Abstract
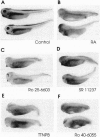
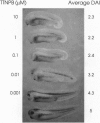
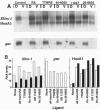
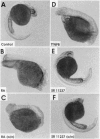
Retinoids exert pleiotropic effects on the development of vertebrates through the action of retinoic acid receptors (RAR) and retinoid X receptors (RXR). We have investigated the effect of synthetic retinoids selective for RXR and RAR on the development of Xenopus and zebrafish embryos. In Xenopus, both ligands selective for RAR and RXR caused striking malformations along the anterior-posterior axis, whereas in zebrafish only ligands specific for RAR caused embryonic malformations. In Xenopus, RAR- and RXR-selective ligands regulated the expression of the Xlim-1, gsc, and HoxA1 genes similarly as all-trans-retinoic acid. Nevertheless, RXR-selective ligands activated only an RXR responsive reporter but not an RAR responsive reporter introduced by microinjection into the Xenopus embryo, consistent with our failure to detect conversion of an RXR-selective ligand to different derivatives in the embryo. These results suggest that Xenopus embryos possess a unique response pathway in which liganded RXR can control gene expression. Our observations further illustrate the divergence in retinoid responsiveness between different vertebrate species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel C., Bauer F., Crettaz M., Forni L., Kamber M., Kaufmann F., LeMotte P., Pirson W., Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aström A., Pettersson U., Krust A., Chambon P., Voorhees J. J. Retinoic acid and synthetic analogs differentially activate retinoic acid receptor dependent transcription. Biochem Biophys Res Commun. 1990 Nov 30;173(1):339–345. doi: 10.1016/s0006-291x(05)81062-9. [DOI] [PubMed] [Google Scholar]
- Bernard B. A., Bernardon J. M., Delescluse C., Martin B., Lenoir M. C., Maignan J., Charpentier B., Pilgrim W. R., Reichert U., Shroot B. Identification of synthetic retinoids with selectivity for human nuclear retinoic acid receptor gamma. Biochem Biophys Res Commun. 1992 Jul 31;186(2):977–983. doi: 10.1016/0006-291x(92)90842-9. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Mangelsdorf D. J., Dyck J. A., Bittner D. A., Evans R. M., De Robertis E. M. Multiple retinoid-responsive receptors in a single cell: families of retinoid "X" receptors and retinoic acid receptors in the Xenopus egg. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2321–2325. doi: 10.1073/pnas.89.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. C., 3rd, Kazmer S., Levin A. A., Yen A. Myeloid differentiation and retinoblastoma phosphorylation changes in HL-60 cells induced by retinoic acid receptor- and retinoid X receptor-selective retinoic acid analogs. Blood. 1996 Jan 1;87(1):227–237. [PubMed] [Google Scholar]
- Dawid I. B., Otani H., Curtiss P., Taira M. Regulatory interactions during embryogenesis in Xenopus laevis. C R Acad Sci III. 1993 Sep;316(9):945–958. [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Dreyer C. The pattern of retinoic acid receptor gamma (RAR gamma) expression in normal development of Xenopus laevis and after manipulation of the main body axis. Mech Dev. 1993 Apr;41(1):33–46. doi: 10.1016/0925-4773(93)90053-z. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Umesono K., Chen J., Evans R. M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995 May 19;81(4):541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Joore J., van der Lans G. B., Lanser P. H., Vervaart J. M., Zivkovic D., Speksnijder J. E., Kruijer W. Effects of retinoic acid on the expression of retinoic acid receptors during zebrafish embryogenesis. Mech Dev. 1994 May;46(2):137–150. doi: 10.1016/0925-4773(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Keeble S., Maden M. The relationship among retinoid structure, affinity for retinoic acid-binding protein, and ability to respecify pattern in the regenerating axolotl limb. Dev Biol. 1989 Mar;132(1):26–34. doi: 10.1016/0012-1606(89)90201-7. [DOI] [PubMed] [Google Scholar]
- Kolm P. J., Sive H. L. Regulation of the Xenopus labial homeodomain genes, HoxA1 and HoxD1: activation by retinoids and peptide growth factors. Dev Biol. 1995 Jan;167(1):34–49. doi: 10.1006/dbio.1995.1005. [DOI] [PubMed] [Google Scholar]
- Kraft J. C., Schuh T., Juchau M., Kimelman D. The retinoid X receptor ligand, 9-cis-retinoic acid, is a potential regulator of early Xenopus development. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3067–3071. doi: 10.1073/pnas.91.8.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., DiRenzo J., Boehm M., Sugarman J., Gloss B., Rosenfeld M. G., Heyman R. A., Glass C. K. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994 Oct 6;371(6497):528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Jong L., Fanjul A., Cameron J. F., Lu X. P., Haefner P., Dawson M. I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992 Dec 18;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Medin J. A., Minucci S., Driggers P. H., Lee I. J., Ozato K. Quantitative increases in DNA binding affinity and positional effects determine 9-cis retinoic acid induced activation of the retinoid X receptor beta homodimer. Mol Cell Endocrinol. 1994 Oct;105(1):27–35. doi: 10.1016/0303-7207(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Minucci S., Zand D. J., Dey A., Marks M. S., Nagata T., Grippo J. F., Ozato K. Dominant negative retinoid X receptor beta inhibits retinoic acid-responsive gene regulation in embryonal carcinoma cells. Mol Cell Biol. 1994 Jan;14(1):360–372. doi: 10.1128/mcb.14.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello S., Scita G., Mantovani G., Cavazzini D., Rossi G. L. Retinol bound to cellular retinol-binding protein is a substrate for cytosolic retinoic acid synthesis. J Biol Chem. 1993 Dec 25;268(36):27133–27142. [PubMed] [Google Scholar]
- Papalopulu N., Lovell-Badge R., Krumlauf R. The expression of murine Hox-2 genes is dependent on the differentiation pathway and displays a collinear sensitivity to retinoic acid in F9 cells and Xenopus embryos. Nucleic Acids Res. 1991 Oct 25;19(20):5497–5506. doi: 10.1093/nar/19.20.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T., Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995 Apr 1;9(7):769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. M. Retinoic acid modifies the pattern of cell differentiation in the central nervous system of neurula stage Xenopus embryos. Development. 1991 Aug;112(4):945–958. doi: 10.1242/dev.112.4.945. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Cheng P. F. Retinoic acid perturbs the expression of Xhox.lab genes and alters mesodermal determination in Xenopus laevis. Genes Dev. 1991 Aug;5(8):1321–1332. doi: 10.1101/gad.5.8.1321. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990 Jun;4(6):932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Taira M., Otani H., Jamrich M., Dawid I. B. Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development. 1994 Jun;120(6):1525–1536. doi: 10.1242/dev.120.6.1525. [DOI] [PubMed] [Google Scholar]
- Thaller C., Hofmann C., Eichele G. 9-cis-retinoic acid, a potent inducer of digit pattern duplications in the chick wing bud. Development. 1993 Jul;118(3):957–965. doi: 10.1242/dev.118.3.957. [DOI] [PubMed] [Google Scholar]
- Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995 May 1;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]