Significance
Isoniazid preventive therapy (IPT) is a key tuberculosis prevention strategy in high-HIV-prevalent settings. While randomized controlled trials have shown IPT provides protection against tuberculosis disease in HIV-positive patients during therapy, protection was quickly lost after cessation of therapy. It was not known whether this loss of protection is due to reinfection and rapid progression to disease, or lack of cure. Using mathematical modeling, fitted to trial data, we show IPT does not cure Mycobacterium tuberculosis infection in the majority of HIV-infected individuals. These results contrast with long-held beliefs about the working mechanism of IPT, but explain the empirical results. These results are important for determining appropriate clinical guidelines for IPT use in varying epidemiological settings.
Abstract
Trials of isoniazid preventive therapy (IPT) for people living with HIV in southern Africa have shown high rates of tuberculosis disease immediately after cessation of therapy. This could be due to the lack of cure following preventive therapy or reinfection with rapid progression to disease. Using a model fitted to trial data, we estimate the degree to which preventive therapies cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-tuberculosis-burden settings. We identified randomized controlled trials that compared IPT to placebo or alternative regimen in HIV-positive, tuberculin skin test positive individuals. A mathematical model describing tuberculosis transmission in a closed cohort of HIV-positive, M. tuberculosis infected, antiretroviral therapy naive individuals following completion of preventive therapy (or placebo) was fitted to posttherapy tuberculosis rates to estimate the annual risk of M. tuberculosis reinfection and the proportion of individuals whose latent infection was cured after therapy. Three trials met our inclusion criteria. Estimated annual risks of reinfection ranged between 3.7 and 4.9%. Our results suggest 6 mo of isoniazid cured in a small proportion [estimated proportion cured = 0% (interquartile range 0–30.9%)]. The proportion cured for 3-mo regimens containing rifampicin or rifapentine was 19–100%. IPT alone does not cure existing infections in the majority of HIV-infected individuals. In high-incidence settings, continuous IPT should be integrated with HIV care. Where the risk of reinfection is lower, preventive therapy with more curative drugs should be preferred for HIV-positive individuals to achieve durable patient benefit.
Isoniazid preventive therapy (IPT) is an important part of tuberculosis (TB) prevention in high-HIV–prevalent settings (1) and has been shown to provide protection against TB disease in HIV-positive patients during therapy (2). The effect is most clearly seen in individuals with presumed Mycobacterium tuberculosis infection, as indicated by a positive tuberculin skin test (TST) (2, 3). However, long-term follow-up of TB-preventive therapy trials set in sub-Saharan Africa have shown high rates of TB post IPT (2, 4–8), and the results of the recently published Thibela study (9) showed a rapid loss of protection following completion of IPT. It is unclear what drives this lack of durable protection from TB disease.
Following infection or reinfection with M. tuberculosis an individual can progress rapidly to disease (i.e., within the first 1–5 y), or the infection can be contained and the individual remains at a continuous, albeit low risk of active TB disease (10).
In individuals with M. tuberculosis infections, TB disease postpreventive therapy can therefore follow from one of two mechanisms; acquisition of new infections which can then progress to disease (reinfection disease) or reactivation of a persisting infection (reactivation disease).
If preventive therapy “cures” an existing M. tuberculosis infection (defined here as preventing subsequent disease from that infection), then these cured patients must first be reinfected before they can be at risk for developing TB disease. If preventive therapy does not cure, all noncured patients will immediately be at risk for developing TB disease through reactivation of their M. tuberculosis infection in addition to the risk of disease following reinfection. The latter scenario, where IPT does not cure latent M. tuberculosis infections (LTBI) in this population, could explain the high TB rates observed immediately post IPT (2, 4–8).
Understanding the extent to which preventive therapy cures M. tuberculosis infection in HIV-positive individuals is critical for any valid estimation of potential individual and population-level posttherapy benefits in high-HIV-prevalence countries. Although the curative ability of preventive regimens may depend on immune status of the individual, and therefore be affected by antiretroviral therapy (ART) cotherapy, it is important to quantify the impact of IPT for a clear assessment of the biological mechanism. In addition, ART is still not available for many HIV-positive individuals, for whom the results of this analysis will be directly relevant.
In this paper we use a model, fitted to trial data, to estimate the proportion of LTBI cured by preventive therapies in HIV-positive, TST-positive individuals not taking ART in high-tuberculosis-burden settings.
Methods
Data Extraction.
We searched the literature for trials on TB-preventive therapy. Eligible studies published before April 2008 were selected from a Cochrane systematic review (2). Medline was searched for trials published between April 2008 and December 15, 2012, using a modified version of the Cochrane literature search [HIV AND (tuberculosis OR TB)] AND (preventive therapy OR chemoprophylaxis OR treatments) including trials and reviews.
Studies were included if they were set in sub-Saharan Africa (as a proxy for generalized TB and HIV epidemics), reported at least 2 y of postpreventive therapy TB incidence in a HIV-positive TST-positive population not taking ART, and compared a preventive therapy regimen versus placebo or at least one alternative regimen.
For each selected study, the relevant figure was identified. Using graph digitizer software (GraphDigitizerScout, www.bytescout.com), data on the TB-free survival or cumulative hazard curves were extracted. For nonplacebo arms, we wished to explore the impact of preventive therapy on the TB dynamics following the completion of therapy. To minimize bias we included data from the entire trial period in the placebo arms but only data from the posttreatment period from the intervention arm(s). We account for potential differences in CD4 counts between arms by explicitly including a previously parameterized time-dependent model of decline in CD4 (11, 12). This approach minimizes bias due to the differential risk of TB between the trial arms during the intervention period which could not be directly adjusted for, because we did not have access to the individual-level data.
A 95% confidence interval for the TB incidence rate during each year was calculated as ±rate/exp(1.96/√number of events) (13). The number of events was extracted by multiplying the total person years in the given period by the proportion of the population at risk that developed TB.
Model Structure.
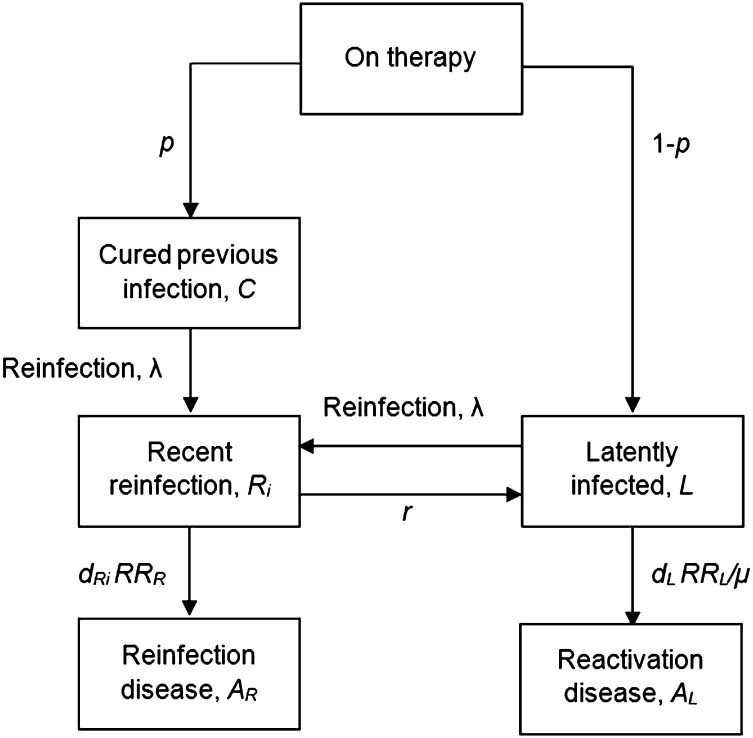
A simple deterministic compartmental model was created to reflect a closed cohort of individuals during and after TB-preventive therapy (Fig. 1).
Fig. 1.
Structure of the model. The main text provides a description of the model, and Tables 1 and 2 provide explanation of the model parameters.
The model describes M. tuberculosis infection and TB disease dynamics in a closed population of TST-positive individuals following a course of preventive therapy. We assume that in these populations a positive TST result is an indicator of M. tuberculosis infection and therefore assume that at the start of the intervention all individuals are latently infected with M. tuberculosis. This is consistent with a systematic literature review which estimated the specificity of TST to be 97% (95–99%) in nonbacillus Calmette–Guérin-vaccinated populations (14), and evidence that the effect of bacillus Calmette–Guérin vaccination in infancy on false-positive TST results is minimal beyond adolescence (15). We assume following isoniazid or other preventive therapy a proportion p are cured and enter a cured class (C) (Tables 1 and 2). These individuals can then only develop TB disease if they are reinfected with M. tuberculosis (at an annual risk of reinfection, λ). We assume those who do not cure their infection (1 − p) enter a latently infected class (L) and remain at risk for TB reactivation disease.
Table 1.
Model variables
| Description | Variable |
| Individuals who have cured infection following therapy | C |
| Latent M. tuberculosis infected individuals | L |
| Individuals reinfected within past year | Ri=1 |
| Individuals reinfected between 1 and 2 y ago | Ri=2 |
| Individuals experiencing reinfection disease | AR |
| Individuals experiencing reactivation disease | AL |
Variables are proportions of total population.
Table 2.
Model parameters
| Description | Value | Distribution | Source/Remarks |
| p, proportion cured by preventive therapy | 0–1 | — | Estimated by fitting |
| λ, annual risk of M. tuberculosis infection | 0+ | — | Estimated by fitting |
| dRi=1, risk of disease in first year following reinfection, per year | 5% | Normal (mean = 5, 95%, CI = 1–10) | (10,11) |
| dRi=2, risk of disease in second year following reinfection, per year | 0.41 × dRi=1 | — | (10) |
| dL, risk of disease due to reactivation in Latent stage, per year | 0.13 × dRi=1 | — | (10) |
| RRR, relative risk of disease following recent reinfection in HIV+ vs. HIV−, per year | — | — | Depends on CD4 (SI Appendix) |
| µ, ratio RRR:RRL | 3:1 | Normal (mean = 3, 95%, CI = 1–5) | (14) |
| RRL, relative risk of disease following reactivation in HIV+ vs. HIV−, per year | — | — | = RRR / µ |
CI, confidence interval.
We assume latently infected individuals can develop TB through reactivation of their LTBI at rate dL. Reinfected individuals are assumed to enter a recent reinfection class (Ri) (defined as reinfection in the past 2 y) that is stratified by time since infection (i) and can progress to reinfection disease at rate dRi. Following convention (16, 17), the risk of progressing to disease following reinfection is initially high but declines quickly with time since infection from a maximum value dRi = 1 at reinfection to dL upon return to the latent class L. The estimates of the risk of disease following infection used here are based on modeling studies of data from the United Kingdom. To account for the uncertainty in these estimates and potential differences in the risk of disease between the United Kingdom and the trial settings, we performed sensitivity analysis to explore the robustness of our results to these parameter values.
In HIV-infected individuals, the risk of developing TB is assumed to increase with decreasing CD4 count. Following Williams et al. (11) we assume that upon infection with HIV, an individual’s CD4 count falls by 25% and then declines linearly to zero at the time of death in the absence of ART. The relative risk of TB following reinfection (RRR) increases exponentially with decreasing CD4 cell count at a rate α = 0.36 ± 0.12 for each decline of 100 cells/μL (SI Appendix provides further details). Including a CD4-dependent risk of TB allows us to include potential differences in CD4 counts between arms.
Data also suggest that HIV infection increases the risk of (re)infection disease more than reactivation disease (18–21). From a molecular epidemiological study exploring the impact of HIV on TB following reinfection with M. tuberculosis in a setting with generalized HIV and TB epidemics, the ratio of RRR to the relative risk of reactivation (RRL) was estimated at roughly 3:1 (19). The impact of variation in this parameter was explored in sensitivity analysis. Full details of the model are given in the SI Appendix. Default parameter values and uncertainty ranges are shown in Tables 1 and 2.
Parameter Estimation.
The cumulative incidence predicted by the model was fitted to the cumulative incidence data from each of the TB-preventive therapy trials to estimate the unknown model parameters: the annual risk of M. tuberculosis reinfection (λ) and the proportion of the population whose LTBI was cured by each regimen (p). The former determines the rate of “reinfection” TB disease, and the latter determines the proportion of the cohort at risk for reactivation TB disease immediately postpreventive therapy (1 − p). Fitting was carried out using the “optim” function of the R statistical programming language (22) to minimize the sum of squared residuals between the model and the data.
For placebo-controlled trials, the model was fitted to data from the placebo and intervention arms simultaneously to estimate the annual risk of reinfection in the trial population and the proportion cured for each regimen. This approach makes two assumptions. First, the placebo did not cure LTBI and therefore the proportion cured in the placebo arm could be fixed to zero. Second, given the individual-level randomization in the trial, participants in each arm of the trial would experience the same annual risks of infection.
To allow further validation of the TB epidemiology predicted by the model, we calculated the proportion of disease due to recent transmission. Based on molecular epidemiological studies from sub-Saharan settings (23, 24), we would expect at least 50% of cases to be due to recent transmission in the past 2 y, i.e., following recent (re)infection.
For trials without a placebo arm the above approach was not possible. Instead we used the model to estimate the annual risk of infection in each intervention arm for a range of assumed values for the proportion cured (p). We did this by fitting the model for each intervention arm separately to the observed incidence for each assumed value of the proportion cured. We then made two more assumptions to estimate a proportion cured for each therapy arm: (i) As in placebo-controlled trials, all participants will experience the same annual risk of reinfection, regardless of trial arm. (ii) The proportion cured in the isoniazid group would be similar to the value we estimated for the isoniazid group in the placebo-controlled trials (as above). These two assumptions allowed us to estimate the annual risk of reinfection for the isoniazid arm and then estimate the value of the proportion cured for the alternative preventive therapy arm(s).
Sensitivity Analysis.
We explored the robustness of the model predictions to the uncertainty in both the parameter values and trial data using a combination of a parametric bootstrap procedure with probabilistic sensitivity analysis (25). Sets of parameter values were sampled from appropriate distributions selected to reflect the uncertainty in estimates in the literature (Table 2) and combined with incidence values drawn from the 95% confidence intervals calculated from the trial data. The model was fitted for each combination of parameters and data to estimate the annual risk of infection (ARI) and proportion cured. The number of model fits was not defined a priori; rather, the process was repeated for sufficient combinations of parameters and data to obtain 2,000 model outputs for which 50% or more of all TB disease was due to recent transmission. Results are reported as the median and interquartile range calculated from these 2,000 samples and reflect the uncertainty in the model outputs due to uncertainty in the empirically observed TB incidence and the model parameters.
To assess the sensitivity of the parameter estimates to our assumption about the proportion of disease due to reinfection the analysis was repeated with a lower constraint of 20%. The results of a probabilistic sensitivity analysis can also be influenced by the choice of parameter distributions; therefore we explored the impact of our choice of parameter distributions by repeating our analysis with alternative uncertainty distributions, which did not affect our conclusions (SI Appendix provides details).
An alternative model for the action of isoniazid is to reduce the risk of reactivation in the whole population rather than cure latent TB infection in some proportion of individuals, i.e., analogous to assuming a “degree” vaccine effect rather than a “take” vaccine effect. The sensitivity of our results to this alternative biological model was explored using a modified model structure, details of which are provided in the SI Appendix.
Results
Study Selection.
Out of 14 trials identified in the Cochrane review, two studies met our criteria (4, 5). The Medline search identified 954 papers, from which 15 reported results from prospective studies on TB-preventive therapy, and one met our inclusion criteria (6). In total, three studies were included in the analysis, two placebo-controlled set in Nairobi (Kenya) and Kampala (Uganda) as well as one non-placebo-controlled trial set in Soweto (South Africa), all areas with expected high rates of ongoing M. tuberculosis transmission in the general population (4–6).
Overall these trials evaluated 3,118 HIV-positive, TST-positive participants (Table 3) in eight groups. Apart from two placebo groups, three groups received 6 mo of isoniazid only, two groups received 3 mo or 12 wk of a rifampicin-containing regimen, and one group received 12 wk of a rifapentine-containing regimen. In the Nairobi and Kampala studies, ART was not available, and in the Soweto study all participants were ART-naive at inclusion, and less than 10% of follow-up time was spent on ART.
Table 3.
Summary of included studies
| First author and year of publication | Data source | Study location | Therapy arms | Number of participants* | Post PT follow-up (mo) |
| Johnson 2001 (4) | Fig. 2b | Kampala, Uganda | Placebo | 464 | 36 |
| 6 mo INH (300 mg) | 536 | ||||
| 3 mo INH (300 mg) + RIF (600 mg) OR 3 mo INH (300 mg)+RIF (600 mg) + PYR (2000 mg) | 998 | ||||
| Hawken 1997 (5) | Fig. 1b | Nairobi, Kenya | Placebo | 69 | 24 |
| 6 mo INH (300 mg) | 67 | ||||
| Martinson 2011 (6) | Fig. 2 | Soweto, South Africa | 6 mo INH (300 mg) | 327 | 36 |
| 12 wk INH (900 mg) + RPT (900 mg) | 328 | ||||
| 12 wk INH (900 mg) + RIF (900 mg) | 329 |
Dosing regimens are daily, except 12-wk regimens in Soweto study (given weekly). INH, isoniazid; RIF, rifampicine/rifampin; PYR, pyrazinamide; RPT, rifapentine.
Number of participants at enrollment.
Placebo-Controlled Trials—Proportion Cured.
Table 4 summarizes the model results for the placebo-controlled trials. Median and interquartile ranges are calculated from the sensitivity analysis as described above. The distributions of the estimated parameters are shown in the SI Appendix, Fig. S3.
Table 4.
Estimated annual risk of infection (λ) and proportion cured (p) for the two placebo-controlled trials
| Annual risk of reinfection, %/y (IQR) | Proportion cured latent M. tuberculosis infection, % (IQR) | Proportion of disease due to lack of cure, % (IQR) | |
| Nairobi (Kenya) | |||
| Placebo | 4.9 (3.0–12.2) | 0.0* | — |
| 6 mo INH | 4.9 (3.0–12.2)* | 0.0 (0.0–0.0) | 30.8 (17.3–46.3) |
| Kampala (Uganda) | |||
| Placebo | 3.7 (2.9–5.1) | 0.0* | — |
| 6 mo INH | 3.7 (2.9–5.1)* | 0.0 (0.0–30.9) | 22.7 (8.6–40.1) |
| 3 mo INH + RIF or 3 mo INH + RIF + PYR | 3.7 (2.9–5.1)* | 100 (95.0–100) | 0.5 (0.4–1.0) |
In the Nairobi trial, the estimated annual risk of reinfection was 4.9% (3.0–12.2%), and 6 mo of daily IPT cured 0% (0–0%) of study participants, assuming the percentage of disease due to recent transmission exceeds 50%.
For the Kampala trial, the estimated annual risk of infection was 3.7% (2.9–5.1%); IPT cured 0% (0–30.9%) of study participants; 3-mo regimens containing rifampicin cured 100% (95.0–100%) of study participants.
The proportion of TB disease due to reactivation of uncured latent infections following 6 mo of IPT was estimated to be 30.8% (17.3–46.3) in the Nairobi study and 22.7% (8.6–40.1) in the Kampala study. For rifampicin-containing regimens the estimated proportion of disease due to reactivation was much smaller [0.5% (0.4–1.0)], consistent with the higher estimated proportion cured by these regimens.
When the constraint on the percentage of disease due to recent transmission was relaxed (percentage of disease due to recent transmission exceeds 20%), the model estimated that IPT cured 0% (0–0%) of infections in the Nairobi trial and 14.2% (0–33.6%) of infections in the Kampala study. The results were also robust to the form of the parameter distributions used in the sensitivity analysis (SI Appendix).
When the impact of preventive therapy was modeled as reducing the risk of reactivation in all individuals (i.e., analogous to assuming a degree rather than take vaccine effect) we obtain a similar result—in both trials the median estimated level of protection provided by isoniazid was 0.0.
Non-Placebo-Controlled Trials.
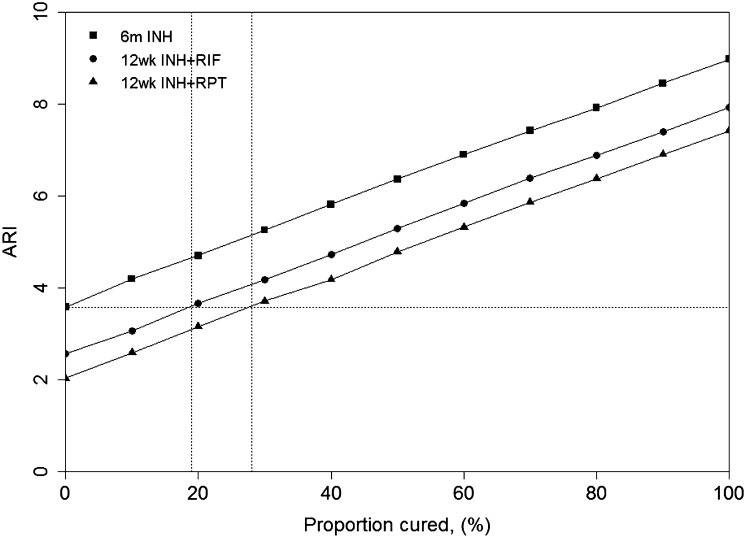
Fig. 2 shows the estimated ARI for each arm in the Soweto trial (6) as the proportion cured (p) is varied. The horizontal dashed line shows the ARI in the population when we assume that isoniazid does not clear latent infection (i.e., p = 0). The vertical dashed lines show the cure rates in the rifampicin and rifapentine arms, respectively, assuming that all arms experienced this same ARI. The model estimates the annual risk of infection is 3.6% if isoniazid does not clear latent infection. Given this ARI, isoniazid plus rifampicin is estimated to cure 19% of M. tuberculosis infections and isoniazid plus rifapentine is estimated to clear 28% of M. tuberculosis infections.
Fig. 2.
Estimated ARI for the Soweto trial. Symbols and solid lines show the estimated ARI (y axis) for each arm in the study as p, the proportion cured (x axis), is varied. Horizontal dashed line shows the estimated ARI assuming p = 0 for INH. Vertical dashed lines show the proportion cured by rifampicin and rifapentine assuming this ARI is the same across all arms. INH, isoniazid; RIF, rifampicine/rifampin; RPT, rifapentine; 6m, 6 mo; 12w, 12 wk.
Discussion
Our results suggest that in HIV-positive, ART-naive individuals with a positive TST, IPT is very unlikely to result in cure of LTBI, whereas rifampicin- or rifapentine-containing regimens cured between 19 and 100% of individuals.
If IPT did not cure, individuals would immediately be at risk for tuberculosis through reactivation of preexisting infections. This large pool of individuals already at risk, in combination with a high risk of reinfection, could explain the rapid bounce back in incidence observed posttherapy in IPT trials in sub-Saharan Africa.
These results contrast with the long and widely held view that a 6–9 mo course of IPT is sufficient to cure infection in most individuals (26), although HIV-positive populations were seldom explicitly included in these discussions. Historical data on HIV-negative populations have suggested a long-term protective effect lasting years after completion of a 6–9 mo course of IPT (27, 28), suggesting that cure was achieved. However, the short interval between therapy completion and TB disease has led to speculation that reactivation of noncured infections was responsible for some of the posttherapy disease episodes occurring in trials in HIV-positive populations (7, 29). Our results are also consistent with the recently published findings of the Thibela study, whose authors conclude that “reactivation of inadequately treated latent tuberculosis infection” was one factor which may have contributed to the lack of effect on TB incidence in their trial of mass IPT (9), and provide additional evidence that noncure is the underlying mechanism.
Our results fit with the current understanding of the mechanism of action of both isoniazid and rifampicin. Isoniazid’s main activity is bactericidal, highly effective against actively replicating bacteria (30, 31), but unlikely to completely cure a LTBI with few replicating bacteria (29). In vitro and mouse studies have shown that rifampicin and rifapentine can sterilize (31, 32) and would therefore be expected to more often result in a cure of LTBI.
An alternative hypothesis for the lack of long-term protection in HIV-positive individuals following IPT is that isoniazid is effective in curing LTBI but in doing so removes any immunity conferred by previous infection. If this were the case, we would expect higher TB incidence rates in groups taking regimens containing drugs such as rifampicin with higher sterilizing capability. However, this effect has not been observed in clinical trials comparing such regimens (4, 6).
The risk of tuberculosis increases with declining CD4 count; therefore differences in CD4 count distributions between trial arms could have contributed to the differences in observed incidence. Randomization resulted in similar CD4 count distributions across arms at baseline (SI Appendix, Table S1), although no data were available on changes in CD4 distributions during the study. However, a difference in average CD4 counts may have been introduced through the different study periods used in this analysis. For placebo arms, data from the entire trial period were included, whereas for the intervention arms, only the posttherapy period was considered. This would have resulted in lower average CD4 counts in the intervention populations compared with the placebo arms (due to the additional time elapsed). We accounted for the potential difference in TB risk by including a time-dependent CD4 function in our model.
Competing risks of mortality, for example, through deaths from undiagnosed tuberculosis, could have biased our results. However, through randomization these risks will have been similar between study arms, and repeated screening for TB during the trial should have resulted in few unrecorded TB deaths (33).
Drug resistance was not included in the model, and it is possible that resistance to isoniazid may partly explain the low estimated cure rate following IPT. However, estimates of primary resistance to isoniazid in HIV-infected individuals in all three trial settings were low, ranging from 3.8% in Soweto (6), 7.5% in Nairobi (34), and 4–9% in Uganda (35, 36), and unlikely to affect our results. A recent national drug resistance survey in Uganda found that primary isoniazid resistance has remained low at 5% (37). It is also possible that IPT may lead to the selection of isoniazid resistance in individuals undergoing treatment; however, there appears to be no evidence of this in the included trials. In the Nairobi trial, the rate of resistance in the isoniazid arm was not statistically different from the rate in HIV-positive individuals in Kenya (5). More generally, a systematic review of IPT trials which assessed the risk of acquired resistance as a result of IPT (38), while not excluding the possibility of an increased risk, found no statistically significant relationship between isoniazid resistance and completion of IPT.
This work was restricted to HIV-positive, TST-positive populations with no ART. With regard to TST status, there is little reason to assume that IPT would be more likely to cure in HIV-positive, TST-negative individuals, where no effect is seen during therapy (2).
It has been suggested that ART, perhaps through immune reconstitution, would increase the proportion of latent infections cured by isoniazid (7, 29). A recent pragmatic randomized controlled trial in South Africa examined the additional impact of IPT in patients already receiving ART (39). Although not designed or powered to detect posttherapy differences, there was weak evidence to suggest that the effect of IPT persisted during the first year posttherapy, longer than seen in previous trials comparing 6 mo of IPT with an alternative (4–8). Even though this effect appeared to wane subsequently, it could suggest that posttherapy TB incidence was more likely due to rapid reinfection and progression to disease, and less due to suboptimal cure. However, despite the tremendous success of ART roll-out, access to ART for HIV-positive individuals is far from universal, and an estimated 15 out of 20 million HIV-positive individuals in sub-Saharan Africa are not yet receiving ART (40). Initiation of ART is often at low CD4 counts, and even after years of successful ART, an increased risk of TB persists, strongly suggesting that the immune system never fully recovers (41). In short, our results will continue to be relevant for clinical care for a large part of the HIV-positive community for the foreseeable future.
If IPT does not cure, what are the policy implications for TB/HIV care? We suggest that this is dependent on the local epidemiological situation. In areas with high-TB-incidence settings the risk of reinfection is substantial. Even if a preventive regimen would cure LTBI, the effect would not be durable posttherapy as the risk of reinfection disease dominates. Here, continuous preventive therapy would be most appropriate, and IPT is currently the only tested candidate for this purpose (7). If the risk of reinfection is sufficiently low, a more curative regimen, e.g., one including rifampicin or rifapentine as recently implemented in the United States (42), would be more appropriate as curing LTBI would have long-lasting benefits for patient and population. As TB control progresses, it will be key for policy makers to know when to switch between strategies.
Conclusion
Our results suggest that in HIV-positive, TST-positive individuals, IPT does not cure LTBI, whereas rifampicin- or rifapentine-containing regimens show a stronger curative ability. In settings with ongoing high risks of M. tuberculosis reinfection, durable protection from TB will require continuous preventive therapy. Where reinfection rates are lower, preventive regimens with better curative potential may have advantages both for individuals and for populations.
Supplementary Material
Acknowledgments
This work was funded by Bill and Melinda Gates Foundation [TB Modelling and Analysis Consortium: Grants 21675/OPP1084276 (to R.M.G.J.H. and R.G.W.) and Consortium to Respond Effectively to the AIDS/TB Epidemic: Grant 19790.01 (to T.S., A.D.G., and R.G.W.)].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317660111/-/DCSupplemental.
References
- 1.World Health Organization . Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings. World Health Org; 2011. [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;(1):CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins RE, Brennan R, Plant AJ. Tuberculin reactivity and the risk of tuberculosis: A review. Int J Tuberc Lung Dis. 2000;4(10):895–903. [PubMed] [Google Scholar]
- 4.Johnson JL, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15(16):2137–2147. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 5.Hawken MP, et al. Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: Results of a randomized controlled trial. AIDS. 1997;11(7):875–882. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Martinson NA, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samandari T, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: A randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 8. Samandari T, et al. (2012) TB incidence increase after cessation of 36 months’ isoniazid Prophylaxis in HIV. Conference on Retroviruses and Opportunistic Infections (abstr 147). Available at www.croi2014.org/past_croi_conferences. Accessed June 1, 2013.
- 9.Churchyard GJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370(4):301–310. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 10.Styblo K. Epidemiology of Tuberculosis, Selected Papers. The Hague: Royal Netherlands Tuberculosis Assoc; 1991. [Google Scholar]
- 11.Williams BG, et al. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci USA. 2010;107(45):19485–19489. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams BG, et al. HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis. 2006;194(10):1450–1458. doi: 10.1086/508206. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd Ed. Malden, MA: Blackwell Science; 2003. [Google Scholar]
- 14.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection. An update. Ann Intern Med. 2008;149(3):177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10(11):1192–1204. [PubMed] [Google Scholar]
- 16.Vynnycky E, Fine PE. The natural history of tuberculosis: The implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119(2):183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland I, Svandová E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli. 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;63(4):255–268. doi: 10.1016/s0041-3879(82)80013-5. [DOI] [PubMed] [Google Scholar]
- 18.Houben RMGJ, et al. HIV increases the risk of TB due to recent infection more than that due to reactivation of latent infection. Int J Tuberc Lung Dis. 2011;15(1):24–31. [PubMed] [Google Scholar]
- 19.Houben RM, et al. Human immunodeficiency virus increases the risk of tuberculosis due to recent re-infection in individuals with latent infection. Int J Tuberc Lung Dis. 2010;14(7):909–915. [PMC free article] [PubMed] [Google Scholar]
- 20.Crampin AC, et al. Recurrent TB: Relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS. 2010;24(3):417–426. doi: 10.1097/QAD.0b013e32832f51cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonnenberg P, et al. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: A cohort study in South African mineworkers. Lancet. 2001;358(9294):1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team (2013) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna) [Google Scholar]
- 23.Verver S, et al. Transmission of tuberculosis in a high incidence urban community in South Africa. Int J Epidemiol. 2004;33(2):351–357. doi: 10.1093/ije/dyh021. [DOI] [PubMed] [Google Scholar]
- 24.Glynn JR, et al. The importance of recent infection with Mycobacterium tuberculosis in an area with high HIV prevalence: A long-term molecular epidemiological study in Northern Malawi. J Infect Dis. 2005;192(3):480–487. doi: 10.1086/431517. [DOI] [PubMed] [Google Scholar]
- 25.Lord J, Asante MA. Estimating uncertainty ranges for costs by the bootstrap procedure combined with probabilistic sensitivity analysis. Health Econ. 1999;8(4):323–333. doi: 10.1002/(sici)1099-1050(199906)8:4<323::aid-hec431>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3(10):847–850. [PubMed] [Google Scholar]
- 27.Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: A final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979;119(5):827–830. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- 28.International Union Against Tuberculosis Committee on Prophylaxis Efficacy of various durations of isoniazid preventive therapy for tuberculosis: Five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60(4):555–564. [PMC free article] [PubMed] [Google Scholar]
- 29.Esmail H, Barry CE, 3rd, Wilkinson RJ. Understanding latent tuberculosis: The key to improved diagnostic and novel treatment strategies. Drug Discov Today. 2012;17(9-10):514–521. doi: 10.1016/j.drudis.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grosset J, et al. Modeling early bactericidal activity in murine tuberculosis provides insights into the activity of isoniazid and pyrazinamide. Proc Natl Acad Sci USA. 2012;109(37):15001–15005. doi: 10.1073/pnas.1203636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167(10):1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 32.Leung CC, Rieder HL, Lange C, Yew WW. Treatment of latent infection with Mycobacterium tuberculosis: Update 2010. Eur Respir J. 2011;37(3):690–711. doi: 10.1183/09031936.00079310. [DOI] [PubMed] [Google Scholar]
- 33.Grant AD, Fielding KL, Charalambous S, Chaisson RE, Churchyard GJ. Why have trials of isoniazid preventive therapy among people with HIV infection not demonstrated an effect on mortality?: Did close examination of the trees obscure our view of the wood? AIDS. 2010;24(Suppl 5):S15–S18. doi: 10.1097/01.aids.0000391011.40892.ff. [DOI] [PubMed] [Google Scholar]
- 34.Githui W, et al. Cohort study of HIV-positive and HIV-negative tuberculosis, Nairobi, Kenya: Comparison of bacteriological results. Tubercle Lung Dis. 1992;73(4):203–209. doi: 10.1016/0962-8479(92)90087-Z. [DOI] [PubMed] [Google Scholar]
- 35.Lukoye D, et al. Rates of anti-tuberculosis drug resistance in Kampala-Uganda are low and not associated with HIV infection. PLoS ONE. 2011;6(1):e16130. doi: 10.1371/journal.pone.0016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bretzel G, et al. Anti-tuberculosis drug resistance surveillance in Uganda 1996–1997. Int J Tuberc Lung Dis. 1999;3(9):810–815. [PubMed] [Google Scholar]
- 37.Lukoye D, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: Results of the first national survey. PLoS ONE. 2013;8(8):e70763. doi: 10.1371/journal.pone.0070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12(5):744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rangaka MX, et al. (2012) Randomized controlled trial of isoniazid preventive therapy in HIV-infected persons on antiretroviral therapy. XIX International AIDS Conference (abstr THLBB03). Available at http://pag.aids2012.org/Abstracts.aspx?SID=16&AID=21471. Accessed March 11, 2014.
- 40.UNAIDS . Global HIV/AIDS Response—Epidemic update and health sector progress towards Universal Access—Progress Report 2011. Geneva: World Health Org; 2011. [Google Scholar]
- 41.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: Comparison with rates in the community. PLoS ONE. 2012;7(3):e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60(48):1650–1653. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.