigation efforts have altered the global distribution of bycatch intensity. For example, turtle excluder devices in trawl fisheries have been implemented successfully in Australian fisheries, where the bycatch of sea turtles has been reduced by 90% (40). Likewise, a suite of seabird mitigation devices has successfully reduced the bycatch of albatross and petrels in longline vessels in Hawaii, Alaska, and the Southern Ocean (41, 42).
Despite these improvements and other important technological and policy improvements (43), bycatch remains a significant threat to many populations of megafauna, because many nations and regional fisheries management agencies do not require or enforce the use of proven bycatch reduction measures; bycatch mitigation is long on potential solutions and short on effective implementation (44). Furthermore, although targeted, top-down mitigation implementation and enforcement have proven effective for some fishing sectors [e.g., seabird bycatch mitigation measures in the Southern Ocean (45)], community-level engagement is needed to successfully reduce bycatch in less-regulated, small-scale fisheries (46–48).
Bycatch as a Cumulative Threat to Megafaunal Assemblages.
Beyond the taxon- and gear-specific patterns that can be used to inform management and conservation action for a particular species group or region, our analyses highlight potential cumulative impacts of bycatch across gears and taxa. Our findings suggest that traditional management and mitigation efforts that are species-, taxa-, or gear-specific may be necessary but not sufficient. Many species included in the database are wide-ranging, traveling hundreds to thousands of kilometers, and thus, encounter fishing vessels from numerous fleets that deploy many types of fishing gear. Because the loss of marine megafauna has been linked to major changes in marine ecosystem function and process (17), the cumulative effect of bycatch across fisheries sectors and fleets represents a threat to both species viability and ecosystem process. This data synthesis suggests a confluence of species- and ecosystem-level effects for at least two regions—the Mediterranean and the southwest Atlantic—that are experiencing large cumulative bycatch impacts and that are areas of high-intensity bycatch for particular taxa.
Identification of Critical Data Gaps and Needs.
Our analyses also identified several critical knowledge gaps. One data gap is the disparity in information from different fisheries sectors. High-seas and industrial fisheries have been the focus of more data collection than coastal or small-scale fisheries (>50% of all records), a result driven by the infrastructure of the industrial fisheries and the national and regional management organizations that oversee these fisheries (49). The lack of information from coastal and small-scale fisheries is troubling given that bycatch in small-scale fisheries can be substantial (on par with or exceeding bycatch levels in industrial fisheries) (31, 50, 51), suggesting that small-scale fisheries may pose a much greater challenge to marine megafauna than previously thought. Existing data from small-scale fisheries show that this sector requires mitigation (31) in addition to data collection to help guide efforts to develop appropriate conservation strategies.
This assessment also illustrates the disparities in the availability of bycatch data among regions (Fig. 3). The most obvious data gaps are in the Indian Ocean, eastern Atlantic, southeast Asia, and central and western Pacific. Although some bycatch data exist in these ocean areas (Fig. S1), there is relatively little information for these large fishing regions that are known to support highly developed industrial fisheries as well as many millions of small-scale fishers (52, 53). It is likely that other undocumented hotspots of bycatch exist in these regions, where data are simply not available.
In this assessment, we provide a synoptic view of existing records of air-breathing megafauna bycatch interactions. However, a detailed map of global fishing effort is needed to fully contextualize these interaction data. Information on the amount and spatial distribution of fishing effort is rarely reported, because fishing activity is typically reported as landed catch because of the relative ease of data collection at landing sites (54). Catch data provide information on the harvest of target species but do not represent fishing effort because of variable catchability in space and time. As with bycatch data, fishing effort mapping has been hindered by a lack of data, but there have been a number of attempts to map fishing effort in large ocean regions (35, 55–57). Even with the inherent imprecision of such mapping exercises and their inability to capture dynamic changes to fisheries, these maps, together with bycatch data, can serve as the foundation for spatially explicit bycatch risk assessments. Maps that provide gross estimates of total fishing effort can be combined with bycatch data to help frame the potential risk that fisheries pose to megafauna in particular fishing regions.
Conclusions
Our analysis shows that air-breathing megafauna bycatch is widespread at a global level and that the bycatch landscape is complex, because bycatch intensity varies substantially within and among gears and regions. Wide-ranging megafauna species are likely to encounter multiple gears and experience cumulative effects from multiple fisheries across the seascape. This assessment also identifies critical data gaps (most notably, the need for more data from small-scale and coastal fisheries, gillnets and trawls, and large fishing regions that are known to support highly developed industrial fisheries as well as many millions of small-scale fishers).
This comprehensive bycatch assessment can be used to improve our understanding of population-level effects of bycatch (13), develop and strengthen existing dynamic management approaches to minimizing bycatch (58–60), and prioritize management and conservation efforts worldwide (23, 61). It is likely that many hotspots of bycatch of marine megafauna remain to be identified, particularly in small-scale fisheries and data-deficient ocean regions. This synoptic approach also supports the development of fisheries management strategies that focus on cumulative impacts to avoid the transfer effect (i.e., the reduction of bycatch of one species or taxon that leads to bycatch of another species) and address both the species- and ecosystem-level effects of megafauna bycatch.
Given the global nature of megafauna bycatch, there is a need for cross-sectoral and coordinated international action to effectively reduce bycatch of this assemblage in high-seas and coastal waters. Although some national and regional fisheries agencies have initiated bycatch reduction measures (62), these measures have not been globally adopted. Reducing bycatch levels in coastal fisheries sectors will require coordination across national boundaries using integrated approaches that link conservation actions with sustaining human livelihoods, incentives, and commitment to protecting the environment (63–67). Management structures that focus on solutions with fisher communities, cooperatives, and councils taking an active role in developing and testing mitigation measures to reduce bycatch have proven effective in many areas (47, 48, 68, 69). Although megafauna bycatch is pervasive and in some regions, intense, the recovery of depleted populations is possible if threats are mediated (70). There is an urgent need for the development and adoption of participatory and adaptive approaches to promote sustainable harvest practices, reduce the bycatch of air-breathing and other marine megafauna through effective mitigation, and ultimately, protect the health and function of marine ecosystems.
Materials and Methods
We compiled a global bycatch database from peer-reviewed publications, agency and technical reports, and symposia proceedings published between 1990 and 2008 (a complete bibliography of the sources in the database is in Data Sources S1). We chose three taxa (seabirds, marine mammals, and sea turtles), because they represent marine megafauna that are not typically retained or commercially valuable (i.e., in contrast to sharks or large finfish). We focused on the three most globally distributed fishing gear and used gear categories recognized by the Food and Agriculture Organization of the United Nations (www.fao.org/fishery/topic/1617/en) (13). Despite the broad nature of these gear categories, which included several different gear types within each gear category (e.g., trawls includes both bottom and midwater trawls), this classification scheme allowed us to draw general conclusions over two decades, hundreds of studies, and multiple spatial scales, while balancing relevant variation and details. The database represents bycatch information from direct observation, termed observer data, as well as interviews with fishers (∼10% of all records). Because much of the bycatch literature exists outside scientific literature databases, we also directly contacted agencies around the world in charge of collecting and collating bycatch information to ensure that our database was comprehensive (Data Sources S1 has a complete bibliography of the sources in the database). The research team systematically evaluated records for errors by species groups. We did not include logbook data, because such data have been found to underrepresent observed bycatch of marine megafauna (6). Even observer data, while providing the most accurate direct measure of bycatch, have been shown to underestimate bycatch, because bycaught individuals may drop out of fishing gear during hauls or go undetected (42). A record in the database represents a unique bycatch report for a particular species and includes records in which zero bycatch was observed. A single reference may contain many records. The database contains a total of 2,270 records across the three taxa and gear types included in this study (Table S1). More information on the database fields and database access is in SI Text.
Bycatch Intensity Scores.
We solicited input from 17 independent scientists who are established experts and leaders in research on seabirds (n = 5), marine mammals (n = 6), or sea turtles (n= 6) with an established familiarity with fisheries bycatch. Our use of expert knowledge followed guidelines established by existing literature in the field (ref. 26 and references therein). Our selection criteria for panelists were independence (to avoid conflict of interest), research expertise, and years of experience (≥5 y) (26). Experts came from government agencies, research institutions, and academia from different countries (Argentina, Brazil, Taiwan, United States, and United Kingdom). To support the expert knowledge process, participants were given a specific elicitation to help them draw on appropriate data and relevant background information (26). Each participant was given three datasheets: one for each gear category—trawl, gillnet, and longline–for the taxonomic group for which she or he had expertise. Each taxa group had at least five different experts, and every record in the database was reviewed and scored by at least three experts. For each bycatch record, experts were asked to evaluate the intensity represented by each bycatch record from high (five) to low (one) within each gear category type. Bycatch intensity scores integrated all of the information for each record (e.g., bycatch per unit effort, species information, gear type, region, any spatial information, and the amount of observed fishing effort from which the bycatch rate was calculated) and generated a common measure across all records. In this way, bycatch intensity reflects not only the recorded bycatch but also, the spatial and species-specific information. For example, record A could have the same bycatch per unit effort as record B, but if the species caught in record A is a common species and the species caught in record B is endangered, the bycatch intensity score for the two records would reflect those differences. A high-intensity bycatch score (i.e., five) may reflect both the reported bycatch rate as well as the status of the population or stock caught, because the experts were given all of the species and spatial information that was provided with the bycatch record. Experts were instructed to score records within but not among gear categories, such that a record with high-intensity bycatch in one gear category (e.g., gillnets) would not be considered equivalent to a record with high-intensity bycatch in another gear category (e.g., trawls). Expert responses were aggregated (averaged) to control for differences among experts (26). Intensity scores were analyzed for differences by taxa, gear, and region using generalized linear models in STATISTICA (Statsoft), and therefore, we could test for effects of categorical and continuous predictor variables assuming a Poisson distribution and log canonical link functions.
Bycatch Maps.
All geospatial analysis was performed and all maps were visualized using ArcGIS 10.0 software (ESRI). Individual bycatch records were assigned an initial location based on geographic information in the source material. In some cases, precise latitude and longitude coordinates were provided. More often, only coarse geographic data, such as ocean region or relation to geographic features, were provided. In these instances, records were assigned a location based on the named geographic features in the reference. To visualize individual records at the global scale, overlapping or highly clustered points were exploded out into 0.5° grids centered on the initial locations.
To represent the precision of the bycatch estimates, we determined the proportional fishing effort to reflect the amount of observed fishing effort on which the bycatch estimate was based. Proportional effort was calculated as the ratio of the observed effort for the record to the summed total amount of fishing effort observed for each gear category and each taxon. These proportional values were divided into three classes to represent the lower 5%, middle 90%, and upper 5% of records. This classification yielded scaled fishing effort for each taxon and gear type. Weighting records based on the amount of effort used to generate a bycatch rate is important, because it has been directly linked to the precision of reported rates (i.e., rates based on relatively little observed effort tend to exhibit substantial error vs. rates based on a large amount of fishing effort) (9, 30). Therefore, records based on more fishing effort (larger symbols) are likely to be more precise than those records based on lower levels of effort (smaller symbols). Ranked bycatch records that did not report observed fishing effort were mapped separately (Fig. S2).
Cumulative Bycatch Intensity.
We evaluated cumulative bycatch intensity across all taxa and gears, both graphically and numerically, to consider the total aggregate bycatch using the bycatch intensity scores. Although records from gear categories were scored independently, the cumulative bycatch calculation serves to identify areas where high-intensity bycatch among gear types overlaps. Graphically, bycatch intensity values were used to generate a raster surface from an inverse weighted distance function to consider cumulative bycatch intensity. The extent of the smoothed convex hull was a 2.5° buffer around all points. This spatial extent formed the polygon groups given in the cumulative surface figure and was used to limit the extent. Interpolated values were displayed using the same color ramp as the other visualization methods (dark blue, 1; red, 5). Numerically, we calculated cumulative bycatch intensity for ocean regions with more than 50 records. Cumulative intensity (IC) was calculated as in Eq. 1 (the mean product of Ri, the bycatch intensity score for each record within a region, and OEi, the scaled observed fishing effort for each record within a region, divided by rc, the total number of data records per region).
Supplementary Material
Acknowledgments
We thank the many researchers who participated as expert panelists for their time and commitment to a time-consuming process. This work was also supported by numerous student research assistants at Duke University and San Diego State University, notably K. James. This work was part of Project Global Bycatch Assessment of Long-Lived Species (GLoBAL), supported by the Gordon and Betty Moore Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A.E. is a guest editor invited by the Editorial Board.
Data deposition: The information reported in this paper has been deposited in the OBIS SeaMAP database (ID 1117).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318960111/-/DCSupplemental.
References
- 1.Lotze HK, Coll M, Dunne JA. Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean ecosystem. Ecosystems. 2011;14:198–222. [Google Scholar]
- 2. Food and Agriculture Organization (2012) The State of World Fisheries and Aquaculture. Available at www.fao.org/docrep/016/i2727e/i2727e00.htm. Accessed March 6, 2014.
- 3.Worm B, et al. Rebuilding global fisheries. Science. 2009;325(5940):578–585. doi: 10.1126/science.1173146. [DOI] [PubMed] [Google Scholar]
- 4.Davies RWD, Cripps SJ, Nickson A, Porter G. Defining and estimating global marine fisheries bycatch. Marine Policy. 2009;33:661–672. [Google Scholar]
- 5.Hall MA. On by-catches. Rev Fish Biol Fish. 1996;6:319–352. [Google Scholar]
- 6.Baum JK, et al. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299(5605):389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- 7.Rivalan P, Barbraud C, Inchausti P, Weimerskirch H. Combined impacts of longline fisheries and climate on the persistence of the Amsterdam Albatross Diomedea amsterdamensis. Ibis (Lond 1859) 2010;152(1):6–18. [Google Scholar]
- 8.Crowder LB, Heppell SS. The decline and rise of a sea turtle: How Kemp’s Ridleys are recovering in the Gulf of Mexico. Solutions. 2011;2:67–73. [Google Scholar]
- 9.Wallace BW, et al. Global patterns of fisheries bycatch of marine turtles: Implications for research and conservation. Conserv Lett. 2010;3:1–12. [Google Scholar]
- 10.Lewison RL, et al. In: Biology of Sea Turtles. Vol 3. Wyneken J, Lohmann K, Musick J, editors. Boca Raton, FL: CRC; 2013. pp. 329–352. [Google Scholar]
- 11.D'agrosa C, Lennert-Cody CE, Vidal O. Vaquita bycatch in Mexico's artisanal gillnet fisheries: Driving a small population to extinction. Conserv Biol. 2000;14(4):1110–1119. [Google Scholar]
- 12.Weir CR, Van Waerebeek K, Jefferson TA, Collins T. West Africa's Atlantic humpback dolphin (Sousa teuszii): Endemic, enigmatic and soon endangered? Afr Zool. 2011;46(1):1–17. [Google Scholar]
- 13.Wallace BW, et al. Impacts of fisheries bycatch on marine turtle populations worldwide: Toward conservation and research priorities. Ecosphere. 2013;4(3):1–12. [Google Scholar]
- 14.Hamer DJ, et al. The endangered Australian sea lion extensively overlaps with and regularly becomes by-catch in demersal shark gill-nets in South Australian shelf waters. Biol Conserv. 2013;157:386–400. [Google Scholar]
- 15.Robertson BC, Chilvers BL. The population decline of the New Zealand sea lion Phocarctos hookeri: A review of possible causes. Mammal Rev. 2011;41(4):253–275. [Google Scholar]
- 16.Bearzi G, Politi E, Agazzi S, Azzellino A. Prey depletion caused by overfishing and the decline of marine megafauna in eastern Ionian Sea coastal waters (central Mediterranean) Biol Conserv. 2006;127:373–382. [Google Scholar]
- 17.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 18.Soykan CU, et al. Why study bycatch? Endanger Species Res. 2008;5:91–102. [Google Scholar]
- 19.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319(5865):948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 20.Watson JW, Epperly SP, Foster D, Shah AK. Fishing methods to reduce sea turtle mortality associated with pelagic longlines. Can J Fish Aquat Sci. 2005;62(5):965–981. [Google Scholar]
- 21.Barcelona SG, Ortiz de Urbina JM, de la Serna JM, Alot E, Macias D. Seabird bycatch in Spanish Mediterranean large pelagic longline fisheries, 2000–2008. Aquat Living Resour. 2010;23(4):363–370. [Google Scholar]
- 22.Forney KA, Koboyashi DR, Johnston DW, Marchetti JA, Marsik MG. What’s the catch? Patterns of cetacean bycatch and depredation in Hawaii-based pelagic longline fisheries. Mar Ecol (Berl) 2011;32(3):380–391. [Google Scholar]
- 23.Croxall JP, et al. Seabird conservation status, threats and priority actions: A global assessment. Bird Conserv Int. 2012;22(1):1–34. [Google Scholar]
- 24.Reeves RR, McClellan K, Werner TB. Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endanger Species Res. 2013;20:71–97. [Google Scholar]
- 25.Read AJ, Drinker P, Northridge S. Bycatch of marine mammals in U.S. and global fisheries. Conserv Biol. 2006;20(1):163–169. doi: 10.1111/j.1523-1739.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- 26.McBride MF, Burgman MA. In: Expert Knowledge and Its Application in Landscape Ecology. Perera AH, Drew CA, Johnson SJ, editors. New York: Springer Science; 2012. pp. 11–38. [Google Scholar]
- 27.Gobbi M, Riservato E, Bragalanti N, Lencioni V. An expert-based approach to invertebrate conservation: Identification of priority areas in central-eastern Alps. J Nat Conserv. 2012;20:274–279. [Google Scholar]
- 28.Iglecia MN, Collazo JA, McKerrow AJ. Use of occupancy models to evaluate expert knowledge-based species-habitat relationships. Avian Conservation & Ecology. 2012;7(2):5. [Google Scholar]
- 29.Martin TG, et al. Eliciting expert knowledge in conservation science. Conserv Biol. 2012;26(1):29–38. doi: 10.1111/j.1523-1739.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- 30.Sims M, Cox TC, Lewison RL. Modeling spatial patterns in fisheries bycatch: Improving bycatch maps to aid fisheries management. Ecol Appl. 2008;18(3):649–661. doi: 10.1890/07-0685.1. [DOI] [PubMed] [Google Scholar]
- 31.Peckham SH, et al. Small-scale fisheries bycatch jeopardizes endangered Pacific loggerhead turtles. PLoS ONE. 2007;2(10):e1041. doi: 10.1371/journal.pone.0001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weimerskirch H, et al. GPS tracking of foraging albatrosses. Science. 2002;295(5558):1259. doi: 10.1126/science.1068034. [DOI] [PubMed] [Google Scholar]
- 33.Block BA, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475(7354):86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- 34.Lewison RL, Nel D, Taylor F, Croxall JP, Rivera K. Thinking big—taking a large-scale approach to seabird bycatch. Mar Ornithol. 2005;33:1–5. [Google Scholar]
- 35.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 36.Tittensor DP, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466(7310):1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 37.Stewart KR, et al. Characterizing fishing effort and spatial extent of coastal fisheries. PLoS ONE. 2010;5(12):e14451. doi: 10.1371/journal.pone.0014451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns DK, Gaston AJ, Huettmann F. Endothermy, ectothermy and the global structure of marine vertebrate communities. Mar Ecol Prog Ser. 2008;356:239–250. [Google Scholar]
- 39.Middleton JF. Wind forced upwelling: The role of the surface mixed layer. J Phys Oceanogr. 2000;30(5):745–763. [Google Scholar]
- 40. Haine OS, Garvey JR (2005) Northern Prawn Fishery Data Summary 2005. Logbook Program, Australian Fisheries Management Authority, Canberra, Australia. Available at www.afma.gov.au/wp-content/uploads/2010/06/NPF-Data-Summary-2005-2.pdf?afba77. Accessed March 6, 2014.
- 41.Croxall JP, Nicol S. Management of Southern Ocean fisheries: Global forces and future sustainability. Antarct Sci. 2004;16(4):569–584. [Google Scholar]
- 42.Gilman E, Brothers N, Kobayashi DR. Principles and approaches to abate seabird by-catch in longline fisheries. Fish and Fisheries. 2005;6(1):35–49. [Google Scholar]
- 43.Sales G, et al. Circle hook effectiveness for the mitigation of sea turtle bycatch and capture of target species in a Brazilian pelagic longline fishery. Aquat Conserv Mar Freshwater Ecosyt. 2010;20(4):428–436. [Google Scholar]
- 44.Cox TM, et al. Comparing effectiveness of experimental and implemented bycatch reduction measures: The ideal and the real. Conserv Biol. 2007;21(5):1155–1164. doi: 10.1111/j.1523-1739.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- 45.Croxall JP, Trathan PN, Murphy EJ. Environmental change and Antarctic seabird populations. Science. 2002;297(5586):1510–1514. doi: 10.1126/science.1071987. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins LK. Profile and influence of the successful fisher-inventor of marine conservation technology. Conservation & Society. 2010;8(1):44–54. [Google Scholar]
- 47.Hall MA, et al. Working with fishers to reduce bycatches. In: Kennelly SJ, editor. Bycatch Reduction in the World’s Fisheries. Berlin: Springer; 2007. pp. 235–286. [Google Scholar]
- 48.Peckham SH, Maldonado Díaz D. In: Sea Turtles of the Eastern Pacific. Seminoff JA, Wallace BP, editors. Tuscon, AZ: Univ of Arizona Press; 2012. pp. 279–301. [Google Scholar]
- 49.Lewison RL, Crowder LB. Putting longline bycatch of sea turtles into perspective. Conserv Biol. 2007;21(1):79–86. doi: 10.1111/j.1523-1739.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- 50.Alfaro-Shigueto J, Mangel JC, Bernedo F. Small-scale fisheries of Peru: A major sink for marine turtles in the Pacific. J Appl Ecol. 2011;48(6):1432–1440. [Google Scholar]
- 51.López-Barrera EA, Longo GO, Monteiro-Filho ELA. Incidental capture of green turtle (Chelonia mydas) in gillnets of small-scale fisheries in the Paranaguá Bay, Southern Brazil. Ocean Coast Manag. 2012;60:11–18. [Google Scholar]
- 52.Walmsley SA, Leslie RW, Sauer WHH. Managing South Africa's trawl bycatch. ICES J Mar Sci. 2006;64:405–412. [Google Scholar]
- 53.Bhathal B, Pauly D. Fishing down marine food webs’ and spatial expansion of coastal fisheries in India, 1950–2000. Fisheries Research. 2008;91(1):26–34. [Google Scholar]
- 54.McClusky SM, Lewison RL. Quantifying fishing effort: A synthesis of current methodsand their applications. Fish and Fisheries. 2008;9:188–200. [Google Scholar]
- 55.Tuck GN, Polacheck T, Bulman C. Spatio-temporal trends of longline fishing effort in the Southern Ocean and implications for seabird bycatch. Biol Conserv. 2003;114(1):1–27. [Google Scholar]
- 56.Lewison RL, Freeman SA, Crowder LB. Quantifying the effects of fisheries on threatened species: The impact of pelagic longlines on loggerhead and leatherback sea turtles. Ecol Lett. 2004;7(3):221–231. [Google Scholar]
- 57.Waugh SM, Filippi DP, Kirby DS, Abraham E, Walker N. Ecological risk assessment for seabird interactions in Western and Central Pacific longline fisheries. Mar Policy. 2012;36(4):933–946. [Google Scholar]
- 58.Howell E, Kobayashi D, Parker D, Balazs G. Turtlewatch: A tool to aid in the bycatch reduction of loggerhead turtles, Caretta caretta, in the Hawaii-based pelagic longline fishery. Endanger Species Res. 2008;5:267–278. [Google Scholar]
- 59.Hobday AJ, Hartog JR, Spillman CM, Alves O. Seasonal forecasting of tuna habitat for dynamic spatial management. Can J Fish Aquat Sci. 2011;68(5):898–911. [Google Scholar]
- 60.Dunn DC, et al. Empirical move-on rules to inform fishing strategies: A New England case study. Fish and Fisheries. 2013 10.1111/faf.12019. [Google Scholar]
- 61.Wallace BP, et al. Global conservation priorities for marine turtles. PLoS ONE. 2011;6(9):e24510. doi: 10.1371/journal.pone.0024510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Small CJ. Regional Fisheries Management Organisations: Their Duties and Performance in Reducing Bycatch of Albatrosses and Other Species. Cambridge, United Kingdom: BirdLife International; 2005. [Google Scholar]
- 63.Ban NC, Alidina HM, Ardon JA. Cumulative impact mapping: Advances, relevance and limitations to marine management and conservation, using Canada’s Pacific waters as a case study. Mar Policy. 2013;34(5):76–886. [Google Scholar]
- 64.Pomeroy RS. Managing overcapacity in small-scale fisheries in Southeast Asia. Mar Policy. 2012;36(2):520–527. [Google Scholar]
- 65.Lewison RL, et al. Ingredients for addressing the challenges of fisheries bycatch. Bull Mar Sci. 2011;87(2):235–250. [Google Scholar]
- 66.Shester GG, Micheli F. Conservation challenges for small-scale fisheries: Bycatch and habitat impacts of traps and gillnets. Biol Conserv. 2011;14(5):1673–1681. [Google Scholar]
- 67.Davis JL, Le B, Coy AE. Building a model of commitment to the natural environment to predict ecological behavior and willingness to sacrifice. J Environ Psychol. 2011;31(3):257–265. [Google Scholar]
- 68.Piovano S, Basciano G, Swimmer Y. Evaluation of a bycatch reduction technology by fishermen: A case study from Sicily. Mar Policy. 2012;36(1):272–277. [Google Scholar]
- 69.Alfaro-Shigueto J, et al. Where small can have a large impact: Structure and characterization of small-scale fisheries in Peru. Fisheries Research. 2010;106(1):8–17. [Google Scholar]
- 70.Salomon AK, et al. Bridging the divide between fisheries and marine conservation science. Bull Mar Sci. 2011;87(2):251–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.