Significance
We have resolved a century-long scientific controversy and demonstrated with a 3D biophysical model that infants suck breast milk by subatmospheric pressures and not by mouthing the nipple–areola complex to induce a peristaltic-like extraction mechanism. Analysis of ultrasound (US) movies demonstrated that the anterior tongue, which is wedged between the nipple–areola complex and the lower lips, moves as a rigid body with the cycling motion of the mandible, while the posterior section of the tongue undulates like a peristaltic wave, which is essential for swallowing. The computational simulations of breast-feeding successfully mimicked the dynamic characteristics observed in US imaging and also predicted the subatmospheric pressure required to draw the nipple–areola complex into the infant mouth during latch-on.
Keywords: submental ultrasound, sucking pressure, computational model, fluid-structure interaction
Abstract
How do infants extract milk during breast-feeding? We have resolved a century-long scientific controversy, whether it is sucking of the milk by subatmospheric pressure or mouthing of the nipple–areola complex to induce a peristaltic-like extraction mechanism. Breast-feeding is a dynamic process, which requires coupling between periodic motions of the infant’s jaws, undulation of the tongue, and the breast milk ejection reflex. The physical mechanisms executed by the infant have been intriguing topics. We used an objective and dynamic analysis of ultrasound (US) movie clips acquired during breast-feeding to explore the tongue dynamic characteristics. Then, we developed a new 3D biophysical model of the breast and lactiferous tubes that enables the mimicking of dynamic characteristics observed in US imaging during breast-feeding, and thereby, exploration of the biomechanical aspects of breast-feeding. We have shown, for the first time to our knowledge, that latch-on to draw the nipple–areola complex into the infant mouth, as well as milk extraction during breast-feeding, require development of time-varying subatmospheric pressures within the infant’s oral cavity. Analysis of the US movies clearly demonstrated that tongue motility during breast-feeding was fairly periodic. The anterior tongue, which is wedged between the nipple–areola complex and the lower lips, moves as a rigid body with the cycling motion of the mandible, while the posterior section of the tongue undulates in a pattern similar to a propagating peristaltic wave, which is essential for swallowing.
Breast-feeding is strongly publicized and encouraged by many societies and communities. It is well accepted that breast milk provides the infant both nutrients and immunities required for growth and development during the first months after birth. It is less known that breast-fed infants exercise and prepare their orofacial muscles for future tasks of speaking and chewing (1), and also have higher oxygen saturation than bottle-fed infants (2). Breast-feeding is the outcome of a dynamic synchronization between oscillation of the infant’s mandible, rhythmic motility of the tongue, and the breast milk ejection reflex that drives maternal milk toward the nipple outlet. First, the infant latches onto the breast and nipple so that the nipple, areola, and underlying mammary tissue and lactiferous ducts are drawn into the infant’s mouth with the nipple tip extended as far as the hard–soft palate junction (HSPJ). Then, the infant moves its mandible up and down, compressing the areola and the underlying lactiferous ducts with its gums in a suckling process that extracts the milk into its mouth (3, 4). Simultaneous with compression, spontaneous undulating motions of the infant tongue channel the milk posteriorly and trigger the swallowing reflex (5). During breast-feeding, suckling, swallowing, and breathing are coordinated by the central nervous system in a way that allows for the infant’s continuous feeding without breathing interruptions (2, 6, 7).
The physical mechanisms that enable the infant to extract milk from the breast have intrigued scientists for more than a century (8). The two proposed mechanisms that have been a subject of scientific controversy to this day are (i) sucking—emptying of the nipple–breast contents by development of subatmospheric pressures within the infant oral cavity (9–12) and (ii) mouthing—squeezing out of the nipple–areola contents by compression between the jaws or other mouth parts (3). With the appearance of cine–X-ray and ultrasound (US) imaging modalities, a significant role was also attributed to tongue undulation which was naturally referred to as “tongue peristalsis” while chewing the nipple (13, 14). However, advanced computational modeling has not yet been used along with imaging data to perform hypothesis testing on the underlying explanations of the suckling behavior during breast-feeding.
We have explored the physical aspects of infant feeding via noninvasive visualizations of the moving components in the oral cavity and a biophysical model. An objective dynamic analysis of submental US imaging of the midsagittal cross-section of the oral cavity during infant feeding was used to study the dynamic characteristics of tongue motion with respect to the rigid upper palate. A 3D fluid–structure interaction (FSI) biophysical model was developed to simulate milk extraction during breast-feeding.
Results
Tongue Motion During Breast-Feeding.
Midsagittal submental US movie clips recorded during infant breast-feeding were dynamically analyzed. The tracked contours representing the palate (in red in Fig. 1A) and the upper surface of the tongue (in green in Fig. 1A) were tracked on each frame (Figs. S1–S3) and are demonstrated for several frames (Fig. 1A). The tip of the tongue is not visible on submental US images of breast-feeding. Assembling the results from all frames (about 150) of the movie clip on a single plane revealed that both contours moved with time within the image plan (Fig. 1B). These movements may be due to time-dependent deformations of the palate and tongue, as well as movements of the mother, infant, and technician during data acquisition. Because the hard palate is rigid, its contour does not change during US imaging and can be used for rigid registration of all of the images (15) to eliminate the noise induced by movements not related to breast-feeding. After rigid registration, the tongue and palate contours are depicted in Fig. 1C. A movie clip of the original US recording and the final identified tongue and palate outlines can be found in Movie S1. More examples can be found at www.eng.tau.ac.il/~elad/Lab/movies.html.
Fig. 1.
Tongue motility of a healthy infant during breast-feeding. (A) Submental US images with traced contours of the palate and the tongue’s upper surface in different frames of the movie clip (subject 24). (B) Contours of all of the palate (red) and the tongue (green) from 150 frames of subject 24. (C) The contours of B after rigid registration around the rigid palate and the imposed polar coordinates. (D) Motility of the anterior tongue around anterior coordinates 1–8 after scaling (data from subject 41). The pattern fits the motility of a rigid body. (E) Motility of the posterior tongue around polar coordinates 8, 13, 17, and 22 after scaling (data from subject 41). The pattern fits the motility of a peristaltic wave. (F) Frequency distribution of the tongue contours around all of the polar coordinates. All regions of the tongue have the same dominant frequency of 1.56 Hz (i.e., 0.64 s per suckling cycle) (data from subject 41). The original and processed movie is provided in Movie S1. HSPJ, hard–soft palate junction.
Dynamic analysis of tongue motility during breast-feeding was performed with respect to a system of polar coordinates that was imposed on the registered data in the spatial coordinate system (Fig. 1C). The origin of this system was set at the bottom of the image in the vicinity of the chin surface (16, 17). Tracing the intersections between the palate and tongue contours and the polar coordinates in consecutive images yielded the local time-dependent motility of each structure. Tracing the movements within the hard palate region revealed significant and periodic motility for the tongue (i.e., about 30 pixels), while the palate was randomly oscillating within a band of 4–5 pixels, which represented the noise resulting from the system. The location of the HSPJ on the polar coordinates was identified at a location where the band became wider than 5 pixels as shown by the blue circle on Fig. 1C.
The time-dependent motility of the anterior tongue around polar lines 1–8 collapsed on each other, which denoted a rigid body motion in a direction perpendicular to the hard palate (Fig. 1D). On the other hand, drawing the motility of a more posterior section of the tongue around polar lines 8, 13, 17, and 22 (Fig. 1E) clearly demonstrated an ordered posteriorly spreading of the curves with a phase shift, similar to a pattern of propagating peristaltic waves toward the posterior end of the tongue.
An important outcome of this dynamic analysis is that the anterior part of the tongue oscillated like a rigid body against the rigid palate. This was further supported by analyzing the time delay between lines 1–8, which revealed zero phase shifts. According to the classic theory of breast-feeding, the infant draws the nipple into its mouth, with the areola and the underlying enlarged and branching lactiferous ducts held between the infant’s upper gums. Then, a roller-like peristaltic wave of contraction throughout the surface of the tongue squeezes milk from the ampullae into the esophagus (3, 13). Simultaneous recordings of orokinetogram and pressure near the nipple during breast-feeding similarly led to conclusions that oral activity was associated with nipple squeezing (18, 19). The results shown in Fig. 1D provide objective support that movement of the anterior part of the tongue, which lies under the nipple, is controlled by the periodic movement of the mandible and definitely does not represent a peristaltic wave of contraction. That is to say, the whole nipple is periodically compressed against the hard palate.
Analysis of posterior tongue motility further revealed a peristaltic-type motion toward the esophagus with time delays of about 0.14 s between lines 8 and 22 (Fig. 1E). The time delay was computed with Matlab (MathWorks) “finddelay” function that performed a cross-correlation between two signals of a similar pattern. This peristalsis motility was similar for all subjects, but varied in size and pattern between subjects and was essential for swallowing of the milk extracted from the breast. In this work we tracked the anterior section of the soft palate (i.e., velum) which plays an important role in swallowing (Fig. 1C), but did not analyze its motility. Observation of movie clips of breast-feeding infants revealed mandible movements without motions of the buccinator muscles, which may alter mouth volume and pressure. Hence, the peristalsis of the posterior tongue, as well as changes in mouth volume due to mandible oscillations, are most likely the generators of the pressure fluctuations measured near the nipple tip (12, 19, 20), which was located a few millimeters anterior to the HSPJ (21).
The frequency domain of tongue motility was analyzed by using a fast Fourier transform algorithm on the local time-dependent movements around each polar line (Fig. 1F). The distribution of frequencies was almost the same for all polar lines and the dominant frequency was 1.56 Hz, corresponding to an average time length of 0.64 s per suckling cycle. This means that the whole tongue was undulating with the same frequency; however, the displacements differ along the tongue to yield different types of motions in the anterior and posterior sections. The dynamic characteristics computed from US movie clips recorded from nine healthy infants during nutritive breast-feeding were similar to those depicted in Fig. 1 (Table S1). The dominant frequencies for all of the infants (i.e., aged 14–120 d) revealed suckling rates in the 1.17–1.95 Hz range. It should be noted that the age differences between infants does not change the physical principles used by the infant to extract milk from the breast. It certainly has an effect on the efficiency and process characteristics due to development of the relevant muscles. Measurements with a Doppler US flow transducer installed in the tip of a latex nipple shield in infants aged 5–9 d showed frequencies under 1 Hz for continuous (i.e., nutritive) suckling and about 2 Hz for intermittent (i.e., nonnutritive) suckling (22). It is obvious that using a latex shield between the nipple and the infant mouth does not represent natural breast-feeding and it has been known to cause disturbances (23). Acquisition of orokinetogram signals in 1- to 2-mo-old infants revealed oral movements at frequencies under 1 Hz during continuous suckling and a band of 4–8 Hz for intermittent suckling (19). The differences may be the result of the different experimental techniques.
Nipple Movement During Breast-Feeding.
Observation of cine–X-ray film (13) and US movies recorded during breast-feeding clearly revealed an anterior–posterior movement of the nipple with respect to infants’ mandible oscillations. The nipple is moving posteriorly (i.e., toward the inside of the mouth) while the tongue and mandible are lowered, and anteriorly (i.e., toward the outside of the mouth) when the tongue and mandible are moving upward and compressing the nipple against the hard palate (3, 24). The US movie clips recorded from subjects 4, 6, and 41 (Table S1) clearly revealed the nipple motion, and accordingly we applied the same tracking algorithm and extracted the outline of the nipple tip in each frame, in addition to the tongue and palate contours. The results for subject 41, after registration of the images with respect to the hard palate, are shown in Fig. 2. Aiming to measure the time-dependent displacement of the nipple, we first represented the band of the registered hard palate by a single averaged curve (i.e., black curve in Fig. 2A) and marked the HSPJ obtained earlier. We then identified the nipple tip (i.e., the deepest point the nipple reached in the infant’s mouth) and calculated its distance from the HSPJ as shown in Fig. 2B for eight frames of a complete suckling cycle. A clip of nipple movement during breast-feeding can be found in Movie S2. More examples can be found at www.eng.tau.ac.il/~elad/Lab/movies.html.
Fig. 2.
Nipple movements in the anterior–posterior direction during breast-feeding (data from subject 41). (A) Contours of the tongue (green) and nipple distal surface (purple) after rigid registration around the rigid palate. The black curve represents the average of all contours of the rigid palate. (B) Contours of the palate (red), tongue (green), and nipple (purple) of several frames during a single suckling cycle. The black arrow represents the distance between the nipple tip and the HSPJ (blue circle). (C) The scaled motility of the nipple tip (purple, length of black arrows in B) in comparison with the rigid motility of the anterior tongue (green, average of the motility over lines 1–8 as in Fig. 1D). Nipple movement is provided in Movie S2. HSPJ, hard–soft palate junction.
Once the time-dependent motion of the nipple tip with respect to the HSPJ is known, one can evaluate its dynamics with respect to the anterior part of the tongue, which was found to undulate as a rigid structure. The anterior tongue motility curves were averaged with respect to the hard palate (i.e., lines 1–8 in Fig. 1D), as shown by the green curve in Fig. 2C, and in comparison with the purple curve of the nipple tip motility (i.e., the distance between the nipple tip and HSPJ in Fig. 2B). It should be noted that identification of the nipple tip in US images is sometimes difficult because of increased echogenicity as a result of milk expressed from the breast (24), which may explain the noisy sections on the nipple movement curve. Nevertheless, a very similar pattern between the tongue and nipple movements was also obtained for two other subjects (subjects 4 and 6). The important facts emerging from the results depicted in Fig. 2 were (i) the range of nipple tip motion was about 3.7 mm (25 pixels) with respect to the HSPJ, whereas the range of rigid movement of the anterior tongue was 4.4 mm (30 pixels) with respect to the rigid palate; and (ii) the nipple tip lags about 0.08 s after the anterior tongue motion, which might be due to the viscoelastic properties of the nipple and tongue tissues.
Tongue Motion with Respect to the Mandible.
Tongue undulation during breast-feeding has been the topic of many studies (4–14). Submental US imaging revealed the complex motion of the tongue, as was also demonstrated here. Observation of movie clips of breast-feeding infants revealed steady mandible oscillations without motions of the buccinator muscles. Because both the tongue and the mandible are oscillating during breast-feeding, we were interested in exploring their relative motions. We recently received a movie clip of a cinefilm of radiographic exposure during breast-feeding from the study of Ardran et al. (13), which clearly demonstrated the movement of the nipple, tongue, and mandible. With permission from the Nuffield Department of Obstetrics and Gynecology (University of Oxford, Oxford) we used dynamic analysis to explore the tongue motion with respect to the mandible during breast-feeding. Specifically, we performed rigid registration with respect to the mandible and the tracked outlines of the tongue can be found in Movie S3. The results demonstrated that the rigid movement of the anterior tongue (as demonstrated in Fig. 1D) was dictated by mandible oscillations, while the posterior tongue was undulating as shown in Fig. 1E to facilitate swallowing and coordination with breathing. The anterior tongue, which is wedged between the nipple–areola complex and the lower lips, is continuously moving with the mandible and also slightly moves anteriorly (i.e., outside the mouth) as the mandible moves down (i.e., the mouth opens) and vice versa. This anterior motility complies with the babies’ tongue thrust reflex and may be of major concern in infants with tongue tie.
Physical Model of Milk Extraction During Breast-Feeding.
The physical process by which the infant extracts milk from the breast is still unclear and two theories have been proposed in the absence of noninvasive techniques for experimental validation. The results obtained in this study clearly demonstrated that the anterior tongue moves as a rigid body under the nipple, ruling out the hypothesis of a peristaltic squeezing of the nipple. Other unexplained physical issues are related to the deformation of the nipple and the proximal areola. During latch-on, the infant closes its mouth on the nipple and part of the areola to form a teat in the mouth, which is believed to be two to three times longer than the free lactating nipple (3). Also unexplained is the retraction of the nipple when the mandible and tongue move up and compress the nipple against the palate, and vice versa when the mandible moves down and the volume of the infant’s mouth increases. In the absence of negative mouth pressure, one would expect a reversed pattern of nipple deformation during mandible oscillations.
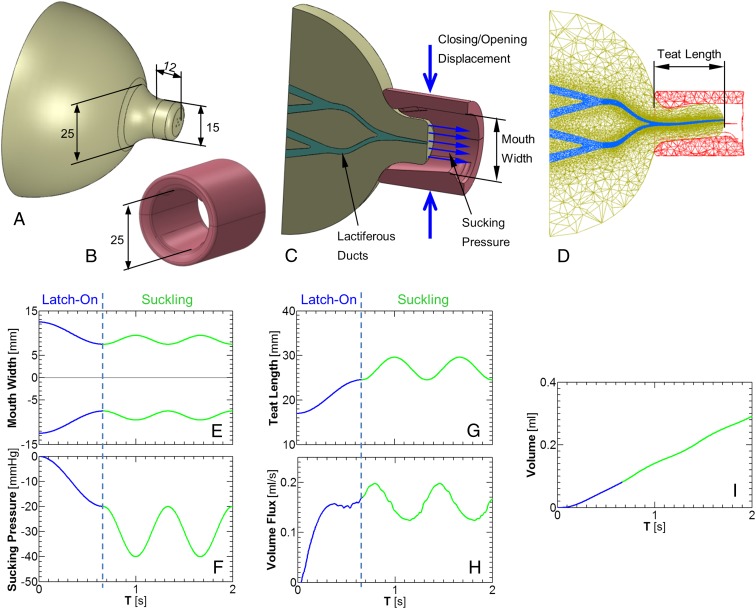
A 3D FSI breast-feeding model was developed to obtain better insights into the mechanical aspects occurring during breast-feeding. For this purpose we developed an elastic breast model (Fig. 3A) that represented a lactating breast with an areola and a 12-mm long nipple with a diameter of 15 mm (25). A complete lobe of branching lactiferous tubes running from the proximal alveoli toward a single outlet in the nipple tip was simulated by a branching network of tubes that ended at an outlet 0.7 mm diameter (Fig. 3C and Fig. S4) (26–29). The material elasticity of the breast, including nipple and areola, was assumed to behave as a Mooney–Rivlin hyperelastic material with coefficients that fit in vitro force-deformation tests of breast tissue (30). The infant mouth, including the tongue and gum, at maximal opening before starting to latch-on the breast was modeled as an elastic cylinder made of two halves with 25-mm inner diameters and 30 mm long (Fig. 3B). The gum was assumed to bulge 1.9 mm from the hard palate. Following the analysis of the cine–X-ray movie clip, the tongue was assumed to be connected to the lower jaw, whereas the proximal part of the tongue was 3 mm higher than its distal part. The closure of the mouth was obtained by applying vertical forces via the compression of the cylinder by two opposing flat plates (Fig. 3C). Mouth elasticity was much stiffer than that of the nipple–areola complex but allowed partial closure when subjected to the opposing vertical forces. We assumed that milk is available in the breast or supplied as needed in the simulation of breast-feeding as a result of let-down reflux.
Fig. 3.
Physical model of milk extraction during breast-feeding. (A) The breast model. (B) The infant mouth model at maximal opening. (C) Sagittal cross-section of the infant mouth attached to the breast just at the beginning of latch-on with arrows to represent the closing–opening displacements and the subatmospheric sucking pressure. (D) Sagittal cross-section of the infant mouth and the breast just at the end of latch-on. (E) Vertical maximal width of the infant mouth during latch-on and suckling. (F) Sucking subatmospheric pressure during latch-on and suckling. (G) The teat length (as shown in D) during latch-on and suckling. (H) Volume flux of milk extracted by the infant during latch-on and suckling. (I) Accumulated milk during the simulation shown here (i.e., latch-on and two suckling cycles), obtained by integration of the data in H.
Simulation of the infant feeding on the mother’s breast was performed in two stages: first, latch-on to the nipple–areola region to extend the nipple–areola complex into the infant’s oral cavity, followed by periodic suckling maneuvers by the infant’s mandible while holding the skull and palate stationary and the mouth sealed to the breast. Explorations of the physical forces that the infant exerts on the breast to extract milk were performed for a reference breast model with a single lobe of branching lactiferous tubes and at a suckling rate of 1.5 Hz. For simulation of latch-on, we assumed mouth closure from a vertical opening of 25 mm to 15 mm with simultaneous generation of a subatmospheric sucking pressure of −20 mmHg in the infant mouth (blue curves in Fig. 3 E and F). Then, for nutritive suckling, we assumed periodic cycles of mouth opening/closing within a range of 5 mm (i.e., between 15 and 20 mm) and simultaneously synchronized with the subatmospheric pressure cycling between −20 and −40 mmHg (green curves in Fig. 3 E and F). Throughout the simulations we assumed full contact between the mouth model “lips” and the breast areola to ensure the mouth sealing observed during in vivo breast-feeding.
Simulations of the latch-on phase during mouth closure on the nipple and part of the areola revealed the generation of a 24.5-mm-long teat within the infant’s mouth, which is twice the length of the free nipple (i.e., 12 mm), for a subatmospheric pressure of −20 mmHg (Fig. 3 D and G). Reducing the subatmospheric pressure to −10 mmHg reduced the teat length to 21.5 mm and a larger subatmospheric pressure of −30 mmHg increased the teat length to 27.4 mm. For a more flexible nipple–areola tissue, the length of the teat will be larger and vice versa for stiffer tissue.
The simulation of breast-feeding for the reference case, while the infant performed cyclic mouthing (i.e., mandible oscillations) and periodic sucking (i.e., oscillatory subatmospheric pressure) depicted by the green curves in Fig. 3 E and F, revealed anterior–posterior cyclic displacements of the nipple tip in the 4.9 mm range and a breast milk flow rate of 0.105 mL per cycle of suckling (Fig. 3 G and H and Movie S4). The simulated motion of the nipple tip with respect to mouth opening/closing was similar to the observations in US imaging (Fig. 2C and Movie S1). The accumulation of milk volume with time is demonstrated in Fig. 3I, which is similar to Woolridge’s measurements (22).
Several simulations with single changes with respect to the reference case were conducted to explore the relative contribution of mouthing and sucking. Simulation of mouthing only (as in Fig. 3E) while a latch-on subatmospheric pressure of −20 mmHg was held constant revealed a flow of 0.116 mL per cycle. Leaving the mouth closed at 15 mm (as at the end of latch-on) while a latch-on subatmospheric pressure of −20 mmHg was held constant yielded a flow of 0.114 mL per cycle. On the other hand, simulation of mouthing only (i.e., between 25 and 15 mm) without any subatmospheric mouth pressure but with a let-down oscillating pressure of 1 mmHg revealed the significantly lower flow rate of only 0.023 mL per cycle. This analysis clearly demonstrated that the infant can extract milk from the breast simply by applying subatmospheric pressure in its mouth. However, this does not take into account the natural reflexes of swallowing and breathing which require mandible oscillation and tongue undulation. This analysis also mimicked the periodic anterior–posterior displacement of the nipple tip observed in US imaging.
The contribution of breast elasticity and lactiferous tube size were also analyzed. Simulations with a 20% softer breast increased the teat length to 32 mm (i.e., 2.65 times the free nipple length) and reduced the milk flow rate to 0.086 mL per cycle (i.e., 18% reduction) due to reduction of the lactiferous ducts cross-sectional areas. Assuming a 20% stiffer breast elasticity yielded a milk flow of 0.116 mL per cycle, which is 10% greater than the reference case. This pattern was expected due to the collapse of the lactiferous tubes when the mouth closed and because of subatmospheric pressures. Simulating a model with a branching network of tubes that ended at an outlet of 0.5 mm diameter (instead of 0.7 mm) yielded a milk volume of 0.068 mL per cycle during a single nutritive suckling. It should be noted that the milk flow rate for a rigid model of the lobe of lactiferous tubes subjected to the same subatmospheric pressure oscillations was almost three times greater, which clearly indicated that the major resistance to breast milk flow is in the deformed region of the areola–nipple complex.
Repeating the simulation for a breast model with two branching networks that simulated two lobes of lactiferous tubes yielded a milk flow of 0.208 mL per cycle, which was almost twice the flow rate obtained with a single lobe. A more complex simulation of a more realistic breast with five lobes of branching lactiferous tubes with five outlets in the nipple tip (Fig. S5 and Movie S5) yielded the same anterior–posterior motion of the nipple and milk outflow rate of 0.513 mL per cycle, which is almost five times the flow obtained with a single lobe. The existing data on the flow rate of milk intake by infants during breast-feeding were measured either with a special Doppler US device (22) or by weighing the infants before and after breast-feeding (11, 31) and varied in the range of 0.03 to 0.5 mL per cycle of sucking. Obviously, a wide variability of the amount of the extracted milk is expected due to the functional variability of the breast (e.g., elasticity, number and structure of lobes, and number and size of outlets at the nipple tip), as well as the mode and level of driving forces exerted by the infant during breast-feeding. We clearly demonstrated that changing the characteristics of the lactiferous network, breast elasticity, and the infant’s suckling forces leads to the range of variability that was measured in vivo.
Discussion
Breast-feeding requires synchronized manipulation of the jaws, tongue, and lips. The employment of an objective and dynamic analysis of US movie clips acquired during breast-feeding, as well as a physical simulation with a complex biomechanical model, revealed the dynamic characteristics of mouth structures and the exerted forces required for milk extraction during breast-feeding. It has been known for decades that breast-feeding required an infant’s latch-on to the nipple–areola complex, which is then transformed into a long teat in the infant’s mouth, and then suckling, during which the teat moves posteriorly into the mouth during the mouth’s opening (i.e., mandible moving down) and vice versa during the mouth’s closure (i.e., mandible moving up). We demonstrated that transforming the nipple–areola complex into a teat twice as long as the free lactating nipple requires closure of the infant’s lips on the breast and the development of a subatmospheric pressure of about −20 mmHg. This pressure must be maintained throughout breast-feeding to continuously keep the teat in the infant’s mouth. Once the infant starts suckling on the nipple, its anterior tongue moves like a rigid body due to the cycling motion of the mandible. We have shown via the complex biophysical simulation that milk extraction from the breast was the result of cycling subatmospheric pressures within the infant’s mouth in the range of −20 to −40 mmHg and not due to the chewing of the breast nipple.
In view of the results of the present study it is worth noting the valuable observations and critical speculations reported in earlier studies (9, 13). Hytten (9) showed that sucking was associated with a relatively small swing of subatmospheric mouth pressures of about 10 mmHg, with maximal sucking pressure lower than 50 mmHg. Hytten (9) further demonstrated that using an undamped manometer and a pin-hole teat yielded violent pressure oscillations when the infant’s tongue sealed off the teat (or tube) and that a valve-like action caused negative pressures that greatly exceeded the true intraoral pressure. Ardran et al. (13) studied many cineradiographic films via observation and provided accurate descriptions of tongue and nipple motions. The present study further supports his observations and his proposed mechanism with an accurate physical model.
Our study demonstrated a new method that allows exploration of the tongue kinematics from recorded US images and then modeling milk extraction from the breast during breast-feeding. The important outcomes of this work are the subatmospheric pressure oscillations to suck milk and the motility pattern of the anterior tongue. Visual observation of infants during breast-feeding revealed very relaxed oscillations of the mandible without buccal motions and almost no effort. Thus, the subatmospheric pressure oscillations required to extract milk from the breast are most likely generated by changes in mouth volumes due to the mandible oscillations and the posterior tongue peristalsis. The anterior tongue is moving as a rigid body that follows posterior undulations.
Methods
US Imaging.
Midsagittal submental US imaging of infants during breast-feeding was performed with a portable GE Vivid i US system using a high multifrequency (5–8 MHz) transducer with a single focal zone and a small convex curved face. The study was approved by the Ethical Review Board of the Tel Aviv Sourasky Medical Center (0498–10-TLV). The parents were informed of all aspects of the procedure, and signed a written informed consent form. The best observable sequence of four to six suckling cycles of nutritive breast-feeding was selected for the analysis. The palate and tongue contours in each frame were tracked using an active contour model (32, 33). Dynamic analysis was performed after rigid registration of all of the images (15) and by superimposing a polar grid system (16, 17).
Biophysical Model.
Simulations of infant breast-feeding were performed with computational tools for FSI that consider the physical deformation of the nipple–breast complex simultaneously with milk flow in the lactiferous tubes. The physics of the problem is controlled by the simultaneous solution of the dynamic governing equations of the motion and deformation of both the fluid and the structure while coupled with boundary conditions. The 3D models were discretized into thousands of elements and simultaneously solved on a high-speed computer using Automatic Dynamic Incremental Nonlinear Analysis (ADINA) commercial software. Details of the model are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank A. Jaffa for establishing the connection between the clinicians and bioengineers who enabled this study, S. Einav for continuous support and productive discussions, and U. Zaretsky for helping design the complex 3D model of the breast and lactiferous tube networks. We also thank D. Flohr and B. Gambash for conducting the movie analysis at the preliminary stages of the project and Y. Nadlin-Carmeli for editorial assistance with some sections in the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319798111/-/DCSupplemental.
References
- 1.Palmer B. The influence of breastfeeding on the development of the oral cavity: A commentary. J Hum Lact. 1998;14(2):93–98. doi: 10.1177/089033449801400203. [DOI] [PubMed] [Google Scholar]
- 2.Goldfield EC, Richardson MJ, Lee KG, Margetts S. Coordination of sucking, swallowing, and breathing and oxygen saturation during early infant breast-feeding and bottle-feeding. Pediatr Res. 2006;60(4):450–455. doi: 10.1203/01.pdr.0000238378.24238.9d. [DOI] [PubMed] [Google Scholar]
- 3.Woolridge MW. The ‘anatomy’ of infant sucking. Midwifery. 1986;2(4):164–171. doi: 10.1016/s0266-6138(86)80041-9. [DOI] [PubMed] [Google Scholar]
- 4.Neville MC. Anatomy and physiology of lactation. Pediatr Clin North Am. 2001;48(1):13–34. doi: 10.1016/s0031-3955(05)70283-2. [DOI] [PubMed] [Google Scholar]
- 5.Wagner CL. 2009. Counseling the Breastfeeding Mother. eMedicine. Available at http://emedicine.medscape.com/article/979458-overview. Accessed March 9, 2014.
- 6.Bu’Lock F, Woolridge MW, Baum JD. Development of co-ordination of sucking, swallowing and breathing: Ultrasound study of term and preterm infants. Dev Med Child Neurol. 1990;32(8):669–678. doi: 10.1111/j.1469-8749.1990.tb08427.x. [DOI] [PubMed] [Google Scholar]
- 7.Koenig JS, Davies AM, Thach BT. Coordination of breathing, sucking, and swallowing during bottle feedings in human infants. J Appl Physiol (1985) 1990;69(5):1623–1629. doi: 10.1152/jappl.1990.69.5.1623. [DOI] [PubMed] [Google Scholar]
- 8.Kron RE, Litt M. Fluid mechanics of nutritive sucking behaviour: The suckling infant’s oral apparatus analysed as a hydraulic pump. Med Biol Eng. 1971;9(1):45–60. doi: 10.1007/BF02474404. [DOI] [PubMed] [Google Scholar]
- 9.Hytten FE. Observations on the vitality of the newborn. Arch Dis Child. 1951;26(130):477–486. doi: 10.1136/adc.26.130.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith WL, Erenberg A, Nowak A, Franken EA., Jr Physiology of sucking in the normal term infant using real-time US. Radiology. 1985;156(2):379–381. doi: 10.1148/radiology.156.2.3892576. [DOI] [PubMed] [Google Scholar]
- 11.Prieto CR, et al. Sucking pressure and its relationship to milk transfer during breastfeeding in humans. J Reprod Fertil. 1996;108(1):69–74. doi: 10.1530/jrf.0.1080069. [DOI] [PubMed] [Google Scholar]
- 12.Geddes DT, Kent JC, Mitoulas LR, Hartmann PE. Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum Dev. 2008;84(7):471–477. doi: 10.1016/j.earlhumdev.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Ardran GM, Kemp FH, Lind J. A Cineradiographic study of breast feeding. Br J Radiol. 1958;31(363):156–162. doi: 10.1259/0007-1285-31-363-156. [DOI] [PubMed] [Google Scholar]
- 14.Weber F, Woolridge MW, Baum JD. An ultrasonographic study of the organisation of sucking and swallowing by newborn infants. Dev Med Child Neurol. 1986;28(1):19–24. doi: 10.1111/j.1469-8749.1986.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 15.Song T, Lee VS, Rusinek H, Kaur M, Laine AF. Automatic 4-D registration in dynamic MR renography based on over-complete dyadic wavelet and Fourier transforms. Med Image Comput Comput Assist Interv. 2005;8(Pt 2):205–213. doi: 10.1007/11566489_26. [DOI] [PubMed] [Google Scholar]
- 16.Bressmann T, Uy C, Irish JC. Analysing normal and partial glossectomee tongues using ultrasound. Clin Linguist Phon. 2005;19(1):35–52. doi: 10.1080/02699200410001669834. [DOI] [PubMed] [Google Scholar]
- 17.Stone M. A guide to analysing tongue motion from ultrasound images. Clin Linguist Phon. 2005;19(6-7):455–501. doi: 10.1080/02699200500113558. [DOI] [PubMed] [Google Scholar]
- 18.Lau C, Schanler RJ. Oral motor function in the neonate. Clin Perinatol. 1996;23(2):161–178. [PubMed] [Google Scholar]
- 19.Voloschin LM, Althabe O, Olivé H, Diena V, Repezza B. A new tool for measuring the suckling stimulus during breastfeeding in humans: The orokinetogram and the Fourier series. J Reprod Fertil. 1998;114(2):219–224. doi: 10.1530/jrf.0.1140219. [DOI] [PubMed] [Google Scholar]
- 20.Luther EC, Arballo JC, Sala NL, Cordero Funes JC. Suckling pressure in humans: Relationship to oxytocin-reproducing reflex milk ejection. J Appl Physiol. 1974;36(3):350–353. doi: 10.1152/jappl.1974.36.3.350. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs LA, Dickinson JE, Hart PD, Doherty DA, Faulkner SJ. Normal nipple position in term infants measured on breastfeeding ultrasound. J Hum Lact. 2007;23(1):52–59. doi: 10.1177/0890334406297184. [DOI] [PubMed] [Google Scholar]
- 22.Woolridge MW, How TV, Drewett RF, Rolfe P, Baum JD. The continuous measurement of milk intake at a feed in breast-fed babies. Early Hum Dev. 1982;6(4):365–373. doi: 10.1016/0378-3782(82)90074-3. [DOI] [PubMed] [Google Scholar]
- 23.McKechnie AC, Eglash A. Nipple shields: A review of the literature. Breastfeed Med. 2010;5(6):309–314. doi: 10.1089/bfm.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClellan HL, Sakalidis VS, Hepworth AR, Hartmann PE, Geddes DT. Validation of nipple diameter and tongue movement measurements with B-mode ultrasound during breastfeeding. Ultrasound Med Biol. 2010;36(11):1797–1807. doi: 10.1016/j.ultrasmedbio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Smith WL, Erenberg A, Nowak A. Imaging evaluation of the human nipple during breast-feeding. Am J Dis Child. 1988;142(1):76–78. doi: 10.1001/archpedi.1988.02150010086031. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay DT, Kent JC, Hartmann RA, Hartmann PE. Anatomy of the lactating human breast redefined with ultrasound imaging. J Anat. 2005;206(6):525–534. doi: 10.1111/j.1469-7580.2005.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusby JE, Brachtel EF, Michaelson JS, Koerner FC, Smith BL. Breast duct anatomy in the human nipple: Three-dimensional patterns and clinical implications. Breast Cancer Res Treat. 2007;106(2):171–179. doi: 10.1007/s10549-006-9487-2. [DOI] [PubMed] [Google Scholar]
- 28.Taneri F, et al. Microanatomy of milk ducts in the nipple. Eur Surg Res. 2006;38(6):545–549. doi: 10.1159/000096775. [DOI] [PubMed] [Google Scholar]
- 29.Hassiotou F, Geddes D. Anatomy of the human mammary gland: Current status of knowledge. Clin Anat. 2013;26(1):29–48. doi: 10.1002/ca.22165. [DOI] [PubMed] [Google Scholar]
- 30.Samani A, Plewes D. A method to measure the hyperelastic parameters of ex vivo breast tissue samples. Phys Med Biol. 2004;49(18):4395–4405. doi: 10.1088/0031-9155/49/18/014. [DOI] [PubMed] [Google Scholar]
- 31.Bowen-Jones A, Thompson C, Drewett RF. Milk flow and sucking rates during breast-feeding. Dev Med Child Neurol. 1982;24(5):626–633. doi: 10.1111/j.1469-8749.1982.tb13673.x. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Acton ST. Active contour external force using vector field convolution for image segmentation. IEEE Trans Image Process. 2007;16(8):2096–2106. doi: 10.1109/tip.2007.899601. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Acton ST. Automatic active model initialization via Poisson inverse gradient. IEEE Trans Image Process. 2008;17(8):1406–1420. doi: 10.1109/TIP.2008.925375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.