Significance
The accompanying paper describes the precise, in situ replacement of six megabases of mouse immune genes with the corresponding human immune genes. This manuscript shows that this genomic engineering feat resulted in a unique kind of “HumAb” mouse. Dubbed VelocImmune, these mice efficiently generate antibodies that can be rapidly reformatted into therapeutics. VelocImmune mice have proven to be extraordinarily efficient and productive, generating over a dozen therapeutic candidates that have already progressed into human clinical trials for a variety of important diseases.
Keywords: antibody generation, immunoglobulin loci
Abstract
Mice genetically engineered to be humanized for their Ig genes allow for human antibody responses within a mouse background (HumAb mice), providing a valuable platform for the generation of fully human therapeutic antibodies. Unfortunately, existing HumAb mice do not have fully functional immune systems, perhaps because of the manner in which their genetic humanization was carried out. Heretofore, HumAb mice have been generated by disrupting the endogenous mouse Ig genes and simultaneously introducing human Ig transgenes at a different and random location; KO-plus-transgenic humanization. As we describe in the companion paper, we attempted to make mice that more efficiently use human variable region segments in their humoral responses by precisely replacing 6 Mb of mouse Ig heavy and kappa light variable region germ-line gene segments with their human counterparts while leaving the mouse constant regions intact, using a unique in situ humanization approach. We reasoned the introduced human variable region gene segments would function indistinguishably in their new genetic location, whereas the retained mouse constant regions would allow for optimal interactions and selection of the resulting antibodies within the mouse environment. We show that these mice, termed VelocImmune mice because they were generated using VelociGene technology, efficiently produce human:mouse hybrid antibodies (that are rapidly convertible to fully human antibodies) and have fully functional humoral immune systems indistinguishable from those of WT mice. The efficiency of the VelocImmune approach is confirmed by the rapid progression of 10 different fully human antibodies into human clinical trials.
Monoclonal antibodies (mAbs) are a rapidly growing class of therapeutics that combine high binding affinities and specificities with long in vivo half-lives. A large number of mAbs have been approved for therapeutic use or are in development (1–6). Early therapeutic mAbs were derived from mouse sources, retained mouse sequences, and were thus immunogenic when used in human patients, limiting the ability to dose repeatedly. The use of humanized and/or fully human antibodies avoided immunogenicity problems and allowed long-term treatment of chronic diseases. Thus, a variety of systems were developed to create humanized or fully human therapeutic antibodies (7–18). One popular approach involves the in vitro isolation of antigen-specific antibodies using display strategies involving human antibody fragments expressed on the surface of phage, bacteria, or yeast (12). Although these synthetic approaches can be quite powerful and can rapidly generate leads, they potentially result in increased immunogenicity in vivo, and the initial display-derived antibody fragments can subsequently require extensive post hoc protein reengineering efforts when reformatted into conventional antibody formats to overcome issues such as insolubility, aggregation, and proteolysis (1, 12, 19). Natural selection of antibodies in vivo within mammalian systems tends to optimize desirable biochemical and pharmacokinetic properties, avoiding the need for extensive post hoc reengineering. Thus, as first proposed based on the finding that human Ig genes efficiently rearrange when introduced into mouse pre-B cells (20), another popular approach for generating human therapeutic mAbs was developed using transgenic mice genetically engineered to produce fully human antibodies (15–18). These so-called HumAb mice were engineered using a “KO-plus-transgenic” strategy in which the endogenous murine Ig genes were disrupted to eliminate the endogenous mouse immune response, whereas transgenic introduction of human Ig loci at different random genetic loci drove production of fully human antibodies.
Although HumAb mice generated using this KO-plus-transgenic approach represented a transforming advance in the field, they suffered, however, from partial immune deficiencies compared with WT mice, limiting their ability to produce robust Ab responses to some antigens (21–23). The immune deficiencies of these first-generation HumAb mice may be due to the manner in which they were genetically engineered. First, the genomic context of the randomly inserted human transgenes may contribute to their inefficient functionality, as they may lack extended locus control regions such as the 3′ enhancers (24) and regulatory region (25) of the Ig heavy (IgH) locus, which have been shown to play critical roles in Ig expression and class switching or even alter the 3D location of the Ig genes within B-cell nuclei (26, 27) and thus perturb function in unanticipated ways. In addition, the immune deficiencies of the first-generation HumAb mice may be partly explained by their use of human constant regions. Immunoglobulins interact with other components of the B-cell receptor (BCR) signaling complex via their constant regions, and such interactions are required for appropriate signaling required for antigen-independent B-cell development in bone marrow, as well as antigen-dependent natural selection processes in the periphery (28–36). Others previously noted that interactions between constant regions and BCR coreceptors do not operate efficiently across species (29). Thus, we reasoned that the immune deficiencies in first-generation HumAb mice may in part be due to inefficient interspecies protein-protein interactions between human constant regions and the mouse coreceptors. Finally, the secreted human immunoglobulins produced in the HumAb mice may also interact inefficiently with various mouse Fc receptors, further adversely affecting the humoral immune response (37, 38).
As described in the companion article (39), we attempted to exploit the advantages and overcome the limitations of the first-generation HumAb approaches by precisely replacing the entire mouse germ-line variable region gene repertoire with the equivalent human germ-line variable sequences in situ, while maintaining all mouse constant regions and all known gene expression control elements within the natural mouse genomic location. We reasoned that the introduced human Ig variable gene segments would rearrange normally, be linked to mouse constant regions, and furthermore be expressed from the endogenous mouse Ig loci at physiologically appropriate levels. Because these “reverse chimeric” antibody molecules would bear human antigen-binding variable domains fused to mouse constant domains, we presumed they would interact in a species appropriate way with mouse BCR coreceptors and mouse Fc receptors, resulting in a fully functional immune system. We refer to these humanized Ig variable domain mice as VelocImmune mice because they were generated using VelociGene technology (40).
In this paper, we show that VelocImmune mice have a humoral immune system indistinguishable from that of WT mice, with normal cell populations at all stages of B-cell development and normal lymphoid organ structures. Sequences of antibodies derived from the humanized Ig loci exhibit normal variable segment rearrangement, somatic hypermutation, and class switching. Immunizations of VelocImmune mice generate robust humoral responses from which a large diversity of monoclonal antibodies can be isolated. Thus, VelocImmune mice are a unique platform for the efficient production of fully human antibodies, several of which have already entered clinical development.
Results
Humanization of the Mouse Germ-Line Ig Heavy Chain and Kappa Light Chain Variable Regions.
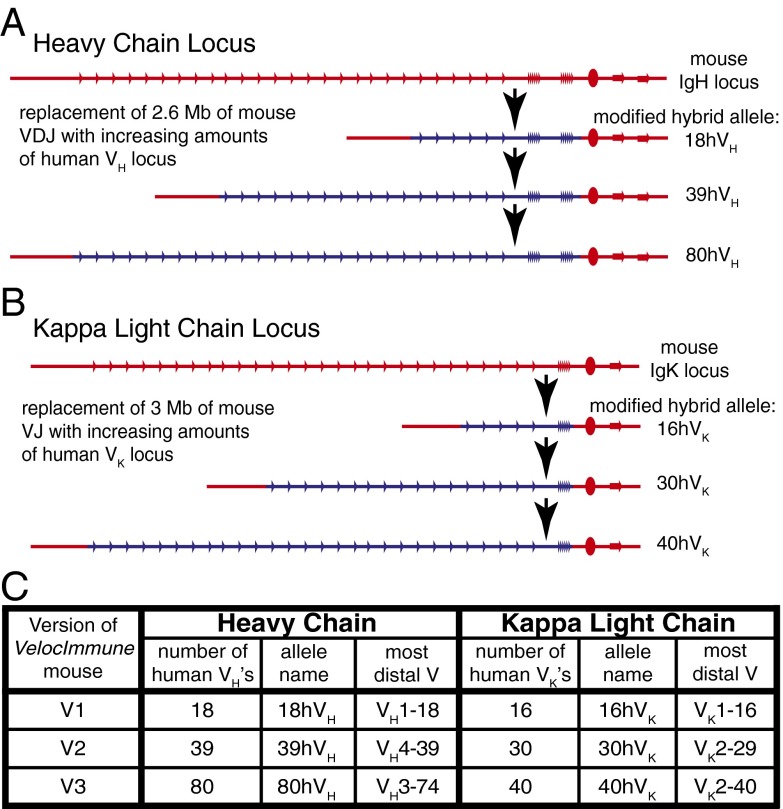
As described in the companion paper (39), the human IgH and kappa light chain (IgK) variable sequences were introduced via stepwise insertion of 13 large compound bacterial artificial chromosome (BAC) targeting vectors (LC-BACvecs) bearing overlapping fragments of the human germ-line variable loci into mouse ES cells. At several points, ES cells bearing a portion of the human IgH or IgK variable repertoire were microinjected and the resulting mice bred to create multiple versions of VelocImmune mice with progressively larger fractions of the human germ-line Ig repertoires (Fig. 1). Because the genomic regions encoding the mouse VH, DH, and JH segments, and VK and JK segments, have been completely replaced, VelocImmune mice generate only antibodies with human variable regions linked to mouse constant regions. The mouse Ig lambda loci remain intact in all versions of the VelocImmune mice described here and serve as a comparator for efficiency of expression of the various VelocImmune kappa loci (see below).
Fig. 1.
Sequential modifications leading to three versions of VelocImmune mice. Schematic representation (not to scale) of sequential modifications of the mouse IgH (A) and IgK (B) chain loci with increasing amounts human variable sequences. Double homozygous mice were made from three different stages of heavy and light chain humanization (C).
Mature B-Cell Populations.
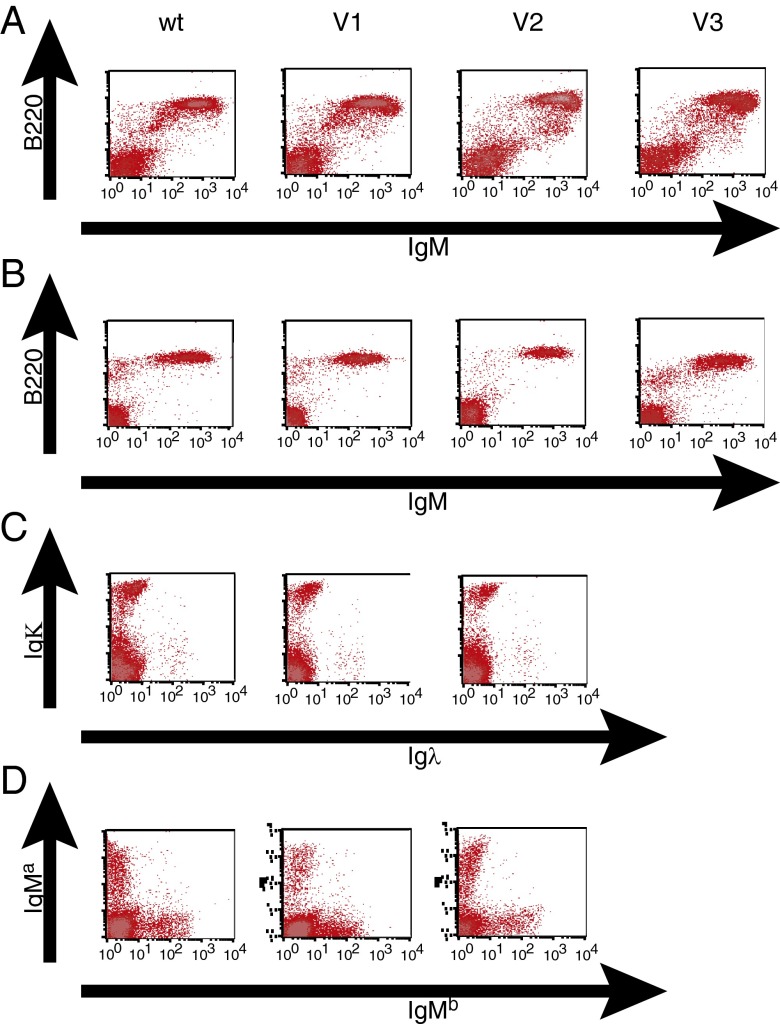
We first evaluated the mature B-cell populations in the three different double homozygous versions (Fig. 1C) of VelocImmune mice. Lymphocytes isolated from spleen or lymph node of homozygous VelocImmune mice were stained for surface expression of the markers B220 and IgM and analyzed using flow cytometry (Fig. 2 A and B). The sizes of the B220+ IgM+ mature B-cell populations in all versions of VelocImmune mice were virtually identical to those of WT mice, regardless of the number of VH segments they possess. In addition, mice containing homozygous hybrid humanized IgH loci, even those with only three Vh segments, but normal mouse IgK loci or mice containing homozygous hybrid humanized IgK loci with normal mouse IgH loci also had normal numbers of B220+ IgM+ cells in their peripheral compartments. The mice with only three human Vh segments did show a defect in pro-B to pre-B transition in the bone marrow, but no such deficit was detected in VelocImmune 1 and subsequent strains containing 18 or more human Vh gene segments. Taken together, these results indicate that chimeric loci with human variable segments and mouse constant regions can fully populate the mature B-cell compartment. These data also suggest that the number of variable segments at either the IgH or IgK loci, and thus the theoretical diversity of the antibody repertoire, does not correlate with the ability to generate WT populations of mature B cells. In contrast, mice with randomly integrated fully human Ig transgenes and inactivated mouse Ig loci had reduced numbers of B cells in these compartments, with the severity of the deficit depending on the number of V regions included in the transgene (41). This finding was the first indication that the “in situ genetic humanization” strategy used for generating VelocImmune mice seemed to result in a fundamentally different functional outcome than previous KO-plus-transgenic approaches.
Fig. 2.
B-cell populations in WT and VelocImmune mice. Cells from spleen (A, C, and D) or inguinal lymph node (B) of WT or VelocImmune 1 (V1), 2 (V2), or 3 (V3) mice (column headings) were stained for surface IgM expressing B cells (A and B), surface Ig containing either kappa or lambda light chains (C), or surface IgM of specific allotypes (D); populations are separated by FACS.
Allelic Exclusion and Locus Choice.
The mechanisms for establishing and maintaining allelic exclusion, which causes individual B cells to produce only one heavy and one light chain, have not yet been fully elucidated. Therefore, we examined mice heterozygous for different versions of humanized IgH loci for allelic exclusion. The humanization of the Ig loci was carried out in a 129S6:C57BL/6N F1 ES line [F1H4 (40)]. The human IgH germ-line variable sequences are targeted to the 129S6 allele, carrying the IgMa haplotype, whereas the unmodified mouse C576BL/6N allele bears the IgMb haplotype. The allelic forms of IgM can be distinguished by flow cytometry using specific antibodies.
B cells found in mice heterozygous for each version of the humanized IgH locus only express a single allele (Fig. 2D), either the humanized IgMa allele or the WT IgMb allele. Therefore, the mechanisms involved in allelic exclusion are intact in VelocImmune mice. In addition, the fraction of B cells positive for IgMa expressed from the humanized IgH allele is roughly proportional to the number of VH segments inserted. The humanized Ig locus is expressed in ∼30% of the B cells in V1 (VelocImmune 1: 18 human VH segments, 16 human Vk segments) heterozygotes and 50% of the B cells in V2 (39 human VH, 30 human Vk) and V3 (80 human VH, 40 human Vk) heterozygotes. Interestingly, the ratio of cells expressing the humanized vs. WT mouse allele (0.5 for V1 and 0.9 for V2) is greater than the ratio of the number of variable segments contained in the humanized vs. WT loci (0.2 for V1 and 0.4 for V2), implying that the probability of allele choice is intermediate between a random choice of one or the other chromosome and a random choice of any particular V segment RSS. This finding may mean that there is a fraction of B cells, but not all, in which one allele becomes accessible for recombination, completes the process, and shuts down recombination before the other allele becomes accessible. In addition, the even distribution of cells that have sIgM derived from either the hybrid humanized IgH locus or the WT mouse IgH locus is strong evidence that the hybrid locus is operating at a completely normal level. In contrast, randomly integrated human Ig transgenes compete poorly with WT mouse Ig loci (15, 42, 43), further supporting the notion that the functional outcome using the VelocImmune approach is much different from that obtained using prior KO-plus-transgenic approaches.
Polymorphisms of the kappa constant regions are not available in 129S6 or C57BL/6N to examine allelic exclusion. However, VelocImmune mice all possess WT mouse lambda light chain loci; therefore, it is possible to observe whether rearrangement and expression of humanized IgK loci can prevent mouse lambda expression. The ratio of the number of cells expressing humanized IgK relative to the number of cells expressing the mouse lambda chain was relatively unchanged in VelocImmune mice compared with WT mice, regardless of the number of human VK segments inserted at the kappa locus (Fig. 2C). In addition, there is no increase in the small number of double positive (kappa plus lambda) cells, indicating that productive recombination at the hybrid kappa loci results in appropriate suppression of recombination of the mouse lambda loci. In contrast, mice containing randomly integrated IgK transgenes with inactivated mouse kappa loci but WT mouse lambda loci show dramatically increased lambda/kappa ratios (21), implying that the introduced IgK transgenes do not function well in these previous-generation HumAb mice and once again highlighting the very different functional outcome obtained using the VelocImmune approach compared with prior KO-plus-transgenic approaches.
B-Cell Development.
Although the mature B-cell populations in VelocImmune mice resemble those of WT mice, it is possible that defects in early B-cell differentiation are compensated for by the expansion of mature B-cell populations. We therefore examined various stages of B-cell differentiation by flow cytometry. Early B-cell development occurs in the bone marrow, with different stages characterized by changes in types and amounts of cell surface marker expression correlating to molecular changes occurring at the Ig loci. The pro-B to pre-B-cell transition requires the successful rearrangement and expression of functional IgH protein, whereas movement from the pre-B to mature B stage is governed by the correct rearrangement and expression of a kappa or lambda light chain. Inefficiencies in transitioning between stages of B-cell differentiation would be reflected in changes in the relative populations of B cells at a given stage. We examined these populations by flow cytometry using combinations of antibodies to cell surface markers (Table 1).
Table 1.
Proportion of sizes of cell populations in VelocImmune mice compared with WT littermates
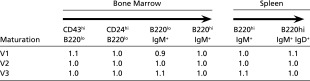 |
The ratio of the fraction of cells in each B-cell lineage population, defined by FACS using the indicated markers, in VelocImmune compared with WT mice.
No major defects were observed in B-cell differentiation in any of the VelocImmune mice. The introduction of at least 18 human IgH variable segments does not appear to affect either the pro-B to pre-B transition, nor do human IgK variables affect the pre-B to B transition. These data also indicate that reverse chimeric Ig molecules possessing human variables and mouse constants are able to function normally in the context of B-cell signaling and coreceptor molecules leading to appropriate B-cell differentiation. In contrast, the balance between the different populations during B-cell differentiation are perturbed to varying extents in mice containing randomly integrated Ig transgenes and inactivated IgH or IgK loci (41).
Variable Repertoire Analysis.
B cells from nonimmunized WT and V3 mice were separated by flow cytometry based on surface expression of B220 and IgM or IgG. The B220+ IgM+ or sIgG+ cells were pooled, and VH and VK sequences were obtained following RT-PCR amplification and cloning. The sequence results were analyzed using V-Quest (44) to identify human VH, DH, JH, and VK, JK segment use. Nearly all of the functional human VH and VK and all of the human DH, JH, and JK segments are used (Table S1), indicating that rearrangement of the human loci occurs efficiently in mice and that human VH domains can couple with mouse surrogate light chain. Of the functional variable segments described but not detected in the VelocImmune mice, several have been previously noted to possess defective recombination signal sequences and therefore would not be expected to be expressed (45). Although the human variable segment 7–81 has been identified in the analysis of the human IgH genomic locus sequences (46), it is not present in the VelocImmune mice as confirmed by resequencing of the entire V3 mouse genome.
Somatic Hypermutation.
Additional diversity is added to the variable regions of rearranged Ig genes during the germinal center reaction by a process called somatic hypermutation. B cells expressing somatically mutated variables compete with other B cells for access to antigen presented by the follicular dendritic cells. Those B cells with higher affinity for the antigen will further expand and undergo class switching before exiting to the periphery (47). Therefore, B cells expressing switched isotypes typically have encountered antigen and undergone germinal center reactions and will have increased numbers of mutations relative to naïve B cells. Thus, variable sequences from predominantly naïve sIgM+ B cells would be expected to have relatively fewer mutations than variable sequences from sIgG+ B cells that have been antigen selected.
Sequences from random VH or VK clones from sIgM+ or sIgG+ B cells from nonimmunized VelocImmune mice or sIgG+ B cells from immunized mice were compared with their germ-line variable segments, and changes relative to the germ-line sequence were annotated. The resulting nucleotide sequences were translated in silico, and mutations leading to amino acid changes were also annotated. The data were collated from all of the variables, and the percent change at a given position was calculated (Fig. S1). The human heavy chain variable regions derived from sIgG+ B cells from nonimmunized VelocImmune mice exhibit many more nucleotides relative to sIgM+ B cells from the same splenocyte pools, and heavy chain variable regions derived from immunized mice exhibit even more changes. The number of changes is increased in the complementarity-determining regions (CDRs) relative to the framework regions, indicating antigen selection. The corresponding amino acid sequences from the human heavy chain variables regions also exhibit significantly higher numbers of mutations in IgG vs. IgM and even more in immunized IgG. These mutations again appear to be more frequent in the CDRs compared with the framework sequences, suggesting that the antibodies were antigen selected in vivo. A similar increase in the number of nucleotide and amino acid mutations is seen in the kappa variable sequences derived from IgG+ B cells from immunized mice.
The CDR3 of the IgH variable is created by recombination of the VH, DH, and JH segments and generates a significant amount of repertoire diversity. During the recombination process, there are both nucleotide deletions via exonuclease activity and non–template-encoded additions via terminal deoxynucleotidyl transferase (TdT). Non–template-encoded nucleotide additions (N-additions) are observed at both the VH-DH and DH-JH joint in antibodies from VelocImmune mice (Table S2), indicating proper function of TdT with the human segments. The end points of the VH, DH, and JH segments relative to their germ-line counterparts indicate that exonuclease activity has also occurred. Unlike the IgH locus, the human IgK variable rearrangements exhibit little or no TdT additions at CDR3, which is formed by the recombination of the VK and JK segments. This finding is not unexpected because the mouse TdT gene is not expressed during light chain rearrangements at the pre-B- to B-cell transition. The diversity observed at the human kappa variable region CDR3 is introduced predominantly through exonuclease activity during the recombination event.
Lymphoid Structures and Serum Isotypes.
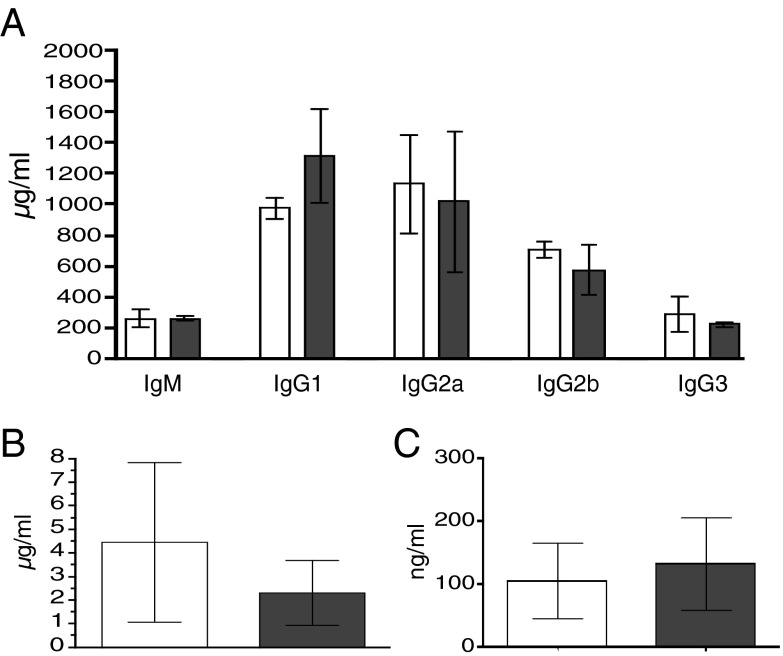
The structure and function of the lymphoid tissues are in part dependent on the proper development of hematopoietic cells. A defect in B-cell development or function may be exhibited as an alteration in the structure of the lymphoid tissues. Therefore, we examined the gross structures of the spleen, inguinal lymph nodes, and Peyer’s patches by light microscopy for any obvious defects. There is no significant difference in appearance of secondary lymphoid organs between the WT and VelocImmune mice (Fig. S2). The levels of various Ig isotypes in serum were analyzed using Luminex technology. The level of expression of each isotype is similar in WT and VelocImmune mice (Fig. 3), suggesting that humanization of the variable segments had no effect on class switching or Ig expression and secretion and therefore maintains all of the murine sequences necessary for these functions.
Fig. 3.
Serum Ig levels. Levels of serum Ig in WT (white bars) or VelocImmune mice (gray bars) for IgM and IgG isotypes (A), as well as IgA (B) and IgE (C).
Immunization and Antibody Production.
To examine the humoral response to foreign antigen challenge in VelocImmune mice, we immunized a cohort of seven VelocImmune mice and five WT littermates with the extracellular domain of the human IL6 receptor (IL6R). Both sets of mice mounted a strong Ab response toward the IL6R with similar titer ranges (Fig. S3A) and IgG isotypes (Fig. S3B), suggesting that humoral response and class switching is similar in mice of each type. Antibodies derived from VelocImmune mice exhibit a wide range of affinity in binding to antigen in solution (Fig. S4A) with a similar number of high-affinity IL6-blocking antibodies derived from VelocImmune mice as their WT littermates (Fig. S4B).
One of these high-affinity IL6R blocking antibodies, after exchange with the human constant domain, is currently undergoing clinical testing in humans, as are more than 10 other VelocImmune-derived antibodies including ones that block PCSK9 (for LDL cholesterol reduction), IL4Rα (for atopic dermatitis and allergic asthma), angiopoietin 2 (for oncology and antiangiogenesis indications), Dll4 (for oncology), and myostatin. The robust nature of VelocImmune mice has allowed antibodies to each of these targets to be brought into clinical development very rapidly—in as little as 18 mo—which are time frames that significantly surpass industry standards.
Discussion
As discussed in the companion paper (39), megabase engineering empowered by VelociGene and represented in the VelocImmune mouse significantly increases the size and precision of which the mouse genome can be manipulated. New projects in which megabases of genomic sequences can be precisely introduced into the mouse genome can now be undertaken for the first time. Although mice with randomly integrated human Ig transgenes (hIgTGx mice) have produced several high-affinity fully human monoclonal therapeutics with great promise for treating important diseases (1, 16, 48, 49), the increase in efficiency in generation of fully human antibodies in VelocImmune mice is likely to dramatically increase the diversity of fully human antibodies that can feasibly be obtained after immunization with any particular antigen. VelocImmune loci with limited variable repertoires exhibit completely normal levels of mature and immature B cells, unlike hIgTGx mice containing limited variable repertoires, which exhibit significantly reduced B-cell populations at various stages of differentiation. Increasing the numbers of V segments in hIgTGx mice reduced the deficits in B-cell development, although even the hIgTGx mice with the most extensive variable repertoires had 40% fewer mature B cells than WT mice (21, 22). Although direct comparison of actual antibody diversity has not been possible, it is likely that the maintenance of normal B-cell populations that we see in VelocImmune mice containing limited V gene repertoires, compared with the dramatically reduced B-cell numbers reported for hIgTGx mice, indicates that an equally dramatic improvement in ultimate diversity occurs in VelocImmune mice with complete V gene repertoires. The development of B1 and marginal zone B cells using human variable repertoires should be possible in these mice.
We identified three reasons why direct replacement of the mouse Ig variable regions may result in more efficient antibody generation than random integration of human Ig transgenes. (i) The chromosomal context may be important for optimal regulation of Ig transcription and recombination. The VelocImmune IgH locus maintains the two mouse IgH enhancers, the intronic enhancer (50) and the 3′ enhancer (25), in their native location. In contrast, hIgTGx IgH transloci rely on human versions of these elements (16, 23) or a combination of human and rodent elements (22) that may not contain the entire 3′ enhancer sequence. Any potential regulatory elements at the 5′ end of the IgH locus are also maintained in the VelocImmune IgH locus and would be missing from hIgTGx loci. In addition, the broader chromosomal context may also affect the efficiency of recombination or transcription. During rearrangement, Ig loci undergo contraction/decontraction, chromatin remodeling, and repositioning within the nucleus (27, 51, 52), which results in heavy and light chain loci becoming spatially linked (26, 52). It seems likely that the chromosomal context and location of the Ig loci have evolved to maximize the efficiency of these chromosomal gymnastics and that randomly integrated Ig transgenes might not operate as efficiently. (ii) Retention of mouse heavy chain constant domains may provide for the formation of more efficient BCRs. Each of the eight mouse constant chain genes (IgM, IgD, IgG3, IgG1, IgG2a, IgG2b, IgE, and IgA) produces a splice variant that encodes a transmembrane version (sIg) that physically associates with the signaling components, Igα and Igβ, to form a BCR (28). Numerous costimulatory and coinhibitory receptors on B cells also modulate the activity of the BCR. These coreceptors are thought to mediate their effects primarily through interaction with Igα and Igβ, but direct interaction between coreceptors and sIg has not been ruled out. (iii) Retention of mouse constant domains on secreted antibodies may allow them to interact more faithfully with the half dozen mouse Fc receptors (FcRs). Antibody:FcR interactions are thought to be involved in the processes by which the B cells expressing the best antibodies are selected in the competitive environment of the germinal center (53–55).
The immediate utility of VelocImmune mice is evident by the more than 10 different fully human antibody therapeutics that have already been produced and entered into clinical development in a short period. In addition, these mice are perhaps the most valuable ever engineered, already having brought in more than $2 billion in committed licensing fees and collaboration revenues.
Materials and Methods
Animal Husbandry.
All mouse studies were overseen and approved by Regeneron’s Institutional Animal Care and Use Committee (IACUC).
Flow Cytometry of Bone Marrow, Spleen, and Thymus.
Flow cytometric analysis was carried out using standard methods and commercially available reagents (SI Materials and Methods).
Sequence Analysis of Human Variable Region and Junctional Diversity.
Variable region use and junctional diversity were assessed by sequencing cDNAs derived from splenocyte or hybridoma RNA (SI Materials and Methods).
Immunizaton and Hybridoma Development.
Seven VelocImmune mice were immunized with the extracellular domain of the IL6R. Antigen-specific antibody titers were determined, hybridomas were developed from the spleens of two mice, and the affinities of antibodies produced from positive hybridomas were determined using an ELISA-based solution competition assay (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Joan Windsor for immunizing mice, David Frendewey for help genotyping mice, and Neil Stahl and Fred Alt for advice.
Footnotes
Conflict of interest statement: Several authors are employed by Regeneron Pharmaceuticals, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324022111/-/DCSupplemental.
References
- 1.Lonberg N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol. 2008;20(4):450–459. doi: 10.1016/j.coi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10(5):301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 4.Leavy O. Therapeutic antibodies: Past, present and future. Nat Rev Immunol. 2010;10(5):297. doi: 10.1038/nri2763. [DOI] [PubMed] [Google Scholar]
- 5.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10(5):345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 6.Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol. 2007;25(10):1134–1143. doi: 10.1038/nbt1337. [DOI] [PubMed] [Google Scholar]
- 7.Traggiai E, et al. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrebaeck CA, Danielsson L, Möller SA. Human monoclonal antibodies produced by primary in vitro immunization of peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1988;85(11):3995–3999. doi: 10.1073/pnas.85.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 11.Brams P, Nguyen ML, Chamat S, Royston I, Morrow PR. Antigen-specific IgG responses from naive human splenocytes: In vitro priming followed by antigen boost in the SCID mouse. J Immunol. 1998;160(5):2051–2058. [PubMed] [Google Scholar]
- 12.Hoogenboom HR, Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992;227(2):381–388. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- 13.Carter P, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89(10):4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Queen C, et al. A humanized antibody that binds to the interleukin 2 receptor. Proc Natl Acad Sci USA. 1989;86(24):10029–10033. doi: 10.1073/pnas.86.24.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green LL, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994;7(1):13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- 16.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23(9):1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 17.Lonberg N, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368(6474):856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- 18.Tomizuka K, et al. Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat Genet. 1997;16(2):133–143. doi: 10.1038/ng0697-133. [DOI] [PubMed] [Google Scholar]
- 19.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature. 1990;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 20.Alt FW, Blackwell TK, Yancopoulos GD. Immunoglobulin genes in transgenic mice. Trends Genet. 1985;1(1):231–236. [Google Scholar]
- 21.Jakobovits A. Production and selection of antigen-specific fully human monoclonal antibodies from mice engineered with human Ig loci. Adv Drug Deliv Rev. 1998;31(1-2):33–42. doi: 10.1016/s0169-409x(97)00092-6. [DOI] [PubMed] [Google Scholar]
- 22.Mendez MJ, et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Genet. 1997;15(2):146–156. doi: 10.1038/ng0297-146. [DOI] [PubMed] [Google Scholar]
- 23.Tomizuka K, et al. Double trans-chromosomic mice: Maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc Natl Acad Sci USA. 2000;97(2):722–727. doi: 10.1073/pnas.97.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi X, Eckhardt LA. Deletional analyses reveal an essential role for the hs3b/hs4 IgH 3′ enhancer pair in an Ig-secreting but not an earlier-stage B cell line. Int Immunol. 2001;13(8):1003–1012. doi: 10.1093/intimm/13.8.1003. [DOI] [PubMed] [Google Scholar]
- 25.Manis JP, Michaelson JS, Birshtein BK, Alt FW. Elucidation of a downstream boundary of the 3′ IgH regulatory region. Mol Immunol. 2003;39(12):753–760. doi: 10.1016/s0161-5890(02)00256-0. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt SL, et al. Association between the Igk and Igh immunoglobulin loci mediated by the 3′ Igk enhancer induces ‘decontraction’ of the Igh locus in pre-B cells. Nat Immunol. 2008;9(4):396–404. doi: 10.1038/ni1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosak ST, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296(5565):158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 28.Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 29.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 30.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev. 2004;197:161–178. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaisho T, Schwenk F, Rajewsky K. The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science. 1997;276(5311):412–415. doi: 10.1126/science.276.5311.412. [DOI] [PubMed] [Google Scholar]
- 33.Casola S, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5(3):317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 34.Cancro MP. Signalling crosstalk in B cells: Managing worth and need. Nat Rev Immunol. 2009;9(9):657–661. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 36.Meffre E, Nussenzweig MC. Deletion of immunoglobulin beta in developing B cells leads to cell death. Proc Natl Acad Sci USA. 2002;99(17):11334–11339. doi: 10.1073/pnas.172369999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 38.Hjelm F, Carlsson F, Getahun A, Heyman B. Antibody-mediated regulation of the immune response. Scand J Immunol. 2006;64(3):177–184. doi: 10.1111/j.1365-3083.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 39.Macdonald LE, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111:5147–5152. doi: 10.1073/pnas.1323896111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valenzuela DM, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21(6):652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 41.Green LL, Jakobovits A. Regulation of B cell development by variable gene complexity in mice reconstituted with human immunoglobulin yeast artificial chromosomes. J Exp Med. 1998;188(3):483–495. doi: 10.1084/jem.188.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brüggemann M, et al. A repertoire of monoclonal antibodies with human heavy chains from transgenic mice. Proc Natl Acad Sci USA. 1989;86(17):6709–6713. doi: 10.1073/pnas.86.17.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuaillon N, Taylor LD, Lonberg N, Tucker PW, Capra JD. Human immunoglobulin heavy-chain minilocus recombination in transgenic mice: Gene-segment use in mu and gamma transcripts. Proc Natl Acad Sci USA. 1993;90(8):3720–3724. doi: 10.1073/pnas.90.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: The highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36(Web Server issue):W503-8. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feeney AJ. Factors that influence formation of B cell repertoire. Immunol Res. 2000;21(2-3):195–202. doi: 10.1385/IR:21:2-3:195. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda F, et al. The complete nucleotide sequence of the human immunoglobulin heavy chain variable region locus. J Exp Med. 1998;188(11):2151–2162. doi: 10.1084/jem.188.11.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 48.Cummings SR, et al. FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 49.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillies SD, Morrison SL, Oi VT, Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- 51.Bolland DJ, Wood AL, Corcoran AE. Large-scale chromatin remodeling at the immunoglobulin heavy chain locus: A paradigm for multigene regulation. Adv Exp Med Biol. 2009;650:59–72. doi: 10.1007/978-1-4419-0296-2_5. [DOI] [PubMed] [Google Scholar]
- 52.Roldán E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6(1):31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuda T, et al. Immunological properties of Fc receptor on lymphocytes. 5. Suppressive regulation of humoral immune response by Fc receptor bearing B lymphocytes. Cell Immunol. 1978;39(2):238–249. doi: 10.1016/0008-8749(78)90100-4. [DOI] [PubMed] [Google Scholar]
- 54.Stockinger B, Lemmel EM. Fc receptor dependency of antibody mediated feedback regulation: On the mechanism of inhibition. Cell Immunol. 1978;40(2):395–403. doi: 10.1016/0008-8749(78)90347-7. [DOI] [PubMed] [Google Scholar]
- 55.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.