Significance
Glioblastoma is one of the most radio-resistant tumors, and the mechanisms of radioresistance are intensely studied. Here, we use a mouse model of proneural glioma to evaluate in vivo radiation-induced gene expression regulation at both the translational and transcriptional levels. We found that p53 and E2F are major regulators of the in vivo radiation response, and that there is little contribution from translational regulation, in contrast to previous in vitro reports. We also found that radiation induces a rapid shift in subtype from proneural to mesenchymal, which may have important implications for attempts to tailor targeted therapy regimens to tumor subtype.
Abstract
Glioblastoma is the most common adult primary brain tumor and has a dismal median survival. Radiation is a mainstay of treatment and significantly improves survival, yet recurrence is nearly inevitable. Better understanding the radiation response of glioblastoma will help improve strategies to treat this devastating disease. Here, we present a comprehensive study of the in vivo radiation response of glioma cells in a mouse model of proneural glioblastoma. These tumors are a heterogeneous mix of cell types with differing radiation sensitivities. To explicitly study the gene expression changes comprising the radiation response of the Olig2+ tumor bulk cells, we used translating ribosome affinity purification (TRAP) from Olig2-TRAP transgenic mice. Comparing both ribosome-associated and total pools of mRNA isolated from Olig2+ cells indicated that the in vivo gene expression response to radiation occurs primarily at the total transcript level. Genes related to apoptosis and cell growth were significantly altered. p53 and E2F were implicated as major regulators of the radiation response, with p53 activity needed for the largest gene expression changes after radiation. Additionally, radiation induced a marked shift away from a proneural expression pattern toward a mesenchymal one. This shift occurs in Olig2+ cells within hours and in multiple genetic backgrounds. Targets for Stat3 and CEBPB, which have been suggested to be master regulators of a mesenchymal shift, were also up-regulated by radiation. These data provide a systematic description of the events following radiation and may be of use in identifying biological processes that promote glioma radioresistance.
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults (1). Current standard of care for patients with GBM is surgical resection followed by local irradiation and temozolomide chemotherapy. Despite aggressive therapy, disease progression is universal and median patient survival is only 15 months (1). The vast majority of tumor recurrence occurs within the field of high-dose irradiation, suggesting that intrinsic radioresistance of glioma cells is the limiting factor in treatment effectiveness (2, 3). A more complete knowledge of the cellular response to radiation in glioma is critically important to understanding the radioresistant biology of this tumor and devising strategies for radiosensitization.
Features of the native GBM microenvironment, such as hypoxia and tumor–stroma interactions, are critical modulators of the radiation response (4–6). Additionally, interactions between GBM cells and the tumor microenvironment have been shown to affect the stem-like character of GBM cells (7), which has been linked to radioresistance (8, 9). Much of the current understanding of the response to radiation of glioma cells comes from work done with glioma cell lines cultured in vitro, but glioma cells cultured in vitro can demonstrate markedly increased radiosensitivity compared with the same cells grown as intracerebral xenografts (10). For these reasons it is critical to develop an understanding of the response of glioblastoma cells to radiation in vivo.
In vivo studies of radiation response are complicated by several factors. Glioblastomas are complex mixes of tumor cells and various stromal cell types, including astrocytes, immune cells, endothelial cells, and vascular smooth muscle cells (11). Meaningful analysis of in vivo gene expression changes after radiation therefore requires techniques for isolating the expression patterns of individual populations of cells. An important development in recent years has been the discovery that high-grade human gliomas actually encompass three to four distinct molecular subtypes of tumors (12, 13). These subtypes are associated with differential activation of oncogenic signaling pathways and correlate with specific genomic alterations. Because many of these pathways have been implicated in the radiation response, in vivo studies of radiation must take care to use relevant models that resemble the subclasses observed human patients.
Furthermore, in vivo expression profiling has been restricted to total mRNA levels. Radiation-induced DNA damage has been reported to regulate translational efficiency both globally and in a transcript-specific manner (14, 15). In glioma cell lines cultured in vitro, irradiation was reported to cause a much larger change in the translating pool of RNA than in total cellular RNA, suggesting a more important role for translational regulation than transcriptional regulation in the GBM radiation response (16). Traditional polysome isolation methods, however, are not amenable to translational profiling in vivo (11).
To study the global radiation response in an in vivo model of a defined GBM subtype, we combined translating ribosome affinity purification (TRAP) methodology (17) with an RCAS/tv-a genetic model of the proneural GBM subtype (18). Irradiation of these tumors induced a rapid and transient cell cycle arrest and asynchronous apoptosis. Interestingly, translational regulation was a relatively minor contributor to overall gene expression changes. Changes in total transcript levels dominated expression changes observed, with genesets for apoptosis and regulation of cell cycle up-regulated. p53 and E2F targets were among the most frequently altered, with p53 appearing to regulate the most highly induced transcripts after radiation. We also observed a shift in glioma subtype from proneural to mesenchymal within 6 h of irradiation, which was highly significant regardless of genetic background. The proneural to mesenchymal shift occurred in tumor cells and was not a result of stromal enrichment after radiation.
Results
Response to Ionizing Radiation is Heterogeneous by Cell Type in GBM.
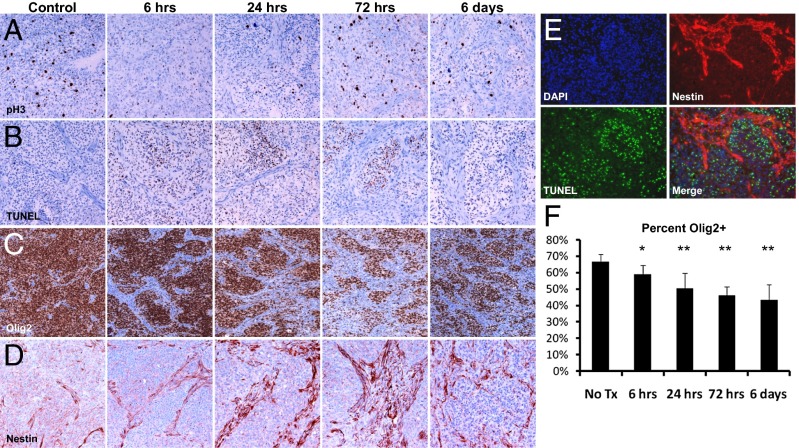
To study the glioma radiation response in vivo, we introduced platelet-derived growth factor B (PDGF-B) retrovirus into mice harboring somatic deletion of the Ink4a/Arf locus to generate highly penetrant high-grade gliomas that closely resemble the proneural subtype of GBM (12, 13, 18–20). We chose to use a single dose of radiation to enable time course studies after radiation, and had previously shown that a single dose of 10-Gy radiation induces cell cycle arrest in these tumors (21). Tumor-bearing mice were subjected to a single dose of 10-Gy total body ionizing radiation (IR), and tumor tissue was collected at time points after treatment for immunohistochemical staining. Radiation caused a complete mitotic arrest, with nearly undetectable levels of phosphorylated-histone H3 (pH3) at 6 h after treatment (Fig. 1A). Proliferating cells reappeared by 24 h after IR and returned to pretreatment levels by 72 h and 6 d after IR (Fig. 1A). By contrast, the apoptosis marker TUNEL was detected in only a small percentage of cells, but apoptosis was observed at all time points measured after IR, demonstrating an asynchronous mode of death (Fig. 1B). This limited, delayed apoptosis in response to IR contrasts sharply with the high levels of apoptosis observed in the more radiosensitive medulloblastoma brain tumor (9). Previous experiments demonstrated that this radiation dose confers only a modest survival benefit of ∼15 d in this model, consistent with the radioresistant phenotype of human GBM and the comparatively minimal apoptosis observed in these experiments (21).
Fig. 1.
Ten gray of IR induces transient cell cycle arrest, asynchronous apoptosis, and depletion of Olig2+ cells in mouse glioma. Staining for phospho-histone H3 (A), TUNEL (B), Olig2 (C), and Nestin (D). (E) TUNEL+ cells predominantly in tumor bulk rather than Nestin+ perivascular region 24 h after IR. (F) Quantification of Olig2+ nuclei in glioma following IR. Error bars are SD. *P < 0.05, **P < 0.005.
Human GBM is a heterogeneous tumor composed of several cell types and displaying multiple histological features. PDGF-driven mouse gliomas recapitulate this complexity, containing tumor bulk (TB) regions that are nucleus dense and stain positive for Olig2 and PDGF receptor α; and perivascular (PVN) regions that have a splindloid morphology and stain positive for nestin, smooth muscle actin, and PDGF receptor β (11). Immunofluorescent costaining for TUNEL and nestin revealed that IR-induced apoptosis occurs almost exclusively in the TB and minimally in the nestin-positive PVN (Fig. 1E). This accumulation of cell death in the TB causes a progressive reduction in the percentage of tumor cells staining positive for Olig2, dropping from 66% before treatment to 43% after 6 d (P = 0.00037) (Fig. 1 C and F). The regions of spindloid cells marked by nestin staining expand concurrently (Fig. 1D).
Tumors are composed of both transformed cells and recruited stroma. The PDGF oncogene that drives this model contains a hemaglutinin (HA) tag, facilitating discrimination between oncogene-expressing tumor cells and nontumor stromal cells. Immunohistochemical staining reveals that most, if not all, PDGF-HA expressing tumor cells reside in the Olig2+ TB both before and after IR (SI Appendix, Fig. S1A). FACS analysis of gliomas generated with a bicistronic retroviral vector expressing PDGF and RFP in mice expressing GFP driven by the Olig2 promoter had confirmed the high degree of coexpression (11).
Taken together, these data suggest that PDGF-driven mouse glioma models the radioresistant phenotype of human GBM. Furthermore, we demonstrate that the cellular response to IR is heterogenous, with oncogene-expressing tumor cells responding differently than nontumor stroma. It is therefore necessary to study the radiation biology of the different cell populations independently, because any differences in radiation response by cell type and changes in cellular composition after radiation would confound interpretation of the radiation biology of GBM studied as homogenous entity.
Regulation of Total Transcript Levels Dominates the Radiation-Induced Changes in Ribosome-Bound RNA.
To study global gene expression changes, we adapted the TRAP methodology to the RCAS-PDGF glioma model, as described (11). Briefly, PDGF-induced gliomas were generated in Olig2 bacTRAP mice (22). Tumors were dissected from untreated control mice and treated mice 6 h after 10-Gy irradiation. Tumor tissue was divided to collect both total RNA from sorted GFP+ cells and translating RNA via immunoprecipitation of GFP-tagged ribosomes from the same cell population. Paired ribosome-bound and total cellular RNA samples were analyzed by expression microarray.
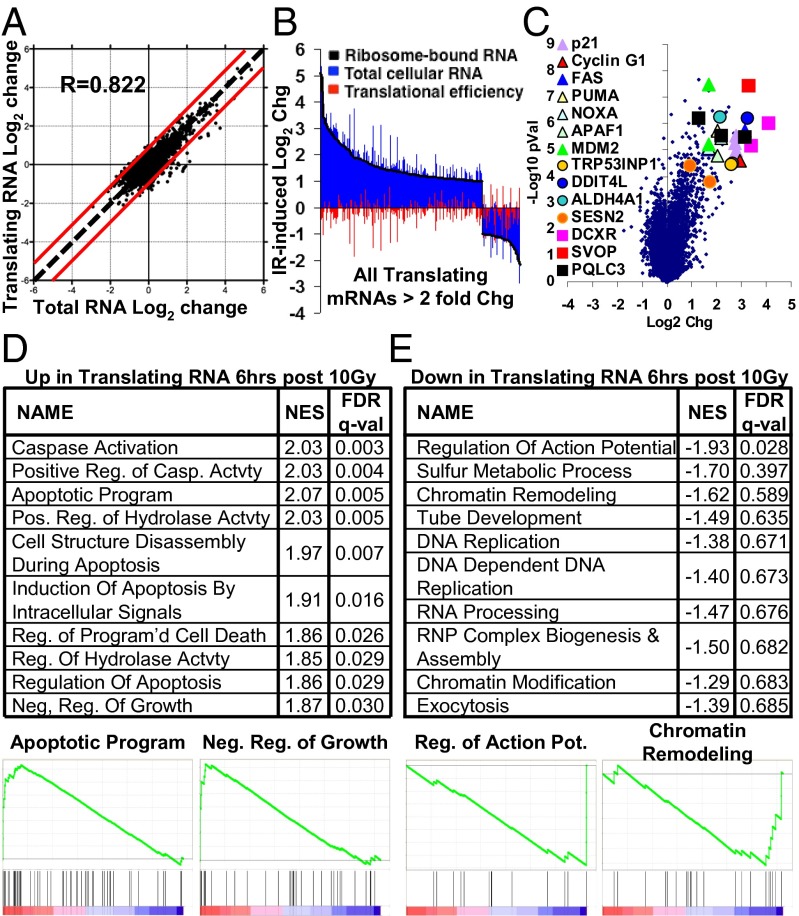
Changes in the levels of individual transcripts present on ribosomes can be divided into changes in the level of each transcript, and changes in the efficiency with which each transcript is recruited to ribosomes for translation, i.e., translation efficiency. Previously, it had been reported that a much larger number of transcripts showed significant changes in translational efficiency than in overall transcript level after IR in vitro (16). In our in vivo system, changes in translation efficiency contributed relatively little to overall changes in the mRNAs present on ribosomes after irradiation (Fig. 2 A and B). Changes in total mRNA and translating mRNAs were highly correlated (R = 0.82) (Fig. 2A). Fifty-eight transcripts showed translational efficiency changes of more than twofold, compared with 429 transcripts showing twofold changes at the total mRNA level. Similarly low levels of translational efficiency changes were seen in irradiated tumors that additionally had PTEN deleted by coinjection of an RCAS-Cre virus (SI Appendix, Fig. S2A) (11). For reference, when comparing normal brain Olig2+ oligodendrocyte precursor cells to their PDGF-transformed tumor counterparts, 2,239 transcripts exhibited greater than a twofold change in translational efficiency (11). We specifically looked at the translational efficiency of mRNAs shown previously to be translationally regulated in vitro in response to radiation and found no significant regulation in our in vivo system (SI Appendix, Fig. S3). Thus, the gene expression response to IR of these gliomas does not appear to have a large translational regulation component in vivo.
Fig. 2.
In vivo gene expression changes induced by IR in RCAS-PDGF Ink4a/Arf−/− gliomas collected 6 h after 10-Gy IR. (A) Log2 change in total mRNA vs. log2 change in ribosome-bound translating mRNA 6 h after 10-Gy IR. R, Pearson correlation. (B) All probes altered more than twofold in translating mRNA, and corresponding changes in total mRNA and translational efficiency. (C) Volcano plot of expression changes 6 h after 10-Gy IR. Genesets most significantly enriched (D) and depleted (E) among translating mRNAs after IR, with selected enrichment plots. FDR q-val, calculated false discovery rate; NES, Normalized Enrichment Score.
IR-Induced Changes in Gene Expression Primarily Involve Genesets Related to Apoptosis and Cell Cycle Arrest.
We next sought to characterize the gene expression changes induced by radiation in vivo. Few genes were highly down-regulated 6 h after 10-Gy IR in the Olig2+ TB, but a number of genes were highly up-regulated (Fig. 2C and SI Appendix, Fig. S2B). The most up-regulated genes by fold change included several genes known to be involved in cell cycle arrest and apoptosis. Fas, Puma, Noxa, and Apaf1 are all associated with induction of apoptosis, whereas cyclin G and p21 are cell cycle inhibitors, and MDM2 and TRP53INP1 have roles in both functions. Additionally, several genes such as Ddit4L, Aldh4A1, and Sestrin2 were up-regulated, which like TRP53INP1, are involved in the response to various stresses including DNA damage and redox imbalances. Ddit4L is not well characterized, but its homolog Ddit4 has been shown to be radioprotective (23) and was also up-regulated. Some genes of unknown relevance were highly up-regulated as well; carbonyl reductase 2 (DCXR) and synaptic vesicle 2-related protein were the most up-regulated genes and have no known function in the radiation response, whereas PQ loop repeat containing 3 (PQLC3) is largely uncharacterized. To validate the reliability of the array results, we selected some of the most highly up-regulated genes, along with some family members, and performed quantitative PCR (qPCR) on RNA extracted from treated and control neurospheres derived from PDGF-driven Ink4a/Arf−/− gliomas. The fold induction in neurospheres at 6 h closely matched the fold change seen on the array for all transcripts examined (SI Appendix, Fig. S4 A and B).
To understand what classes of genes are altered by IR, we performed geneset enrichment analysis (GSEA) (24). In PDGF-driven gliomas with both PTEN intact or deleted, genesets associated with caspase activation and induction of apoptosis were the highest scoring genesets, whereas the geneset for negative regulation of growth also was significantly up-regulated (Fig. 2D and SI Appendix, Fig. S2C). Curiously, the geneset for positive regulation of cellular proliferation was also the 13th most up-regulated geneset. However, an inspection of the leading edge of this geneset revealed that its significance is driven largely by the up-regulation of signaling molecules rather than bona fide cell cycle genes (p21 is also included in this geneset). Olig2+ tumor cells irradiated in vivo increased the expression of a range of growth factors and cytokines, including PDGF-A, CSF1, PGF, LIF, and EGFR.
With relatively few individual transcripts significantly down-regulated, the only geneset highly significantly enriched for down-regulation in PDGF gliomas was “Regulation of Action Potential.” The significance of this geneset was driven by down-regulation of genes associated with oligodendrocyte development and myelination, including MBP, MOG, S100b, and SHH, raising the possibility of a loss of oligodendrocytic differentiation in these cells in response to irradiation (Fig. 2E). PTEN−/− tumors had more significantly down-regulated transcripts and genesets, and genesets associated with mitosis were highly down-regulated (SI Appendix, Fig. S2D). Both PTEN wild-type (WT) and PTEN−/− tumors showed down-regulation of RNA processing and DNA replication genesets. Additionally, during initial experiments, we also generated expression profiles from the PDGF Ink4a/Arf+/− model (19), which showed similar regulation of both individual transcripts and broader genesets after radiation (SI Appendix, Fig. S5 A and B).
To further determine which gene changes are specific for irradiation, and which are general features of cells undergoing arrest, we used an alternative method for generating cell cycle arrest. The mTOR inhibitor temsirolimus is nongenotoxic and induces cell cycle arrest in PDGF-driven gliomas (25). Olig2+ cells from PDGF-induced PTEN−/− gliomas treated with either radiation or temsirolimus shared many of their respective down-regulated genes (SI Appendix, Fig. S6 A and B). These shared down-regulated genes were enriched for genesets associated with cell cycle, nuclear division, and mitosis. Although many of the down-regulated genes were overlapping, the up-regulated genes in each case were largely unique to the given treatment. The genes up-regulated after temsirolimus treatment were not significantly enriched for any genesets (SI Appendix, Fig. S6C). By contrast, the genes that were relatively up-regulated in the irradiated tumors were enriched for apoptosis, DNA damage, and checkpoint-related genesets (SI Appendix, Fig. S6D).
p53 and E2F Activity Are Major Transcriptional Drivers of the Radiation Response.
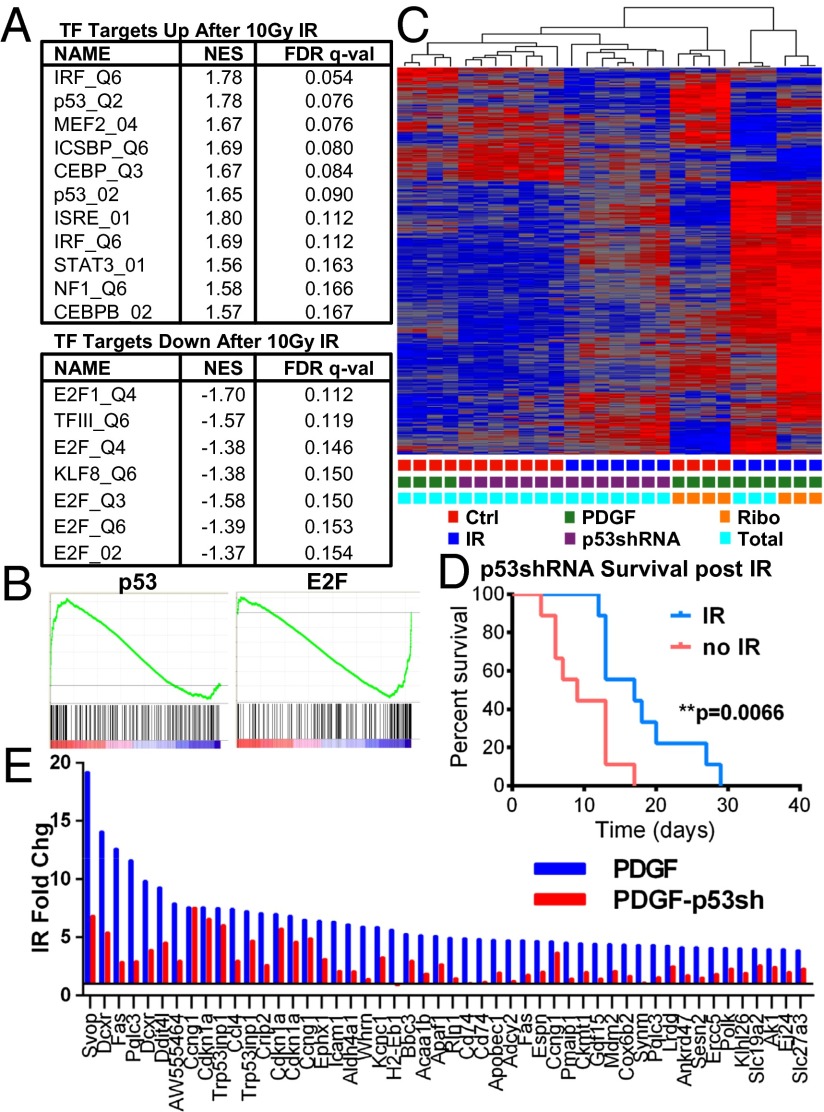
Having established that the in vivo radiation response is largely determined by changes in total mRNA levels rather than translational modulation, we sought to understand which transcription factors might be responsible for the changes in transcript levels observed. GSEA was performed to identify transcription factors whose binding motifs were most commonly found in the promoters of genes regulated by radiation. Genes containing a p53-binding site in their promoter were among the most significantly up-regulated in both PTEN WT and deleted tumors, consistent with our previous observation that several p53 targets were among the most significantly up-regulated transcripts (Fig. 3 A and B and SI Appendix, Fig. S7 A and B). Immunohistochemistry of irradiated and control tumors showed that p53 becomes nuclear localized after irradiation and is restricted to the tumor bulk rather than the perivascular regions (SI Appendix, Fig. S7C). To confirm p53 regulation of specific transcripts, we used irradiated and unirradiated p53−/− tumorspheres as in ref. 21 and performed qPCR for the same panel of genes as in SI Appendix, Fig. S4 A and B. Each gene that was up-regulated after IR in p53-intact neurospheres was unaltered in p53−/− neurospheres (SI Appendix, Fig. S4 A–C). Interestingly, the geneset “Negative Regulation of Programmed Cell Death” was the 18th and 10th most up-regulated geneset in PTEN intact and deleted tumors, respectively. The up-regulation of this set of genes, which includes Fas but also BAX, BCL2L1, NFKB1, and IL-7, may underlie some of the radioresistance of these tumors.
Fig. 3.
p53 and E2F are major drivers of in vivo radiation response. (A) Transcription factors whose targets are most significantly enriched and depleted among translating mRNAs after IR. (B) Enrichment plots for targets of p53 and E2F. (C) Unsupervised hierarchical clustering of genes significantly altered by IR in PDGF and PDGF/p53shRNA gliomas. (D) Kaplan–Meier survival curves of unirradiated and irradiated PDGF/p53shRNA gliomas in Ink4a/Arf−/− mice after postnatal day 18 randomization and single-dose 10-Gy IR. (E) Fold changes of the 50 most induced transcripts 6 h after 10-Gy IR in total RNA from PDGF-driven gliomas, and the corresponding fold change in total RNA from PDGF/p53shRNA tumors.
Several other transcription factor binding motif genesets were significantly up-regulated after 6 h, including genesets for CEBP, myocyte enhancer factor 2, IFN regulatory factor (IRF), and STAT3. IRF is a tumor suppressor that has been shown to enhance the transcriptional activity of p53 (26). Genesets for down-reglated genesets were dominated by those associated with E2F-binding motifs. E2F family transcript levels also fell after irradiation, driven by E2F1, which fell more than twofold by 6 h (SI Appendix, Fig. S7B).
In our preliminary experiments with Ink4a/Arf+/− mice, we collected RNA at both 2 h and 6 h after 10-Gy IR. The Ink4a/Arf+/− dataset was consistent with the Ink4a/Arf−/− dataset; for transcription factors with targets up-regulated in Ink4a/Arf−/− tumors, their targets were already up-regulated by 2 h in Ink4a/Arf+/− tumors and further up-regulated at 6 h as the radiation response progressed (SI Appendix, Fig. S7B). Likewise, E2F targets were down by 2 h and further decreased at 6 h, and E2F transcript levels decreased along a similar time course.
p53 Drives Most of the Highly Induced Genes in the in Vivo Radiation Response.
The gene encoding p53 is altered in 35–50% of proneural tumors, including tumors that exhibit down-regulation of p53 expression without genomic changes (13, 27). These alterations in p53 expression may co-occur with Ink4a/Arf loss and are independent of Ink4a/Arf status. Because p53 is such a large component of the radiation response in our PDGF-Ink4a/Arf−/− glioma model, we compared the radiation response at 6 h of tumors with compromised p53 to p53 WT tumors. We generated gliomas by using a p53shRNA-RFP RCAS retroviral vector in combination with RCAS-PDGF in Ink4a/Arf−/− Olig2-TRAP reporter mice. Consistent with previous work, p53shRNA-containing tumors exhibited shorter latency compared with PDGF-only tumors, and a single dose of 10-Gy radiotherapy conveyed a survival benefit of only 8 d, compared with 15 d for PDGF-only tumors (Fig. 3D; ref. 21). Immunohistochemistry of p53shRNA-PDGF tumors revealed lower levels of nuclear p53 after IR (SI Appendix, Fig. S8B). To perform expression profiling, we collected RFP/GFP double-positive cells via FACS to isolate tumor cells definitively infected with the p53shRNA virus. p53 transcript levels were significantly lower in p53shRNA-PDGF tumors compared with PDGF-only tumors (SI Appendix, Fig. S8A). Interestingly, unsupervised clustering placed p53shRNA tumors 6 h after irradiation with unirradiated tumors rather than irradiated p53 intact tumors, indicating the importance of p53 in shaping the overall radiation response (Fig. 5C).
The most up-regulated transcripts after IR in the p53shRNA-PDGF gliomas were similar to those of the PDGF-only tumors, but the p53 short hairpin markedly inhibited the up-regulation of virtually all of the most highly up-regulated transcripts in p53 intact tumors (Fig. 3E), suggesting that p53 is required for the vast majority of the largest gene expression changes in p53 intact tumors. The induction of each previously ex vivo validated p53 target was also lower in the p53shRNA/PDGF tumors (SI Appendix, Fig. S4A). p53 likely drives much of the caspase pathway up-regulation seen in these tumors, because caspase activation was the most highly enriched geneset when comparing relative expression changes after radiation in PDGF-only and p53shRNA gliomas (SI Appendix, Fig. S8E).
Radiation Triggers a Shift from Proneural Toward Mesenchymal Expression Patterns.
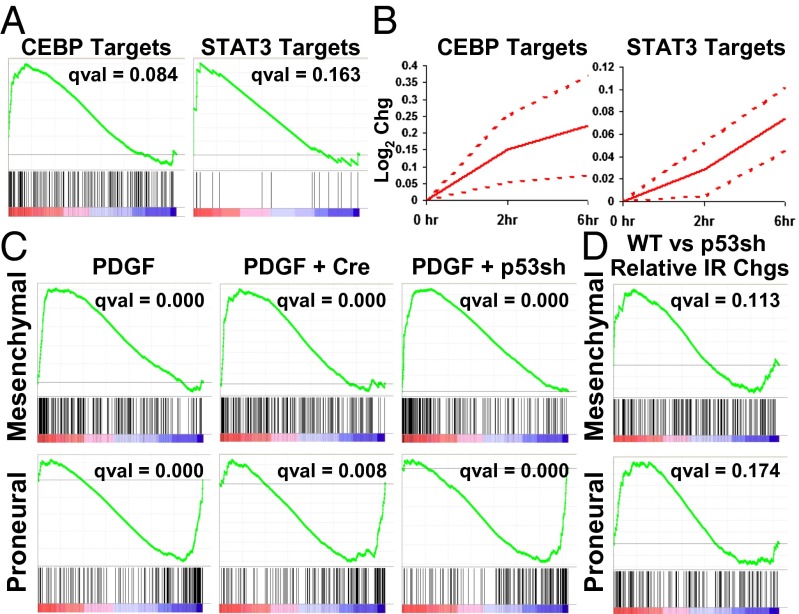
When Phillips et al. first described the major high-grade glioma subtypes, they noted that some proneural tumors tended, upon recurrence, to shift toward a mesenchymal gene signature (12). Because that report and subsequent works have relied on gene expression profiling of whole tumor lysates, and because the mesenchymal subgroup tends to express high levels of markers typical of glioma stromal cell types (11, 12), it is unclear whether any shifts in subtype, and indeed whether significant differences between the groups in general, are due largely to varying amounts of stroma. It was reported that Stat3 and CEBPB acted as master regulators of mesenchymal transformation, whose expression could drive neural stem cells along a mesenchymal lineage, and whose inhibition in tumor cells caused both a loss of mesenchymal signature and tumor aggressiveness (28). As such, we noted with interest the presence of both CEBPB and Stat3 targets high on our list of most up-regulated transcription factor motifs in the Olig2-expressing cells after radiation (Figs. 3A and 4 A and B). Further, pStat3 levels were elevated at 6 h after IR, as detected by IHC (SI Appendix, Fig. S9).
Fig. 4.
IR induces proneural-to-mesenchymal shift in PDGF gliomas. (A) Enrichment plots of CEBP and STAT3 targets in translating mRNA 6 h after 10-Gy IR in PDGF Ink4a/Arf−/− gliomas. (B) Time course of CEBP and STAT3 target up-regulation in PDGF Ink4a/Arf+/− gliomas. Average of total and translating mRNA pools is shown; dotted lines are SE. (C) Enrichment plots of mesenchymal and proneural signature genes in translating mRNA after 10-Gy IR in PDGF-driven Ink4a/Arf−/−, Ink4a/Arf−/−/PTEN−/−, and Ink4a/Arf−/−/p53shRNA gliomas. (D) Enrichment plots of mesenchymal and proneural signature genes in the relative changes of translating mRNA after IR of PDGF Ink4a/Arf−/− tumors vs. PDGF Ink4a/Arf−/−/p53shRNA tumors.
To determine whether radiation did, in fact, induce a shift from proneural to mesenchymal lineages in these tumor cells in vivo, we performed GSEA on proneural and mesenchymal gene signatures from Verhaak et al. (13). Although the Olig2 transcript was reduced by 1.7-fold, 6 h after radiation, the produrance for the GFP-L10a protein is longer than 6 h (SI Appendix, Fig. S10 A and B), allowing expression profiling of the cells that were Olig2 expressing at the time of radiation. Olig2+ tumor cells 6 h after in vivo irradiation did, in fact, exhibit highly significant enrichment of genes in the mesenchymal gene signature, and equally significant loss of proneural signature genes [false discovery rate (FDR) q value of 0.000 for both genesets] (Fig. 4C). The shift is detected in RNA isolated from Olig2+ tumor bulk cells via either cell sorting or cell type-specific TRAP, and also occurred in tumors with PTEN deleted (Fig. 4C). Thus, the proneural to mesenchymal shift induced by IR occurs in the tumor cells themselves and is not an artifact of changing stromal content. In fact, the shift had occurred within 2 h in Ink4a/Arf +/− tumors, before any selection has had time to occur (SI Appendix, Fig. S11 A and B).
Unlike many of the changes observed after IR, the shift from the proneural to mesenchymal character does not appear to be highly sensitive to p53 activity. p53shRNA tumors also showed highly significant loss of proneural markers and gain of mesenchymal markers (Fig. 4C). GSEA on the relative expression changes after irradiation of PDGF-only vs. PDGF+p53shRNA tumors showed nonsignificant enrichment in the PDGF-only tumors for both proneural and mesenchymal genesets after IR (Fig. 4D). Thus, it does not appear that the decrease in p53 activity in the p53shRNA tumors coherently inhibits or reverses the proneural to mesenchymal shift in those tumors.
Previously, we had shown that treatment of a Ras/Akt glioma model with temsirolimus shifted surviving tumor cells toward a more oligodendroglial phenotype, including induction of the proneural marker Olig2 (29). Because mTOR activity as measured by pS6 activity is higher in PTEN−/− PDGF-driven tumors (11), we wondered whether our PTEN-deleted tumors were more mesenchymal. Indeed, PTEN−/− tumor cells are shifted from proneural toward mesenchymal in our expression dataset (SI Appendix, Fig. S11C). However, temsirolimus treatment of PTEN−/− tumors did not trigger a reversal of the proneural to mesenchymal shift (SI Appendix, Fig. S11D), and IHC of irradiated tumors does not suggest an increase in mTOR activity (SI Appendix, Fig. S11E). Taken together, these findings indicate that the IR-induced proneural to mesenchymal shift is not driven by mTOR signaling.
Overall, these results show that radiation rapidly induces a shift in expression profile from proneural to mesenchymal over the course of a few hours. This shift occurs specifically in proneural tumor cells and is not a function of stromal enrichment. Furthermore, unlike much of the response to radiation, the proneural to mesenchymal transformation does not appear to be coherently affected by p53 activity.
Discussion
GBM is a devastating disease for which there is no cure. Radiation is standard therapy for patients with glioma, however, therapeutic response is limited and disease progression occurs universally. A better understanding of the radiation biology of glioma is critical to making progress in treating this destructive disease. Much of our current understanding of the radiation response of glioma is based on data from cultured glioma cells. The in situ tumor microenvironment differs greatly from that provided by cell culture, and work has shown that cells grown in vivo may have a markedly different radiation response than those grown in culture (10). However, interpretation of in vivo glioma radiation studies is complicated by the highly heterogeneous cellular composition of glioma.
In this study, we used a PDGF-driven mouse model of glioma to study the in vivo radiation response in a defined population of proneural tumor cells. It should be noted that although we believe our model is fairly representative of proneural gliomas as a whole, ∼50% of proneural gliomas have Ink4a/Arf intact. Although 35% exhibit PDGFRA amplification, the extent to which PDGF ligand is a driver of this subtype is not known (13).
Radiation of PDGF-driven gliomas with a single 10-Gy dose of radiation resulted in transient cell cycle arrest and asynchronous apoptosis. The effects of radiation vary by cell type, because apoptosis is largely restricted to the Olig2+ tumor cells and is accompanied by a decrease in the proportion Olig2+ cells over time. We used the TRAP system targeted to Olig2+ cells to study both translating and total RNA populations specifically in the Olig2+ proneural tumor bulk before and after radiation. Somewhat surprisingly, and in contrast with previous work done in cell culture, the translational contribution to overall gene expression changes is minimal in this in vivo system.
Consistent with our histological data, we find that apoptotic gene expression programs are highly induced within 6 h by IR and are accompanied by a loss of expression of genes associated with cell cycle progression. Several transcripts are also highly induced that are involved in stress responses, including redox imbalances that may be important in modulating the genotoxic effect of radiation (5). A range of transcripts for secreted growth factors and cytokines are also up-regulated after radiation.
The transcription factors contributing most to these effects appear to be p53 and E2F. p53 activity drives the largest expression differences observed and modulates tumor resistance to IR. The targets of both were among the most significantly altered sets of transcription factor targets in each of the tumor types we studied. The targets of IRF, a transcription factor known to cooperate with p53, were also among the most heavily induced by radiation. The largest expression differences observed are p53 dependent, and suppression of p53 activity by shRNA modulates tumor resistance to IR. p53 controls the overall expression changes after radiation to such a large extent that irradiated p53 knockdown tumors cluster more closely with unirradiated tumors than with irradiated p53 intact tumors.
A shift toward mesenchymal subtype at recurrence in previously proneural gliomas has been noted (12). In this work, we have identified a clear shift in expression pattern from proneural to mesenchymal within 6 h in response to radiation. This shift was observed after radiation in each of the tumor types we examined. The proneural to mesenchymal shift we see is tumor cell specific and is not the result of stromal enrichment via higher rates of cell death in the tumor bulk. Although changes in the stromal compartment may also affect tumor subtype, as measured by profiling of whole tumor lysate, we show here that the tumor bulk cells themselves undergo a subtype shift after radiation treatment. CEBPB and Stat3 have previously been suggested to act as master regulators of a proneural to mesenchymal shift in glioma cells, but the signaling network in that case was inferred from expression data from whole tumor lysates. In RNA isolated from Olig2+ tumor cells, both CEBPB and Stat3 were among the transcription factors whose targets were highly induced by radiation. Our data are evidence that a proneural-to-mesenchymal shift occurs in the same subset of cells in which CEBPB and Stat3 activity is elevated, supporting the reported link between CEBPB and Stat3 and the proneural-to-mesenchymal shift.
The subtype shift we observe is not clearly affected by p53 activity and is also not likely to be driven my mTOR signaling. Stat3 can be phosphorylated through a variety of pathways, including Src, Abl, mTOR, and JAKs. IL-15, LIF, and IL-7 are all among the most up-regulated transcripts after IR in Olig2+ cells themselves, and all are known activators of Stat3 via gp130/JAK (30, 31), suggesting that in irradiated proneural gliomas, cytokine activation of JAKs may drive the proneural-to-mesenchymal shift. Further studies are necessary to fully elucidate the mechanism by which radiation induces a proneural-to-mesenchymal shift.
Recent work indicates that mesenchymally shifted cells are more radioresistant (32). Given that therapeutic radiation is given in fractions over a period of days, a radiation-induced mesenchymal shift may represent an important mechanism of resistance to fractionated therapy, with important consequences for optimal design of radiation schedules.
The recent discernment of distinct subtypes of high-grade glioma and the identification of distinct growth factor pathways active in each subtype suggested that targeted pharmacological inhibition of these pathways on a subtype-specific basis might lead to better clinical outcomes. Subtyping of patient gliomas has already begun in an effort to develop more targeted individualized courses of therapy. However, the shift in subtype from proneural to mesenchymal that we observe here within hours of IR has important implications for this paradigm. The speed of the shift is evidence that the subtypes may not be as hardwired as previously believed. The apparent plasticity of gliomas with regard to subtype may indicate a need to target not only the oncogenic pathways typically associated with a given subtype, but also to identify and target other progrowth pathways that may become activated by treatment. Radiation-induced shifts are of particular importance, because radiation is a nearly universal component of glioma therapy. As various targeted therapies are being tested in combination with radiation, understanding the shifts in signaling patterns in response to radiation will be crucial to interpreting the results of these trials.
Methods
Generation and Treatment of Murine Gliomas.
PDGF-driven gliomas were generated in neonatal mice by RCAS-mediated retroviral transduction as described (18, 19). Symptomatic (lethargy, weight loss, macrocephaly) tumor-bearing mice were subjected to total body irradiation by using a Cs-137 source (Gammacell 40 Exactor; MDS Nordion). For survival studies, 10-Gy single-dose cranial IR was delivered on postnatal day 18 by an X-RAD 320 (Precision X-Ray) as in ref. 21. Temsirolimus was administered for 3 d at 40 mg/kg as in ref. 25.
Collection and Labeling of Ribosome-Bound and Total Cellular RNA.
Tumors generated in Olig2 bacTRAP mice (22) were grossly dissected. Translating RNA was collected by immunoprecipitation, and total RNA was collected via FACS as in ref. 11. RNA was amplified and biotin-labeled according to manufacturer protocol (Ambion AMIL1791) and hybridized to Illumina MouseRef-8 Expression BeadChips. RNA quality and labeling efficiency was assessed by Agilent 2100 Bioanalyzer.
Microarray Analysis and Geneset Enrichment.
Partek Genomics Suite software was used to quantile normalize and log2 transform all data. For translational efficiency analysis, as in ref. 11, a detection P value filter of 0.05 was applied to background subtracted expression values before quantile normalization and log2 transformation. GSEA was performed with the javaGSEA Desktop Application by using 1,000 geneset permutations, the provided gene ontology biological process and transcription factor motif genesets, and all other default settings (24). Genesets for glioma subtype signatures were obtained from ref. 13.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health Grants R01 CA100688, U54 CA126518, U01 CA141502-01, and U54 CA143798. K.H. was a Howard Hughes Medical Institute Medical Research Training Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321014111/-/DCSupplemental.
References
- 1.Huse JT, Holland EC. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 2.Lee SW, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43(1):79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 3.Chan JL, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20(6):1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 4.Davis LW. Malignant glioma—a nemesis which requires clinical and basic investigation in radiation oncology. Int J Radiat Oncol Biol Phys. 1989;16(6):1355–1365. doi: 10.1016/0360-3016(89)90936-x. [DOI] [PubMed] [Google Scholar]
- 5.Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets. 2009;9(3):381–390. doi: 10.2174/156800909788166637. [DOI] [PubMed] [Google Scholar]
- 6.Barcellos-Hoff MH, Newcomb EW, Zagzag D, Narayana A. Therapeutic targets in malignant glioblastoma microenvironment. Semin Radiat Oncol. 2009;19(3):163–170. doi: 10.1016/j.semradonc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles N, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 9.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamal M, Rath BH, Williams ES, Camphausen K, Tofilon PJ. Microenvironmental regulation of glioblastoma radioresponse. Clin Cancer Res. 2010;16(24):6049–6059. doi: 10.1158/1078-0432.CCR-10-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmy K, et al. Identification of global alteration of translational regulation in glioma in vivo. PLoS ONE. 2012;7(10):e46965. doi: 10.1371/journal.pone.0046965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips HS, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Verhaak RG, et al. Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29(21):5645–5656. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powley IR, et al. Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev. 2009;23(10):1207–1220. doi: 10.1101/gad.516509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lü X, de la Peña L, Barker C, Camphausen K, Tofilon PJ. Radiation-induced changes in gene expression involve recruitment of existing messenger RNAs to and away from polysomes. Cancer Res. 2006;66(2):1052–1061. doi: 10.1158/0008-5472.CAN-05-3459. [DOI] [PubMed] [Google Scholar]
- 17.Heiman M, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135(4):738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih AH, et al. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64(14):4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 19.Fomchenko EI, et al. Recruited cells can become transformed and overtake PDGF-induced murine gliomas in vivo during tumor progression. PLoS ONE. 2011;6(7):e20605. doi: 10.1371/journal.pone.0020605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty JD, et al. Candidate pathways for promoting differentiation or quiescence of oligodendrocyte progenitor-like cells in glioma. Cancer Res. 2012;72(18):4856–4868. doi: 10.1158/0008-5472.CAN-11-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Squatrito M, et al. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell. 2010;18(6):619–629. doi: 10.1016/j.ccr.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle JP, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135(4):749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XH, Ha CT, Fu D, Xiao M. REDD1 protects osteoblast cells from gamma radiation-induced premature senescence. PLoS ONE. 2012;7(5):e36604. doi: 10.1371/journal.pone.0036604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitter KL, et al. Perifosine and CCI 779 co-operate to induce cell death and decrease proliferation in PTEN-intact and PTEN-deficient PDGF-driven murine glioblastoma. PLoS ONE. 2011;6(1):e14545. doi: 10.1371/journal.pone.0014545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dornan D, et al. Interferon regulatory factor 1 binding to p300 stimulates DNA-dependent acetylation of p53. Mol Cell Biol. 2004;24(22):10083–10098. doi: 10.1128/MCB.24.22.10083-10098.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerami E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carro MS, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, et al. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7(4):356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J. 2011;438(1):11–23. doi: 10.1042/BJ20102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demoulin JB, Renauld JC. Signalling by cytokines interacting with the interleukin-2 receptor gamma chain. Cytokines Cell Mol Ther. 1998;4(4):243–256. [PubMed] [Google Scholar]
- 32.Bhat KP, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.