Significance
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disease of the central nervous system (CNS) causing paralysis. The most effective treatments for MS aim to block infiltration of inflammatory cells to the brain. However, severe side effects related to the broad-acting specificity of these treatments exist. AZD8797, a unique inhibitor of the chemokine receptor CX3CR1, provides inhibition of subpopulations of peripheral leukocytes with potential for a beneficial effect: side effect ratio. We provide evidence that blocking this receptor results in reduced paralysis, inflammation, and degeneration in the CNS in a disease model for MS. Furthermore, CX3CR1 expression analysis in the MS brain strengthens the evidence for CX3CR1 as a new target for the treatment of MS.
Keywords: fractalkine receptor, inflammation
Abstract
One hallmark of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) is infiltration of leukocytes into the CNS, where chemokines and their receptors play a major mediatory role. CX3CR1 is a chemokine receptor involved in leukocyte adhesion and migration and hence a mediator of immune defense reactions. The role of CX3CR1 in MS and EAE pathogenesis however remains to be fully assessed. Here, we demonstrate CX3CR1 mRNA expression on inflammatory cells within active plaque areas in MS brain autopsies. To test whether blocking CNS infiltration of peripheral leukocytes expressing CX3CR1 would be a suitable treatment strategy for MS, we developed a selective, high-affinity inhibitor of CX3CR1 (AZD8797). The compound is active outside the CNS and AZD8797 treatment in Dark Agouti rats with myelin oligodendrocyte glycoprotein-induced EAE resulted in reduced paralysis, CNS pathology, and incidence of relapses. The compound is effective when starting treatment before onset, as well as after the acute phase. This treatment strategy is mechanistically similar to, but more restricted than, current very late antigen-4–directed approaches that have significant side effects. We suggest that blocking CX3CR1 on leukocytes outside the CNS could be an alternative approach to treat MS.
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating and degenerative disease of the central nervous system (CNS). It was already discovered in the early 1900s that a similar disease could be induced in different animal species by injection of spinal cord extracts or myelin-derived proteins (1–3). This group of animal models for MS, called experimental autoimmune encephalomyelitis (EAE), has provided an experimental platform for building an extensive understanding of the pathology of MS, as well as discovering strategies for intervention of the disease. A typical feature of the pathogenesis in both MS and EAE is the infiltration of leukocytes from the blood stream into the CNS (3). Leukocyte adhesion and extravasation includes several well defined steps and various adhesion molecules, chemokines and their receptors are important mediators for this process. In line with this, the recently developed therapeutic drugs natalizumab and fingolimod, which broadly target leukocyte migration to the brain, exhibit efficacy in EAE models (4, 5), and they are now established therapies in MS (6).
Natalizumab, blocking the interaction between very late antigen-4 (VLA-4) and CD106 (VCAM-1), is an effective treatment both on clinical endpoints and MRI biomarkers (7). Fingolimod, the first oral drug for relapsing remitting MS (RRMS), acts on S1P receptors preventing lymphocytes from moving out of lymphoid tissue (8). Natalizumab is only approved as a second-line monotherapy in RRMS or in patients with very active disease, because it carries increased risk of developing the often fatal progressive multifocal leukoencephalopathy (7, 9). Treatment with fingolimod is associated with side effects such as signs of immune suppression, including increased frequency of infections (8).
Considering the pronounced presence of inflammatory cells in the brain of MS patients, the significant correlation between inflammation and axonal injury (10) and the efficiency of treatments that broadly block infiltration of immune cells, a similar but more restricted therapeutic approach is appealing. Chemokines are synthesized and released at sites of inflammation, where they act on specific receptors expressed by immune cells to mediate directed cell migration in synergy with adhesion molecules, such as VLA-4, from the blood stream and into the sites of inflammation. CX3CR1 is a unique member of the chemokine receptor family (11) and binds with high affinity to its ligand CX3CL1 [fractalkine (FKN)]. FKN is produced in a membrane bound form but can also be released following proteolytic cleavage, making it important for mediating both adhesion and migration of CX3CR1-expressing cells. In contrast to VLA-4, which is broadly expressed on most leukocytes except neutrophils (7, 12), the expression of CX3CR1 is restricted to subpopulations of monocytes, T lymphocytes, and natural killer (NK) cells (13–16). We have previously demonstrated intense accumulation of CX3CR1-expressing microglia/macrophages within inflammatory foci in the spinal cord of Dark Agouti (DA) rats with EAE induced by myelin oligodendrocyte glycoprotein (MOG) (17) and formed the hypothesis that CX3CR1 would be an attractive therapeutic target for treating MS.
To test the hypothesis, we have developed a selective, high-affinity small-molecule inhibitor of CX3CR1 (AZD8797) (18). This molecule has the potential to be administered as an oral drug in humans. However, because of the decrease in potency to rat CX3CR1 and a modest oral bioavailability in rats (39%), we have chosen continuous s.c. dosing for the proof-of-concept studies in rats. The MOG1-125–induced EAE model in DA rats was used, because it exhibits pathology very similar to MS with infiltration of inflammatory cells, demyelination, and axonal degeneration in the CNS, as well as a relapsing remitting disease course (19, 20). To further mimic the human treatment situation, we have not only treated rats before the onset of paralysis but also initiated treatment during ongoing disease. We present efficacy (reduced paralysis) versus exposure data, analysis of CNS pathology, and measurements of functional inhibition of CX3CR1. These data, in combination with an analysis for CX3CR1 expression within MS brain autopsy samples, clearly demonstrate the potential of CX3CR1 inhibition as an alternative and unique approach for treating MS.
Results
CX3CR1 Is Expressed in Active MS Brain Lesions.
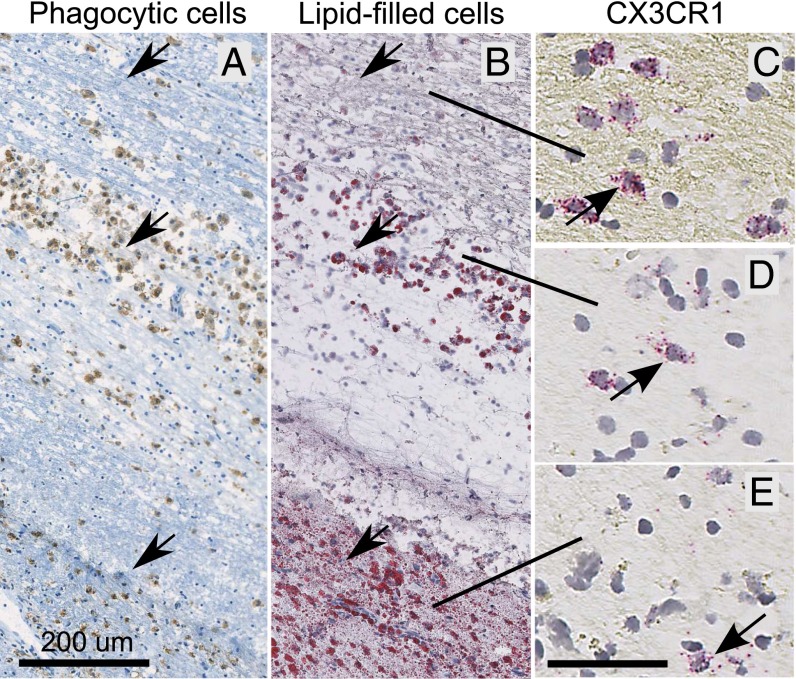
CX3CR1 mRNA-expressing cells were demonstrated within and around inflammatory foci in the MS brain using in situ hybridization histochemistry. Active lesions were identified as dense aggregates of phagocytic and lipid-filled microglia/ macrophages (Fig. 1). Such areas were tightly associated with an abundance of leukocytes and/or resident microglia expressing CX3CR1 mRNA (Fig. 1). This finding supports the hypothesis that CX3CR1 inhibition is a potential approach to treat MS. It is moreover of interest to note that we did not observe any signs of CX3CR1 expression in cells with neuronal like morphology. This is in contrast to what has been claimed by other groups (for example, see ref. 21).
Fig. 1.
CX3CR1 mRNA-expressing cells are present within active lesions of the human MS brain. CX3CR1 expression within a representative, active MS lesion with dense aggregates of macrophages containing myelin breakdown products. (A) Immunostaining for CD68-detecting phagocytic microglia/macrophages. (B) In an adjacent section, increased numbers of microglia/macrophages filled with myelin-derived lipids are readily detected with oil red O staining, hence outlining the active region of the lesion. (D and E) This region also manifested high numbers of CX3CR1-expressing cells, although the highest expression (C) was demonstrated in the adjacent periplaque zone. Harris hematoxylin was used as counterstain.
A Small-Molecule Inhibitor of CX3CR1 (AZD8797) Blocks Development of MOG1–125–Induced EAE in DA Rats.
AZD8797 is a unique CX3CR1 small-molecule inhibitor that has been developed in our laboratory (18) (see Fig. S1). AZD8797 binds selectively with high affinity to human and rat CX3CR1 (Ki of hCX3CR1, 4 nM; Ki of rCX3CR1, 7 nM, respectively). The equilibrium dissociation constant, KB, demonstrates that AZD8797 is a very potent inhibitor for human CX3CR1 (10 nM). The potency is threefold lower for rat CX3CR1 (29 nM) and decreases even further at mouse CX3CR1 (54 nM). AZD8797 appears to be selective versus many other screened receptors including chemokine receptors. In vitro receptor-binding studies showed 246-fold selectivity versus hCCR1 and 187-fold versus hCCR2 and no significant antagonism of the CCR4, CCR5, CCR6, CXCR3, CXCR5, and >65 unrelated receptors (18). The CNS distribution of AZD8797 in orally or s.c. dosed rats is minimal, as judged by direct measurement of the compound in the brain and by studying the 14C-labeled compound using quantitative whole body autoradiography. Hence, we tested the hypothesis that inhibition of CX3CR1-expressing cells in the periphery would block infiltration to the CNS and affect the pathological processes leading to paralysis in MOG-EAE. The studies were performed in DA rats with MOG1-125–induced EAE, which develop a relapsing remitting disease course of paralysis (22). Because of less optimal pharmacokinetic (PK) properties of AZD8797 in the rat for oral treatment [plasma clearance (13 mL⋅min−1⋅kg−1), t1/2 i.v. (1.9 h), t1/2 p.o. (2.7 h), volume of distribution at steady state (1.1 L⋅kg−1), oral bioavailability (39%)], we chose continuous treatment via s.c. osmotic mini pumps as the route of administration to enable sufficient exposure and to avoid periods without exposure between dosings (i.e., “drug holiday”). Furthermore, from our extensive experience with conducting pharmacological treatment studies in EAE, we have learned that the model is very sensitive to stress. Repeated dosings for long periods stresses the animals. Implanted minipumps that are only changed once during the study period cause much less stress to the animals and reduce the risk of study biases.
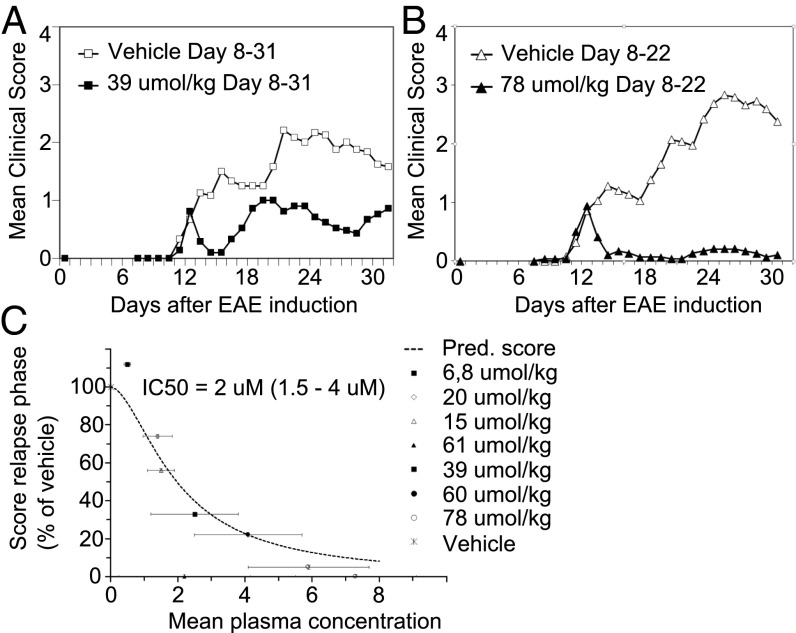
In an initial series of experiments, treatment started at 7–8 d after MOG induction, but before any visible signs of paralysis, and continued for 14–28 d. Treatment at doses between 60–78 μmol/kg (per 24 h) in four studies resulted in an almost complete elimination of clinical symptoms (Table S1). The effect on the relapse phase was reproducible and statistically significant for these doses (P < 0.0008) in all studies (Table S2). Fig. 2 A and B demonstrates the disease course for the different treatment groups in one representative study. In Fig. S2 the distribution of mean clinical score within the different treatment groups is illustrated. Plasma samples were collected twice from each animal for analysis of total compound levels. From this, we calculated an effective plasma concentration for each study. The average in vivo IC50 of the drug was estimated to 2 μM, based on data from all four studies (Fig. 2C). The range of IC50 was estimated to 1.5–4 μM, using population pharmacokinetic/pharmacodynamic modeling and analysis of the mean relapse score vs. steady state plasma concentration. The corresponding free compound IC50 levels were calculated to 4 nM, which is in level with the in vitro Ki of rat CX3CR1 (7 nM).
Fig. 2.
The CX3CR1 inhibitor AZD8797 blocks MOG-EAE in DA rats. AZD8797 was administered through s.c. implanted osmotic minipumps to DA rats at day 8 after EAE induction. (A and B) Daily mean clinical score in groups of rats (n = 25–30) treated with either vehicle or AZD8797 in 39 µmol/kg per day (A) and 78 µmol/kg per day (B). (C) Mean score of EAE expressed as the percentage of vehicle treated rats during relapse phase versus plasma concentration of AZD8797. Data from 4 different EAE studies including 10 different treatment groups, with 25–30 rats per group. The in vivo IC50 was estimated at 2 µM (range, 1.5–4 µM).
To understand the mechanism for AZD8797-mediated inhibition of EAE at a cellular level, histopathology was performed on spinal cord sections from drug-treated EAE rats, using hematoxylin/eosin (H&E) for evaluation of inflammation and Luxol fast blue/periodic acid-Schiff (LFB) for evaluation of demyelination. The 78 μmol/kg dose treatment significantly inhibited both inflammation and demyelination in the spinal cord compared with the vehicle control group (Fig. S3). In another study, we demonstrated that drug treatment at 61 μmol/kg dose and a similar study design also significantly reduced axonal degeneration in the spinal cord (Fig. S4).
AZD8797 Treatment in MOG-EAE Results in Reduced Activation of Microglia and Macrophages in the Spinal Cord.
We next aimed to investigate the correlation between 18-kDa translocator protein (TSPO)-binding sites, as an indicator for the presence of activated microglia and infiltrating macrophages (23) and the density of CX3CR1-expressing cells in active EAE lesions. This would be an independent and complementary measure with potential clinical applicability as a proof-of-mechanism biomarker.
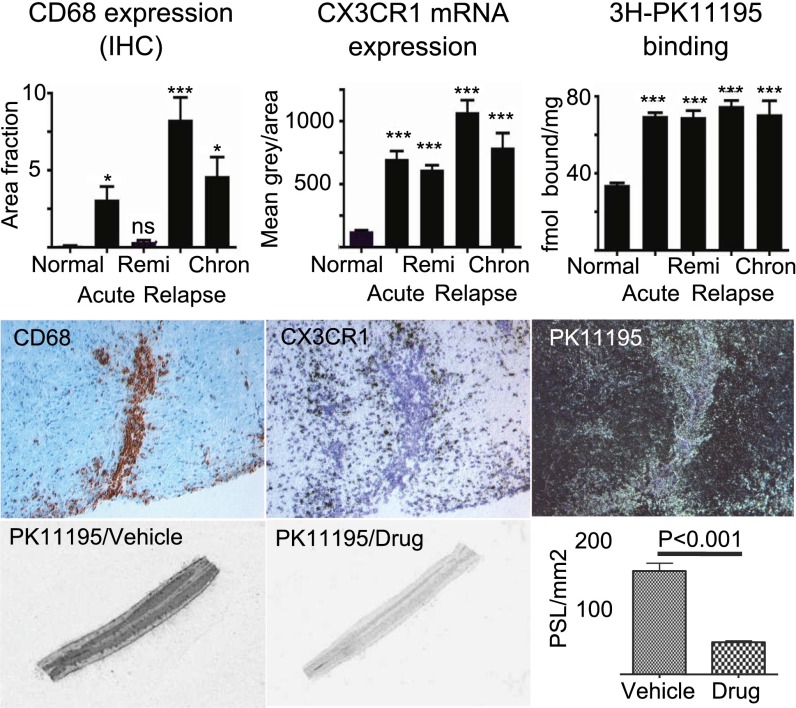
Parallel spinal cord sections, sampled from animals at different phases of the EAE disease course, were subjected to in situ hybridization for CX3CR1 mRNA, high-resolution receptor autoradiography for TSPO and immunohistochemistry (IHC) for CD68. The density of CX3CR1-expressing cells within inflammatory lesions were significantly increased in all phases of EAE compared with normal controls (Fig. 3, Top). The most intense accumulation of CX3CR1-positive cells was detected at the relapse phase of EAE. During remission phase, the animals improved in terms of pathology, as well as clinical score. Moreover, we found that areas with elevated levels of [3H]PK11195 binding to TSPO coincided with regions exhibiting dense aggregates of CD68- and CX3CR1-positive cells. Interestingly, in some plaques surrounding blood vessels, CX3CR1 mRNA was found to be intensively expressed in cells (presumably microglia) in the outer active zone, whereas CD68+ macrophages accumulating within the perivascular space expressed much lower levels of CX3CR1 mRNA. This is in line with our previous data (17), which clearly demonstrated a mean 10-fold lower level of CX3CR1 protein expression in macrophages compared with microglias. [3H]PK11195, on the other hand, labeled both of these cell populations (Fig. 3, Middle). AZD8797 treatment significantly reduced in vitro binding of [3H]PK11195, indicating a direct or indirect effect on microglia/macrophage accumulation and/or activation in MOG-EAE (Fig. 3, Bottom). Importantly, our data suggest that imaging of TSPO expression in the brain with 11C-labeled PK11195 by positron emission tomography may be used as an efficacy marker (proof of mechanism) in clinical studies with a CX3CR1 inhibitor in MS patients.
Fig. 3.
AZD8797 treatment in EAE reduces TSPO binding, a clinically relevant marker for microglia activation. (Top) Quantification of CD68 immunoreactivity, CX3CR1 mRNA (in situ hybridization), and TSPO receptor autoradiography ([3H]PK11195) on tissue sections from the lumbar spinal cord of rats during different phases of EAE; n = 33 rats with EAE (10 acute, 8 remi, 10 relapse, 5 chron) and 9 controls. chron, chronic phase; remi, remission. (Middle) Representative 20× photos of adjacent sections of a perivascular EAE lesion for each of the three markers (CD68, bright field; CX3CR1 bright field; PK11195, dark field). In this plaque, the CD68+ (brown label) cells were most significantly located at a perivascular position, whereas the CX3CR1+ (black silver grain) cells were distributed within the surrounding parenchyma. PK11195 autoradiography (bright silver grains) detected both of these population of cells. (Bottom) Bar graphs illustrates significant treatment effect of AZD8797 at 81.8 µmol/kg per day (from day 10–26 post-EAE induction) on TSPO binding, measured with receptor autoradiography of 1 nM [3H]PK11195. Pictures show representative tissue sections from lumbar spinal cord of AZD8797 or vehicle treated EAE rats (n = 20 rats per group).
AZD8797 Treatment Modulates Disease and Inhibits Development of Relapses in Animals with Established EAE.
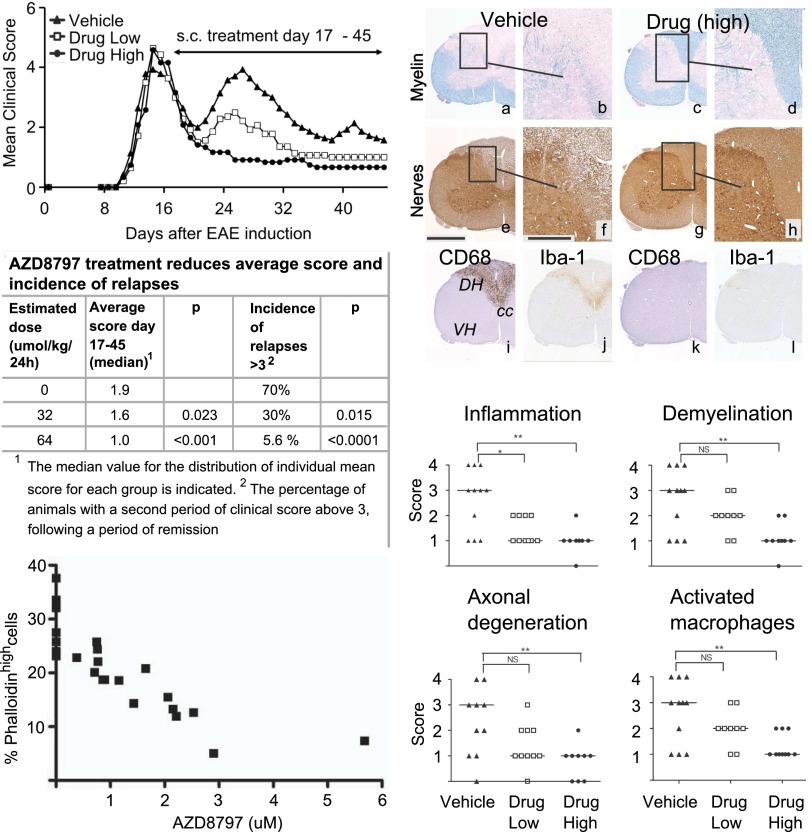
To further examine the therapeutic potential of AZD8797, we treated rats with already established EAE, which would more closely mimic the intervention treatment situation in MS patients. Rats with different levels of disease at day 17 post-EAE induction were evenly distributed into three groups (as outlined in Fig. S5), receiving either vehicle or AZD8797 at 32 or 64 μmol/kg from day 17 until termination (day 45 post-EAE induction). At termination, whole blood was collected from some rats in each treatment group to functionally determine the inhibition of CX3CR1 in blood monocytes by AZD8797, measured by FKN-induced actin polymerization ex vivo. This assay (24, 25) has been modified and applied by us as a pharmacodynamic in vitro assay to assess the relationship between drug concentration and inhibition of CX3CR1 response. As seen in Fig. 4, the drug levels in plasma displayed a clear concentration-dependent inhibition of FKN-induced actin polymerization in the blood monocytes ex vivo with an IC50 value of 1.7 μM. This closely matches the total plasma IC50 value for the EAE studies of 2 μM. The corresponding free fraction of 4 nM in rat plasma agrees with the primary pharmacology in vitro values. Hence, these results demonstrate that AZD8797 inhibits FKN-induced activation of CX3CR1 on blood monocytes in vivo, a cell population of relevance for MS and EAE pathogenesis.
Fig. 4.
AZD8797 treatment in rats with established EAE inhibits development of relapses and all histopathological manifestations of the disease. (Top Left) DA rats with EAE were randomly but evenly distributed into three different groups (n = 20) at day 17 after EAE induction. Groups received AZD8797 or vehicle in s.c. implanted osmotic minipumps (2 × 14 d) at daily doses of either 32 (“drug low”) or 64 µmol/kg (“drug high”). Daily mean clinical score for each group is indicated in the figure. Results in all panels of this figure relates to the same groups of rats. (Bottom Left) Concentration-dependent inhibition of fractalkine-induced actin polymerization in whole blood monocytes from vehicle or AZD8797-treated EAE rats (n = 8). The actin polymerization response was examined by phalloidin-FITC intracellular staining and flow cytometry analysis of CD3−CD4+CD11b/c+ cells. The percentage of cells with induced actin polymerization (phalloidinhigh) versus drug concentration in plasma is presented. The IC50 value was estimated to 1.7 μM. (Middle Left) Statistical comparison between the treatment groups in the EAE study. Therapeutic treatment, starting after the acute phase of EAE, with AZD8797 at 64 µmol/kg resulted in a significant reduction of both clinical score and the incidence of relapses. (Top Right) Representative images of tissue sections from the lumbar spinal cord of rats in the study at termination (Day 45). (a–d) LFB staining of myelin (turquoise), demonstrating normal myelin integrity in the drug-treated rat (c and d), in contrast to the marked loss of myelin present in the spinal cord white matter of the vehicle-treated control (a and b). (e–h) IHC staining using the PGP9.5 antibody specific to ubiquitin–C-terminal hydrolase 1, a protein specifically distributed within neuronal somas, dendrites, and axons. Normal axonal integrity is present in the rat treated with AZD8797 (g and h), whereas the vehicle-treated control displays axonal spheroids in the spinal cord white matter (e and f). (i–l) IHC staining for CD68 (ED-1, phagocytic microglia/activated macrophages) and Iba-1 (microglia/macrophages). Inset rectangles in a, c, e, and g correspond to tissue areas displayed at higher magnification in b, d, f, and h. cc, central canal; DH, dorsal horn; VH, ventral horn. (Middle and Bottom Right) Graphic illustration and statistical analysis of histopathological scoring. Horizontal lines, medians.
AZD8797 treatment in rats with ongoing EAE resulted in dose-dependent reduction of clinical scores of EAE (Fig. 4). Most notably, only 1 out of 20 rats developed a second relapse of paralysis after treatment with the high dose of AZD8797 compared with 16 (out of 20) in the vehicle group, despite all animals showing a prominent acute phase of paralysis before treatment (Fig. 4 and Fig. S6). The high dose significantly reduced all histopathological signs of inflammation, demyelination, and axonal degeneration in the spinal cord, as well as the expression levels of several markers for microglia and macrophage activation (Fig. 4). The lower-dose treatment group had a smaller but evident effect on paralysis and significantly affected some of the spinal cord histopathological scores (inflammation and CX3CR1 expression), as well as the frequency of relapses (Fig. 4 and Fig. S6). Hence, these results clearly demonstrate that blocking infiltration of CX3CR1-expressing leukocytes to the CNS inhibits the progression of EAE, both in terms of CNS pathology and incidence of paralysis.
Discussion
In this study, we have tested the hypothesis that blocking CNS infiltration of CX3CR1-expressing peripheral leukocytes could be an effective treatment strategy for MS. Our results, with the potent and selective CX3CR1 inhibitor AZD8797 in the MOG1-125–induced EAE model in DA rats, support this hypothesis.
Starting treatment with AZD8797 either before or after onset of disease reduced the clinical symptoms and pathological signs of EAE in a concentration-dependent manner. The pharmacological activity of the compound is most likely restricted to the periphery because only very low, subpharmacological levels could be measured in the CNS. We therefore propose that the effect seen with AZD8797 is caused primarily by blocking infiltration of CX3CR1-expressing cells to the CNS from the periphery and, as a consequence, indirectly affecting demyelination, neuronal pathology, microglia activation, and other inflammatory processes in the CNS. The pharmacological effect is likely mediated through direct inhibition of CX3CR1, because AZD8797 has been found selective against different chemokine receptors and unrelated receptors. In addition, peripheral blood monocytes in AZD8797-dosed EAE rats were functionally inhibited to respond to FKN stimulation (Fig. 4), building further confidence to the conclusion that the pharmacological effect is mediated through specific inhibition of peripheral CX3CR1-expressing cells.
The profound reduction of all EAE-related pathology in the CNS, including axonal degeneration, supports the hypothesis that pharmacological inhibition of CX3CR1 is an attractive avenue for treatment of MS. However, our findings stand in stark contrast to the results reported by some other groups (26–28) where CX3CR1 gene-deleted mice were shown to develop enhanced neurodegenerative manifestations in several inflammatory and neurotoxic models. In the case of MOG35–55–induced EAE in these Cx3cr1−/− mice, the more severe disease observed compared with wt littermates was explained by defective accumulation of regulatory NK cells in the CNS (27). Adoptive transfer experiments further demonstrated that the increased EAE severity correlated to the absence of CX3CR1 on peripheral bone marrow-derived cells (28). Hence, in MOG-induced EAE in Cx3cr1−/− mice, the absence of CX3CR1 on peripheral leukocytes led to enhanced migration of myeloid cells into the CNS. In contrast, inhibition of CX3CR1 in the periphery with AZD8797 in MOG-induced EAE in DA rats reduced the migration of leukocytes into the CNS. The mechanistic basis for these divergent results is currently unclear. Studies using gene deletions to evaluate biological functions of proteins are complex in nature, with a potentially confounding impact of constitutive gene deletions during development and influences from back-crossing the gene deletions onto different background strains and/or from environmental factors (such as gut microbial flora). In accordance, no observable effect from the CX3CR1 gene knockout was demonstrated on the EAE pathogenesis in the study by Haskell et al. (29). The different outcomes may also be ascribed to potentially different functional roles of CX3CR1 as a modulator of the EAE disease process in rat and mouse. Unfortunately, the less favorable PK properties of AZD8797 in mice, in combination with dose-limiting solubility, restricted the possibility to achieve sufficient exposure in mice. This prevented us from assessing the effect of AZD8797 treatment in mice. Furthermore, differences between MOG35–55 peptide-induced EAE in C57BL/6 mice and MOG1-125–induced EAE in DA rats (1, 2) may also contribute to the divergent results. The mouse model is more T cell-driven compared with the rat model, and the rat model shows a more relapsing remitting disease course and a more pronounced axonal degeneration [i.e., very common features of the human disease (2, 20)].
Further studies using inhibitors of CX3CR1 with higher brain exposure are warranted to explore possible variant roles of CX3CR1 signaling in brain microglia, as opposed to peripheral immune cells, to fully assess the potential risks of CX3CR1 antagonism, as indicated in the above mentioned gene deletion studies.
Similar to AZD8797, fingolimod has demonstrated efficacy with reduced paralysis and spinal cord pathology in therapeutic treatment of MOG-EAE in DA rats (30, 31). Antibodies directed against the rat VLA-4 were also proven effective in a rat EAE model (5). Fingolimod and natalizumab (the human version of anti-VLA-4) are two of the most effective treatments for MS today. AZD8797 targets a similar mechanism but with a more narrow target cell specificity than fingolimod and natalizumab.
FKN, the ligand for CX3CR1, is constitutively expressed on endothelial and epithelial cells in peripheral tissues and on neurons within the CNS (3). During inflammation in the periphery, membrane-bound FKN is up-regulated and captures circulating CX3CR1-expressing cells, as an important early step in the extravasation of leukocytes to the inflamed tissue (32). This process may not occur in the CNS as vascular endothelial cells in DA rats with MOG-EAE do not express detectable levels of mRNA encoding FKN (32). This highlights the question regarding the mode of action by which the peripherally restricted AZD8797 interferes with infiltration of CX3CR1 cells to the CNS. A potential mechanism may be that CX3CR1 antagonism blocks emigration of leukocytes from lymphoid organs, similar to what has been demonstrated for fingolimod (31). An alternative mechanism is that membrane-bound FKN may be released by proteolytic cleavage from injured neurons and activated astrocytes (32), taken up by vascular endothelial cells and transcytosed in an abluminal-to-luminal direction via the chemokine receptor-like protein DARC (Duffy antigen receptor complex). This would result in functional presentation of FKN by the endothelial cells to blood borne cells and induction of migration toward the gradient. This mechanism has been demonstrated for several chemokines (33), including MCP-1 and IL-8, but has yet to be demonstrated for FKN. Finally, although our analyses strongly argue that AZD8797 remains peripherally restricted after systemic administration, we cannot fully exclude that the compound may penetrate the blood–brain barrier within small discrete brain regions, such as subdivisions of the inflammatory plaques, in pharmacologically relevant quantities.
In conclusion, we have proven that blocking infiltration of CX3CR1-positive leukocytes to the CNS with AZD8797, an inhibitor of CX3CR1, results in reduced paralysis, less CNS pathology, and lower incidence of relapses in DA rats with MOG-induced EAE. This treatment strategy targets a proinflammatory mechanism similar to current anti–VLA-4 therapeutic approaches, but the more restricted pattern of CX3CR1 expression is expected to have less impact on general immune functions. We thus suggest that blocking CX3CR1 on leukocytes outside the CNS could be an alternative and unique treatment approach for MS.
Materials and Methods
Human Brain Tissues.
Fresh frozen human brain tissues from eight patients with MS were analyzed with chromogenic in situ hybridization (QuantiGene ViewRNA; Affymetrix), using probe sets directed to human CX3CR1 (catalog no. VA1-11840-01), human ubiquitin C (catalog no. VA1-10203-01), and dapB (catalog no. VF1-10272-01). Active inflammatory lesions were identified with oil red O staining and IHC detection of CD68. Counterstaining was performed with hematoxylin.
EAE Induction in DA Rats, Scoring, and Treatment.
Female DA rats (Scanbur) were injected intradermal/s.c. at the base of the tail with recombinant rat MOG1-125 (22) in Incomplete Freund’s Adjuvant (Difco). Tail and limb paralysis were manually scored on a nine-grade scale. AZD8797 was formulated in 30–35% (wt/wt) hydroxy-propyl-beta-cyklodextrin and administered s.c. through osmotic minipumps (Alzet). Treatment was blinded to the operator. The plasma concentration of AZD8797 was analyzed twice from each rat. Differences in the average score was analyzed using the Wilcoxon–Mann–Whitney test. Incidence of relapse, defined as the number of animals with clinical signs of EAE above or equal score 3 after a period of remission of 2 or more days, was analyzed by Fisher exact test. A P value of <0.05 was considered statistically significant.
Histopathological Evaluation of EAE.
Perfusion and immersion fixed rat spinal cords were transected in 18–20 serial 3- to 4-mm-long sections, coembedded in one paraffin block, cut at 4–6 µm, and stained with H&E and LFB. IHC was performed using primary antibodies to PGP 9.5, CD68, or Iba-1. The stained tissues were scored semiquantitatively for histopathological lesions, using a three- or four-grade scale. Statistical evaluation was performed with the nonparametric Kruskal–Wallis test, followed by the Mann–Whitney U test, two-tailed distribution. A P value of <0.05 was considered statistically significant.
Evaluation of CX3CR1 mRNA, CD68 Protein Expression, and [3H]PK11195 Binding on Consecutive Rat Spinal Cord Sections.
Rat spinal cords were snap-frozen on dry ice, sectioned, and stored at –70 °C. Actively phagocytizing microglia/macrophages were identified with IHC detection of CD68. CX3CR1 mRNA expressing cells were identified with in situ hybridization histochemistry, using 35S-labeled antisense and sense cRNA probes specific to rat CX3CR1. Counterstaining was performed with hematoxylin. For receptor autoradiographic detection of microglia and macrophages, sections were incubated with 1 nM [3H]PK11195 with or without competing cold ligand. Bound ligand was detected with a FLA-7000 imaging plate reader (Fuji). For high-resolution imaging, slides were dipped in NTB-2 emulsion, exposed for 2–4 wk, and developed. Representative images were captured from the IHC-labeled or emulsion-coated slides by digital microscopy and imported to a computerized image analysis software system for quantitative assessments (Image J for CD68, Multi Gauge for [3H]PK11195 binding and CX3CR1 in situ hybridizations). One-way analysis of variance statistics with Newman–Keuls post hoc test was used to define statistical significance. The analyses were performed on tissue sections sampled from three equally spaced spinal levels per rats.
Actin Polymerization Assay.
Rat blood (in EDTA) was stimulated with 200 nM recombinant human FKN (chemokine domain; Peprotech/Intermedica) for 20 s at room temperature, stopped by fixation in 1% formaldehyde, and stained using primary antibodies (PE-CD11b/c, PE-Cy5-CD4, APC-CD3; all from BD Biosciences). Intracellular staining for actin filaments with phalloidin-FITC (Sigma-Aldrich) was performed in permeabilizing solution after treatment with FACS Lys solution and followed by Cellfix (BD Biosciences). Polymerized actin was recorded and analyzed with a FACSort flow cytometer (BD Biosciences) and CELLQuest software. The percentage of phalloidinhigh-expressing cells among the CD3−CD4+CD11b/c+ monocytes in each FKN-stimulated sample was determined by setting a gate based on the corresponding PBS-stimulated sample.
Supplementary Material
Acknowledgments
Gunilla Ericsson, Lotta Halvarsson, and Daniel Bergström are acknowledged for excellent experimental support with the EAE studies. Anne Petrén and Margareta Berg are acknowledged for excellent experimental in vitro support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316510111/-/DCSupplemental.
References
- 1.Mix E, Meyer-Rienecker H, Hartung HP, Zettl UK. Animal models of multiple sclerosis—potentials and limitations. Prog Neurobiol. 2010;92(3):386–404. doi: 10.1016/j.pneurobio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.’t Hart BA, Gran B, Weissert R. EAE: Imperfect but useful models of multiple sclerosis. Trends Mol Med. 2011;17(3):119–125. doi: 10.1016/j.molmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujino M, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305(1):70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 5.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 6.Barten LJ, Allington DR, Procacci KA, Rivey MP. New approaches in the management of multiple sclerosis. Drug Des Devel Ther. 2010;4:343–366. doi: 10.2147/DDDT.S9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudick R, Polman C, Clifford D, Miller D, Steinman L. Natalizumab: Bench to bedside and beyond. JAMA Neurol. 2013;70(2):172–182. doi: 10.1001/jamaneurol.2013.598. [DOI] [PubMed] [Google Scholar]
- 8.Buck D, Hemmer B. Treatment of multiple sclerosis: Current concepts and future perspectives. J Neurol. 2011;258(10):1747–1762. doi: 10.1007/s00415-011-6101-2. [DOI] [PubMed] [Google Scholar]
- 9.Hellwig K, Gold R. Progressive multifocal leukoencephalopathy and natalizumab. J Neurol. 2011;258(11):1920–1928. doi: 10.1007/s00415-011-6116-8. [DOI] [PubMed] [Google Scholar]
- 10.Frischer JM, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: Why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets. 2010;14(2):207–219. doi: 10.1517/14728220903540265. [DOI] [PubMed] [Google Scholar]
- 12.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 13.Foussat A, et al. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30(1):87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura M, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168(12):6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71(2):173–183. [PubMed] [Google Scholar]
- 16.Ancuta P, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197(12):1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunnemark D, et al. Differential expression of the chemokine receptors CX3CR1 and CCR1 by microglia and macrophages in myelin-oligodendrocyte-glycoprotein-induced experimental autoimmune encephalomyelitis. Brain Pathol. 2003;13(4):617–629. doi: 10.1111/j.1750-3639.2003.tb00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlström S, et al. Substituted 7-amino-5-thio-thiazolo[4,5-d]pyrimidines as potent and selective antagonists of the fractalkine receptor (CX3CR1) J Med Chem. 2013;56(8):3177–3190. doi: 10.1021/jm3012273. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulos D, Pham-Dinh D, Reynolds R. Axon loss is responsible for chronic neurological deficit following inflammatory demyelination in the rat. Exp Neurol. 2006;197(2):373–385. doi: 10.1016/j.expneurol.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Storch MK, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8(4):681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37(4):314–327. [PubMed] [Google Scholar]
- 22.Weissert R, et al. MHC haplotype-dependent regulation of MOG-induced EAE in rats. J Clin Invest. 1998;102(6):1265–1273. doi: 10.1172/JCI3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327(1-2):1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dichmann S, et al. Fractalkine induces chemotaxis and actin polymerization in human dendritic cells. Inflamm Res. 2001;50(11):529–533. doi: 10.1007/PL00000230. [DOI] [PubMed] [Google Scholar]
- 25.Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA. 1979;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 27.Huang D, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20(7):896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 28.Garcia JA, et al. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J Immunol. 2013;191(3):1063–1072. doi: 10.4049/jimmunol.1300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haskell CA, et al. Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection. J Clin Invest. 2001;108(5):679–688. doi: 10.1172/JCI12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balatoni B, et al. FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain Res Bull. 2007;74(5):307–316. doi: 10.1016/j.brainresbull.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos D, et al. FTY720 ameliorates MOG-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses. J Neurosci Res. 2010;88(2):346–359. doi: 10.1002/jnr.22196. [DOI] [PubMed] [Google Scholar]
- 32.Sunnemark D, et al. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: Kinetics and cellular origin. J Neuroinflammation. 2005;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruenster M, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10(1):101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.