Abstract
Distortion-product otoacoustic emission (DPOAE) fine structure and component characteristics are reported between 0.75 and 16 kHz in 356 clinically normal hearing human subjects ages 10 to 65 yr. Stimulus tones at 55/40, 65/55, and 75/75 dB SPL were delivered using custom designed drivers and a calibration method that compensated for the depth of insertion of the otoacoustic emission (OAE) probe in the ear canal. DPOAE fine structure depth and spacing were found to be consistent with previous reports with depth varying between 3 and 7 dB and average spacing ratios (f/Δf) between 15 and 25 depending on stimulus level and frequency. In general, fine structure depth increased with increasing frequency, likely due to a diminishing difference between DPOAE component levels. Fine structure spacing became wider with increasing age above 8 kHz. DPOAE components were extracted using the inverse fast Fourier transform method, adhering to a strict signal to noise ratio criterion for clearer interpretation. Component data from four age groups between 18 and 55 yr old were available for the stimulus levels of 75/75 dB SPL. The age groups could be differentiated with greater than 90% accuracy when using the level of the component presumed to originate from the DPOAE characteristic frequency place. This accuracy held even for frequencies at and below 4 kHz where the age groups exhibited similar average hearing thresholds.
INTRODUCTION
Distortion generated in the cochlea in response to two simultaneous pure tones (f1 and f2, f1 < f2), known as distortion-product otoacoustic emissions (DPOAEs), can be recorded in the ear canal at frequencies mathematically related to the stimulus frequencies (Kemp, 1979). Of these, the most extensively studied and clinically used for detection of hearing loss is the DPOAE at the frequency 2f1-f2 (Dorn et al., 1999). DPOAEs, commonly measured at a few stimulus frequency pairs per octave, provide a generally frequency-specific snapshot of overall outer hair cell function. However, when recorded using more closely spaced stimulus frequencies, a pattern of alternating maxima and minima in both DPOAE level and phase, known as fine structure, is revealed (e.g., Heitmann et al., 1998; Talmadge et al., 1999; Dhar et al., 2002). This fine structure is understood to essentially be an interference pattern between multiple components of the DPOAE at 2f1-f2 (e.g., Talmadge et al., 1999). Advancements in DPOAE measurement techniques, such as the use of stimulus tones continuously swept in frequency (Long et al., 2008), allow time-efficient recording of DPOAE fine structure. The recent development of calibration techniques enabling accurate signal delivery at all frequencies (e.g., Scheperle et al., 2008; Lee et al., 2012) allow the extension of these measures to the upper limit of human hearing. Fine structure allows an examination of the interaction between DPOAE components. The components themselves can be approximately extracted using different experimental or signal processing techniques (e.g., Abdala and Dhar, 2012; Heitmann et al., 1998; Vetesnik et al., 2009; Moleti et al., 2012). In this study we leverage such modern techniques of data recording, calibration, and data analysis to characterize DPOAE fine structure and component characteristics up to 16 kHz in a sizeable population with clinically normal-hearing through 4 kHz.
Several studies have confirmed that two primary regions of the cochlea contribute to the 2f1-f2 DPOAE recorded in the ear canal in humans (Kim, 1980; Siegel and Kim, 1982; Gaskill and Brown, 1996; Heitmann et al., 1998; Talmadge et al., 1999). DPOAE fine structure is believed to arise from the interaction between these two components: one from the region where the activity patterns of the stimulus tones overlap on the basilar membrane, and the other from the characteristic frequency (CF) region of the DPOAE (e.g., Talmadge et al., 1999). There also is evidence of additional contributions to the ear canal DPOAE from areas basal to the f2 CF region. The proportion of these basal contributions appears to vary between species and notable contributions in humans are observed at high stimulus levels and low (f2) frequencies (Martin et al., 2011). There can also be contributions from multiple internal reflections between the DPOAE CF region and the basal boundary of the cochlea under certain stimulus conditions (Dhar et al., 2002). Therefore, the DPOAE recorded in the ear canal is the product of a complex symphony of various sources, often contributing significant proportions with variegated magnitude and phase characteristics.
The two DPOAE components from the region of overlap between mechanical disturbances due to the stimulus tones (overlap component) and the DPOAE CF region (CF component) exhibit significantly different phase behavior as a function of frequency. This difference in phase behavior has led to the development of models which hypothesize different generation mechanisms for the two components from the two cochlear regions (Talmadge et al., 1998, 1999; Mauermann et al., 1999; Shera and Guinan, 1999). The phase of the overlap component is relatively invariant as a function of frequency, whereas the phase of the CF component changes rapidly with frequency (steep phase gradient). This difference in phase characteristics between the two components is ultimately responsible for the observation of fine structure as the two components (overlap and CF) cycle in and out of phase as a function of frequency resulting in alternating peaks and valleys. The diverse phase behavior of the two components is also exploited, as will be done in this report, to segregate the two DPOAE components from within the signal recorded in the ear canal (Talmadge et al., 1999; Dhar et al., 2002).
It could be argued that the complex generation process of the DPOAE makes it difficult to interpret and relate to specific cochlear conditions. In turn, analysis of individual components could potentially allow more detailed and interpretable examination of the DPOAE. For example, notches in DPOAE amplitude due to cancellation between various DPOAE components could be misinterpreted as a sign of cochlear malfunction; however, segregation of components could demonstrate that the notch was not present in the amplitude function of either DPOAE component.1 Furthermore, the components may be differentially vulnerable to different pathologies. For example, Rao and Long (2011) reported a greater but reversible impact on the component from the CF region, in one subject, due to acute salicylate consumption. Subtle trends in the maturation of the human cochlea are also evident via DPOAE components—Abdala and Dhar (2010, 2012) have demonstrated a significantly stronger CF component but comparable overlap components in newborns as compared to normal-hearing young adults.
The examination of the influence of various pathologies on DPOAE fine structure has been more extensive than that on DPOAE components. Changes in DPOAE fine structure have been observed prior to changes in overall DPOAE level and interpreted to be early indicators of cochlear pathology (e.g., Mauermann et al., 2004). Attenuation of fine structure following overexposure to noise (Engdahl and Kemp, 1996) as well as aspirin consumption (Brown et al., 1993; Rao and Long, 2011) or cisplatin exposure (McMillan et al., 2012) provides further support for this notion of sensitivity of fine structure to minor insults to the cochlea. Despite these findings, the sensitivity of fine structure to cochlear insult remains unclear as additional attempts to establish the relationship between fine structure and noise-induced changes in the cochlea have failed to yield clear results (e.g., Reuter and Hammershøi, 2007).
The clinical utility of DPOAE fine structure remains uncertain. It could be argued that evaluating fine structure characteristics is potentially problematic as fine structure is the result of an unknown mix of contributions from various components. Proportional but opposing changes to different components could leave the fine structure characteristics observed in the ear canal unchanged even when the constituent parts have been altered. If the clinical utility of fine structure hinges upon the differential vulnerability of different DPOAE components to various pathological conditions, it could be more efficacious to examine the components independently rather than the result of their interaction. Indeed, recent work has started to focus on methods of extraction and exploration of DPOAE component behavior along with overall fine structure characteristics (e.g., Deeter et al., 2009; Abdala and Dhar, 2010, 2012; Rao and Long, 2011). The overall goal of this investigation was to establish the general behavior of fine structure characteristics (number of fine structure periods per 1/3-octave, depth, and spacing) and DPOAE components in a normal-hearing population. We report these behaviors in a large group of subjects between 10 and 65 yr of age. Further, our data extends to f2 frequencies up to 16 kHz thereby satisfying the need for normative data in a frequency range known to be particularly sensitive to decline in cochlear function due to aging and ototoxicity. These data allow a preliminary comparison of the potential clinical performance of fine structure metrics against individual estimates of DPOAE component characteristics. The results should further our understanding of the age-related changes in the processes responsible for DPOAE generation and also aid the continued development of normative ranges for various DPOAE-related measures that can serve as the benchmark for clinical applications.
METHODS
Subjects
Three hundred fifty-six (356) individuals, ranging in age from 10 to 65 yr, were evaluated. Of the total subject pool, 133 were male and 223 were female. When asked to provide racial information, 298 subjects self-identified as Caucasian, 51 as African-American, 41 as Asian, 1 as Native Hawaiian, and 12 declined to answer. The racial and ethnic distribution of subjects was proportional to the population of Cook County in Illinois. Subjects were grouped according to age and fell into one of five categories (refer to Table TABLE I.): 10–21 yr, 22–35 yr, 36–45 yr, 46–55 yr, or 56–65 yr. The age groups were constructed for ease of comparison to previous work from our group (Lee et al., 2012). As organized in Lee et al. (2012), roughly 10-yr intervals were defined starting from the upper age limit (65 yr) of inclusion with slightly larger age intervals defined in the two youngest groups (10–21 yr and 22–35 yr) in order to provide homogenous groups with comparable gender distribution. Measurements were made in one ear, chosen at random, with a visible and healthy tympanic membrane, as evaluated by otoscopy. Immittance measures and clinical audiometry were performed using an Interacoustics AA220 Audiometer and Middle Ear Analyzer, with study inclusion requiring hearing thresholds equal to or less than 20 dB HL (ANSI, 1996) through 4 kHz. Detailed hearing threshold data (0.125 to 20 kHz) for this population have been previously published in Lee et al. (2012; Fig. 2; Table TABLE I.). All testing was conducted in one of two sound treated audiometric booths that each met the current maximum-allowable ambient noise standard (ANSI S3.1–1999, ANSI, 2008). Subjects were paid for their participation and all measurements were conducted in accordance with the guidelines of the Institutional Review Board at Northwestern University.
TABLE I.
Number of subjects in each age category for the full sample as well as the subset available for DPOAE component analysis. Gender distribution of subjects is presented in parentheses.
| Entire sample | Subset (M/F) | |||
|---|---|---|---|---|
| Age group | (M/F) | 75/75 dB SPL | 65/55 dB SPL | 55/40 dB SPL |
| 10–21 yr | 84 (34/50) | 49 (15/34) | 35 (11/24) | 11 (1/10) |
| 22–35 yr | 101 (35/66) | 62 (21/41) | 42 (13/29) | 10 (3/7) |
| 36–45 yr | 54 (27/27) | 22 (9/13) | ||
| 46–55 yr | 63 (20/43) | 15 (2/13) | ||
| 56–65 yr | 54 (17/37) | |||
| 10–65 yr | 356 (133/223) | 148 (47/101) | 77 (24/53) | 21 (4/17) |
Figure 2.
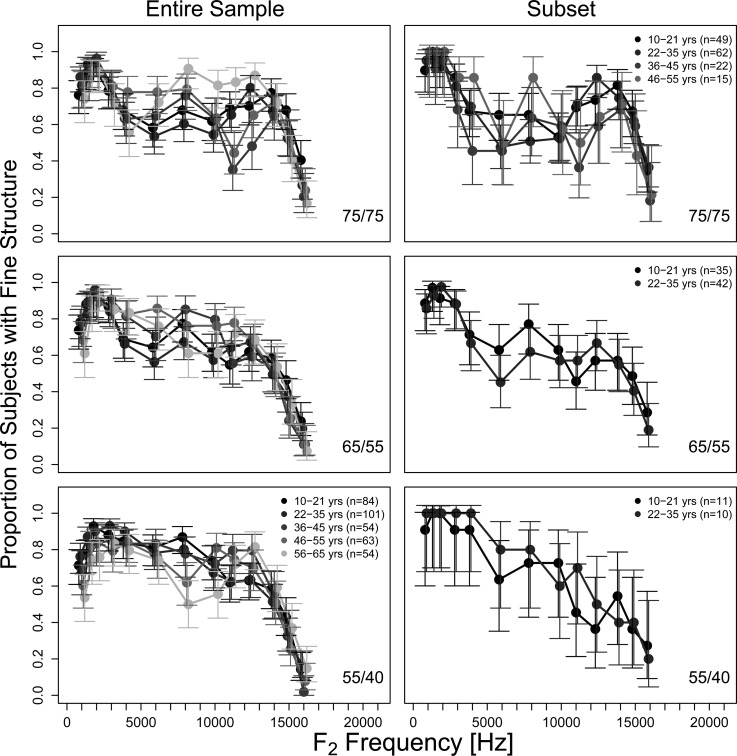
Proportion of the subject pool where fine structure was observable, averaged in 1/3-octave bins and presented as a function of frequency (1 to 16 kHz) for different age groups. Results for the three stimulus level conditions of 75/75, 65/55, and 55/40 dB SPL are displayed in the three rows. The entire sample is displayed in the left column of the figure and the subset of subjects where information was available at least every 6 Hz is in the right column. This resulted in only the youngest two age groups (10–21 yr and 22–35 yr) represented in all three stimulus level conditions. The error bars represent 95% CI for binomial proportions bounded by 0 and 1 [see Eq. 1].
Signal processing and procedure
Signal generation and recording for OAE measurements were done using custom software (developed by C. Talmadge) on an Apple Macintosh computer. A MOTU 828 MkII input/output FireWire device was used for analog-to-digital (44 100 Hz, 24-bits) and digital-to-analog conversion. Generated signals were sent through a custom headphone amplifier to custom-built sound sources containing modified MB Quart 13.01HX tweeters. The sound sources were coupled to the subjects' ear canal via 16 ga plastic tubing connected to an Etymotic Research ER10B+ (Etymōtic Research, Inc., Elk Grove Village, IL) OAE probe and was sealed into the canal using a 13 mm foam ear tip. Signals from the test ear were recorded using the ER10B+ microphone and preamplifier combination, digitized using the MOTU and stored on disk for analysis.
DPOAEs were recorded for a fixed stimulus frequency ratio (f2/f1) of 1.22 for 2f1-f2 frequencies between 0.5 and 10.24 kHz (i.e., f2 between ∼0.75 and 16 kHz). DPOAE recordings were obtained at three stimulus-level combinations (L1 and L2 in dB SPL): 55/40, 65/55, and 75/75. Stimulus tones were swept at 8- and 24-s/octave for 2f1-f2 frequencies below and above 6 kHz with a 2-s silent interval between sweeps consistent with previous work from this laboratory. At least six sweeps were averaged before using a least-squares-fit procedure (Long and Talmadge, 1997; Talmadge et al., 1999; Dhar et al., 2002, 2005; Long et al., 2008) to estimate the level and phase of the DPOAE at the frequency 2f1-f2. The initial analyses resulted in a frequency resolution between 2 and 20 Hz at the lowest and highest frequencies. The noise floor was estimated from an average of the sweeps with every alternate sweep inverted in phase. The median DPOAE level was computed for every three consecutive frequencies and compared against the noise floor estimate for the corresponding center frequency to determine the signal-to-noise ratio (SNR). All data points that did not meet a 6 dB SNR criterion for the three-point median reference were eliminated from further analyses. The use of a three-point median reference helped avoid eliminating points at level minima in order to ensure accurate quantification of DPOAE fine structure depth and spacing.
Calibration
Stimuli used for DPOAE measurements were calibrated using a coupler calibration procedure that allowed for approximate compensation of the depth of insertion in the ear canal (see Lee et al., 2012). In short, the frequency responses of the transducers were measured in a long lossy tube (50 ft long, 0.375 in. outside diameter, copper plumbing tubing) using a slow chirp between 0.2 and 20 kHz. The absence of standing waves in this long tube with its diameter matched to that of the average adult ear canal allows the recording of the combined frequency response of the sound source and the microphone. These frequency responses were also measured in an IEC 711 coupler (Bruel and Kjaer 4157) for various depths of insertion. The response was recorded using the microphone of the OAE probe as well as a Bruel and Kjaer 0.5-in. microphone (BK4134) attached at the distal end of the coupler. The recording obtained using the OAE probe microphone was normalized with that obtained in the long lossy tube at each insertion depth. This normalization resulted in the isolation of the frequency response of the cavity with a half-wave resonance that was related to the depth of insertion. The recording from the BK4134 was used to generate a correction filter for each insertion depth to yield a uniform stimulus level at the distal (“eardrum”) end of the coupler. During experiments, the frequency response for a specific insertion in each subject was measured in the ear canal using a slow chirp between 0.2 and 20 kHz. This response was normalized to that obtained in the long lossy tube and used to detect a half-wave resonance frequency. The compensation function for that particular half-wave frequency was then used to alter the stimuli before delivery to the ear canal.
Characterization of fine structure
DPOAE fine structure features were defined as: number of fine structure periods per 1/3-octave, depth, and spacing. Maxima and minima in DPOAE level were identified by the zero crossings of the first derivative of the level function. The distinction between maxima and minima was made based on the sign of the second derivative of the level function at the zero crossing of the first derivative (see Dhar et al., 2002 for details). Fine structure depth for each period bounded by two maxima or minima was computed as the emission amplitude at the maximum divided by the average amplitude of the two surrounding minima. Only fine structure periods where depth was greater than 2 dB were included in further analyses. Fine structure spacing was quantified by computing the geometric mean (f) between two adjacent minima and dividing by the frequency separation between them (Δf), and referred to as the spacing ratio, f/Δf (Shera, 2003). Only spacing ratios between 4 and 32 (1/3- to 1/20-octave) were included in further analysis. The number of fine structure periods per 1/3-octave was also calculated. Fine structure parameters were averaged by 1/3-octave bands centered at audiometric test frequencies (0.75–16 kHz).
Separation of DPOAE components
Components (overlap and CF) contributing to the DPOAE signal in the ear canal were separated based on group delay from the composite estimate (without additional data processing for SNR) using an inverse fast Fourier transform (IFFT) in a custom matlab analysis program (see Abdala and Dhar, 2012 for details). Once isolated, these individual components were processed through a regular FFT algorithm to obtain independent estimates of magnitude and phase. The DPOAE complex pressure in the frequency domain was multiplied by a moving Hann window in overlapping 50 Hz steps. The Hann window length was adjusted on a logarithmic scale accounting for the cochlear frequency-place map (Greenwood, 1990) and ranged from 400 at the lowest to 1623 Hz at the highest DPOAE frequency. Impulse response (IPR) functions were derived for each window and rectangular time-domain filters applied to each IPR to extract DPOAE components with different delays. Latencies discussed here are based on the phase gradient of the recorded signal, further defined by the analysis filter windows and do not represent true cochlear delays. Specifically, the short-latency (overlap) component was identified as a peak in the amplitude function with two minima within a time window between −2 to 10 ms. Similarly the long-latency (CF) component was identified in a time window between 8 to 15 ms. The filtered windows of data were then transformed back to the frequency domain by FFT and the level and phase of the overlap and CF components were reconstructed.
In order to ensure the validity of the results of the IFFT procedure, only data sets where information was available at least every 6 Hz were included (refer to “subset” in Table TABLE I.). Entire age groups in which there were less than five cases that met this criterion were excluded from this subset analysis (i.e., n = 3 for 36–45 yr at 65/55 and n = 2 for 56–65 yr at 75/75). Data points had been eliminated for failing to meet a 12 dB SNR criterion for the majority of subjects in these age groups. A more stringent 12 dB SNR criterion was adopted for this analysis to avoid ambiguities in the phase data that are particularly sensitive to SNR. Additionally, data at the extreme low and high frequencies were eliminated due to artifactual edge effects inherent to the time-windowing process. IFFT-derived magnitude and phase estimates for the overlap and CF components were averaged into 1/3-octave bins, with these qualification criteria resulting in the data being limited to an f2 range of 1–15 kHz (as opposed to 0.75 and 16 kHz for the fine structure analysis). Additionally, difference scores were calculated by subtracting the CF from the overlap levels in order to examine the relationship between the components.
Statistical procedures
All analyses were carried out using sas version 9.2 (Cary, NC). In figures and data tables throughout, 95% confidence intervals are reported in lieu of standard deviation or standard error, as they are more amenable to clinical comparisons across age groups. Missing error bars in figures represent cases where 95% confidence intervals are too small to observe or could not be calculated due to the availability of only one data point at that particular frequency. Confidence intervals were computed using standard methods except for estimating the proportion of subjects with fine structure. In this case, estimates of the 95% confidence intervals for binomial proportions were calculated using the following formula (Agresti and Coull, 1998):
| (1) |
where
where are the lower and upper bounds of the approximate confidence interval not exceeding 1, z2α/2 is set to 1.96, n1 is the number of cases with observable fine structure, and n is the total number of individuals in the population.
Two-way analyses of variance (ANOVA) (age group × gender), at each frequency and stimulus level combination, were used to evaluate the effect of age and gender on DPOAE fine structure characteristics. A Tukey adjustment was used for multiple comparisons. A mixed effects model was used on DPOAE component factors of level and phase, which accounts for the correlation of individual subjects having repeated measurements for different conditions (i.e., stimulus levels and components).
RESULTS
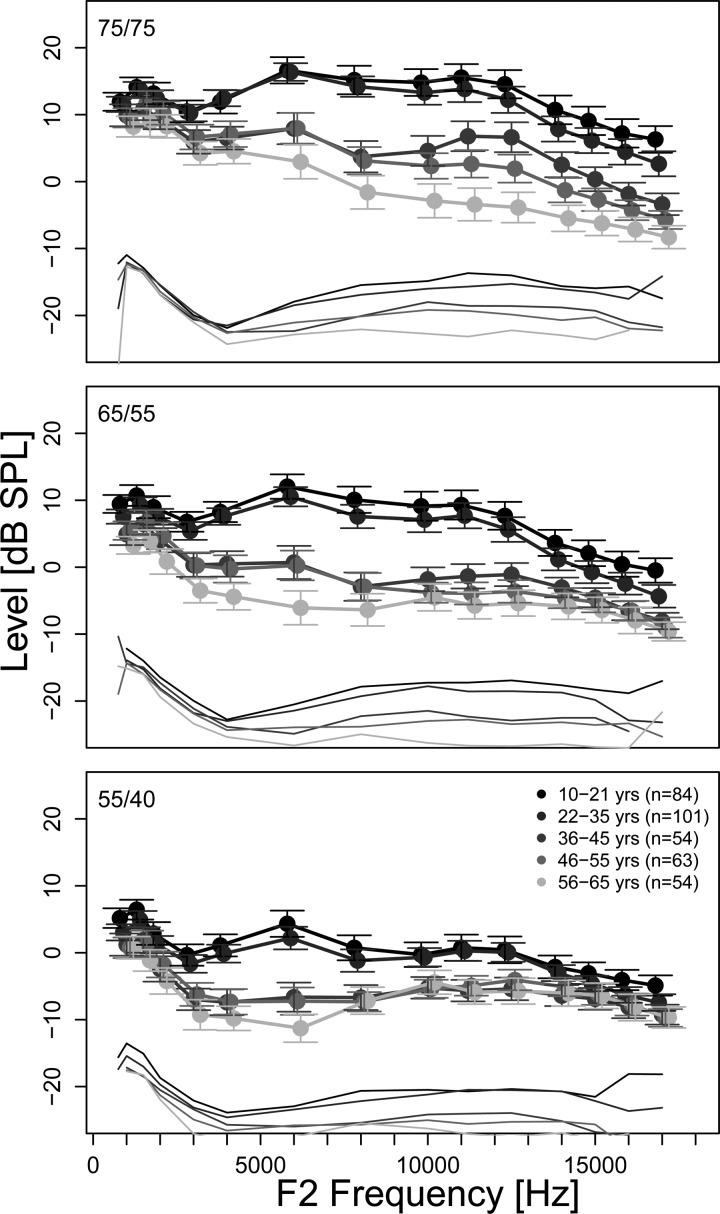
The focus of this investigation was to characterize the 2f1-f2 DPOAE fine structure as well as DPOAE component behavior in a clinically normal hearing population (i.e., hearing thresholds equal to or less than 20 dB HL through 4 kHz). Specifically, the proportion of subjects with observable DPOAE fine structure in the population, number of fine structure periods per 1/3-octave, depth, spacing, and the magnitude and phase of individual DPOAE source components was explored. Average DPOAE level as a function of frequency is shown in Fig. 1 for three stimulus level combinations (75/75, 65/55, and 55/40 dB SPL) for each age group (10–21 yr to 56–65 yr). Overall, higher DPOAE levels were recorded with increasing stimulus levels; but noise floor estimates remained comparable. Higher DPOAE levels were observed for the youngest two age groups (10–21 yr and 22–35 yr) compared to the older age groups (36–45 yr to 56–65 yr) for all stimulus levels (statistical comparisons described later in this section).
Figure 1.
Mean DPOAE level (circles) as a function of frequency for different age groups represented in different shades of gray with average noise floors (think lines). Results for the three stimulus level conditions of 75/75, 65/55, and 55/40 dB SPL are displayed in the three rows. The error bars represent 95% CI. In general, missing error bars signify cases where only one data point was available.
Proportion of subjects with DPOAE fine structure
The proportion of subjects with observable fine structure, for each f2 frequency and stimulus level combination (75/75, 65/55, and 55/40 dB SPL), was calculated in each age group (10–21 yr to 56–65 yr) and is presented in Fig. 2. Only frequencies (1–16 kHz) for which at least one observable fine structure estimate was obtained for each age group were displayed in Figs. 234. No systematic trends in the proportion of subjects with observable fine structure were observed as a function of age. When averaged across frequency, fine structure was observed in approximately half the subjects in each age group. The proportion of subjects with fine structure declined dramatically above approximately 12 kHz in all age groups. For the highest stimulus level (75/75 dB SPL) some differences between the age groups are observable between 5 and 12 kHz. For this stimulus level (75/75 dB SPL) and this frequency range (5–12 kHz), the oldest age group (56–65 yr) appears to have the greatest proportion of subjects exhibiting fine structure (Fig. 2). However, the observed differences were not statistically significant. The data presented in the right column of Fig. 2 represents those subjects who survived the culling criterion of viable data points every 6 Hz. The overall trends seen in the population data (left column) are also observed in the data from this subset.
Figure 3.
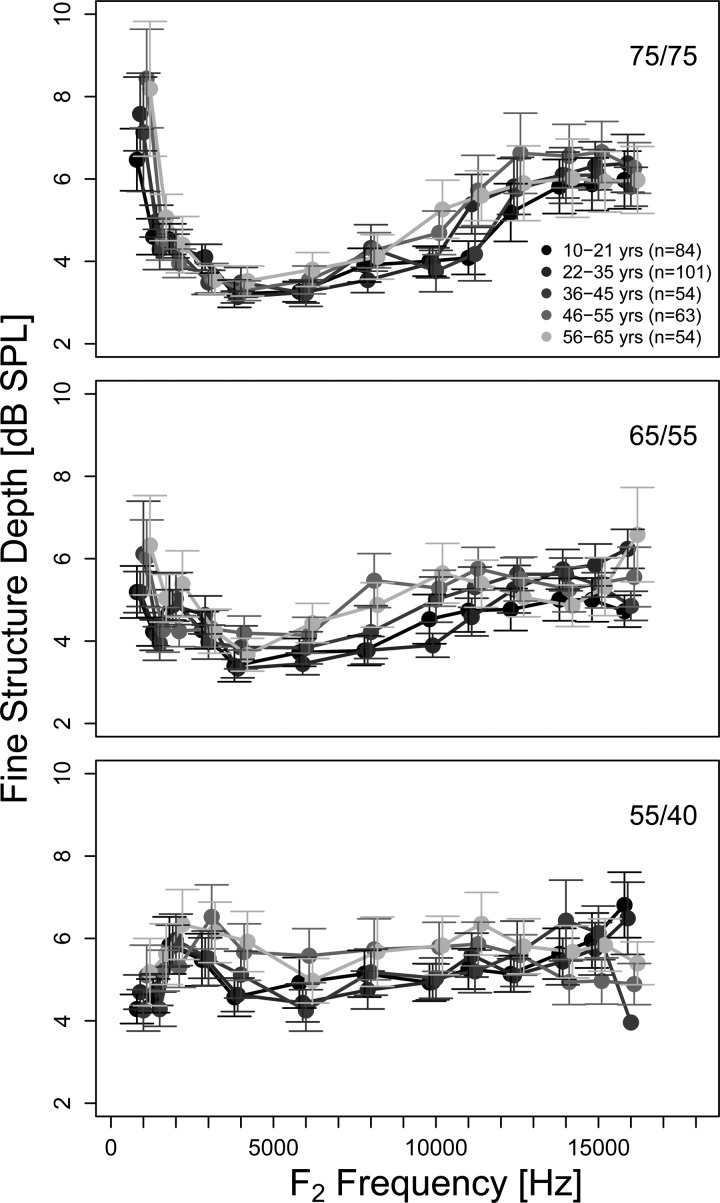
Mean fine structure depth as a function of frequency (1 to 16 kHz) for different age groups presented in a format similar to Fig. 1. In general, missing error bars signify cases where only one data point was available.
Figure 4.
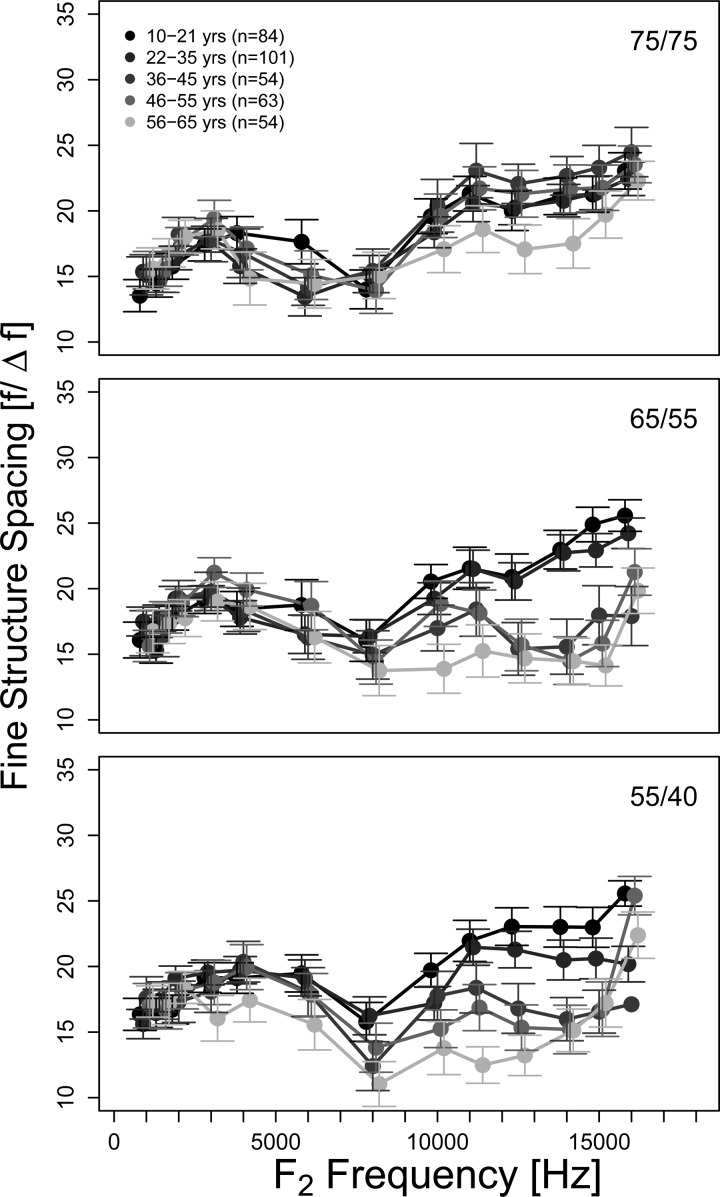
Mean fine structure spacing (f/Δf) as a function of frequency (1–16kHz) for different age groups in a format similar to Fig. 1. The error bars represent 95% CI. In general, missing error bars signify cases where only one data point was available.
DPOAE fine structure characteristics
DPOAE fine structure characteristics: depth, and spacing, and number, were examined as a function of f2 frequency by age group at each stimulus level combination (75/75, 65/55, and 55/40 dB SPL). Average fine structure depth (Fig. 3) across stimulus levels and frequency, was between approximately 4 and 6 dB (range between 2 and 30 dB). Age did not have a statistically reliable effect on fine structure depth at any stimulus level combination. Fine structure depth was generally invariant as a function of frequency in all age groups for the two lower stimulus levels (55/40 and 65/50 dB SPL). A pattern of a peak in fine structure depth at 1 kHz followed by a broad valley and gradually increasing depth as a function of frequency is observed for the highest stimulus level (75/75 dB SPL). However frequency did not have a statistically significant effect on fine structure depth at any stimulus level.
Mean spacing ratio (f/Δf) as a function of f2 frequency within each age group is displayed in Fig. 4, with different stimulus levels represented in each panel. Spacing ratio generally increased with increasing frequency with individual spacing ratio estimates ranging from 4 to 32. Note that this range between 4 and 32 represents the limits of the data accepted for analysis. Therefore the presence of artifacts near the limits of the allowed range cannot be ruled out. Spacing ratio was similar across age groups for all stimulus conditions up to approximately 8 kHz. Some age-dependent trends in spacing ratio were observed above 8 kHz, especially for stimulus levels of 55/40 and 65/55 dB SPL. For these stimulus levels, the spacing ratio was highest (i.e., narrow spacing) for the youngest subjects and gradually decreased with age. The results of statistical comparisons of spacing ratio across age groups are presented below.
The number of DPOAE fine structure periods per 1/3-octave for each age group was examined; however, not plotted here as it is strongly (inversely) correlated to spacing. In general, the number of periods observed peaked at 1 kHz and then gradually decreased with increasing frequency. While as many as four fine structure periods were observed in a 1/3-octave frequency range in some individuals, the mean occurrence across subjects peaked at approximately two periods near 1 kHz and gradually declined to one period near 15 kHz. Neither age nor stimulus level had a significant effect on the number of fine structure periods observed.
The influence of the five age groups and sex (male and female) on DPOAE fine structure characteristics was evaluated with a two-way ANOVA at each frequency (15 frequencies; 0.75–17 kHz) and stimulus level combination (55/40, 65/55, and 75/75 dB SPL). Significant interactions between age group and sex were found for depth of fine structure at 6 (75/75 dB SPL; F4,216 = 3.04, p = 0.0183) and 10 kHz (65/55 dB SPL; F4,224 = 3.60, p = 0.0072), as well as for number of periods at 1.5 kHz (75/75 dB SPL; F4,309 = 3.89, p = 0.0043). Post hoc multiple pairwise comparisons using a Tukey adjustment (p = 0.05) indicated no significant mean difference in fine structure characteristics between male and female subjects within any of the age groups at any stimulus level.
Next, the interaction was removed from the model and the main effect of sex at each stimulus level and frequency combination was examined. Exact p-values comparing main effects of sex at frequencies and stimulus levels that were significant are reported in Table TABLE II.. Differences in fine structure depth, spacing, and the number of periods between the sexes were observed between 1.5 and 11.2 kHz. When statistically significant effects of sex were observed, female subjects generally exhibited deeper, narrower, and a greater number of fine structure periods per 1/3 octave. Although these analyses were performed on data that satisfied a 6-dB SNR criterion (based on a three-point median filter), the influence of differences in SNR between sexes in shaping the fine structure findings cannot be ruled out.
TABLE II.
Significant main effects of gender (p < 0.05) in fine structure characteristics using post-hoc multiple pairwise comparisons after Tukey adjustment. Mean difference scores (females−males) adjusted for age are provided with larger numbers representing greater values for females than males.
| Stimulus level | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 75/75 dB SPL | 65/55 dB SPL | 55/40 dB SPL | |||||||
| Freq. (kHz) | Diff (f−m) | p | Freq. (kHz) | Diff (f−m) | p | Freq. (kHz) | Diff (f−m) | p | |
| DEPTH | 2 | 0.65 | 0.001 | 1.5 | 0.46 | 0.04 | 1.5 | 1.11 | <0.0001 |
| 3 | 0.48 | 0.01 | 2 | 0.93 | 0.001 | 3 | 0.85 | 0.01 | |
| 3 | 0.88 | 0.0003 | 6 | 0.74 | 0.02 | ||||
| SPACE | 4 | 2.06 | 0.03 | 4 | 1.90 | 0.02 | 4 | 1.57 | 0.03 |
| 6 | 2.29 | 0.03 | 6 | 3.01 | 0.001 | ||||
| 8 | 2.17 | 0.02 | |||||||
| NUMBER | 1.5 | 0.18 | 0.02 | 1.5 | 0.33 | 0.0001 | 1.5 | 0.28 | 0.01 |
| 3 | 0.35 | 0.001 | 3 | 0.26 | 0.01 | 3 | 0.22 | 0.03 | |
| 8 | 0.19 | 0.02 | 4 | 0.22 | 0.04 | 4 | 0.33 | 0.002 | |
| 10 | 0.17 | 0.03 | 11.2 | 0.17 | 0.03 | 6 | 0.27 | 0.002 | |
| 8 | 0.21 | 0.01 | |||||||
Comparing fine structure characteristics across age groups using a post hoc multiple pairwise comparison using a Tukey adjustment indicated a significant mean difference in spacing at 55/40 dB SPL at 6 kHz (10–21 yr and 56–65 yr, p = 0.0145; 22–35 yr and 56–65 yr, p = 0.0384) and for the number of periods at 55/40 dB SPL at 3 kHz (22–35 yr and 56–65 yr, p = 0.0224; 10–21 yr and 46–55 yr, p = 0.0419) and 65/55 dB SPL at 4 kHz (36–45 yr and 46–55 yr, p = 0.0302). To draw a general summary, differences in age groups were observed in fine structure spacing and the number of fine structure periods between the two youngest and the oldest age groups between 3 and 8 kHz for the two lower stimulus level combinations (55/40 and 65/55 dB SPL). No significant differences between age groups were observed for depth.
DPOAE components
The ear canal composite DPOAE was separated into the components from the overlap and CF regions and the magnitude and phase estimates of each component were examined. The reader is reminded that in order to ensure the validity of the results of the IFFT procedures, only data sets where information was available at least every 6 Hz were included (refer to Table TABLE I.). This resulted in a smaller number of subjects being evaluated for the component analysis than for the DPOAE fine structure analysis. As another consequence of this strict requirement of data density, a different number of subjects were evaluated for each stimulus level condition (41.6% of the total for 75/75 dB SPL, 21.6% for 65/55 dB SPL, and 5.9% for 55/40 dB SPL). Therefore, not every age group is represented at every stimulus level. In particular, the two youngest groups are represented for all three stimulus levels and the oldest (56–65 yr) is not represented at all.
Component level
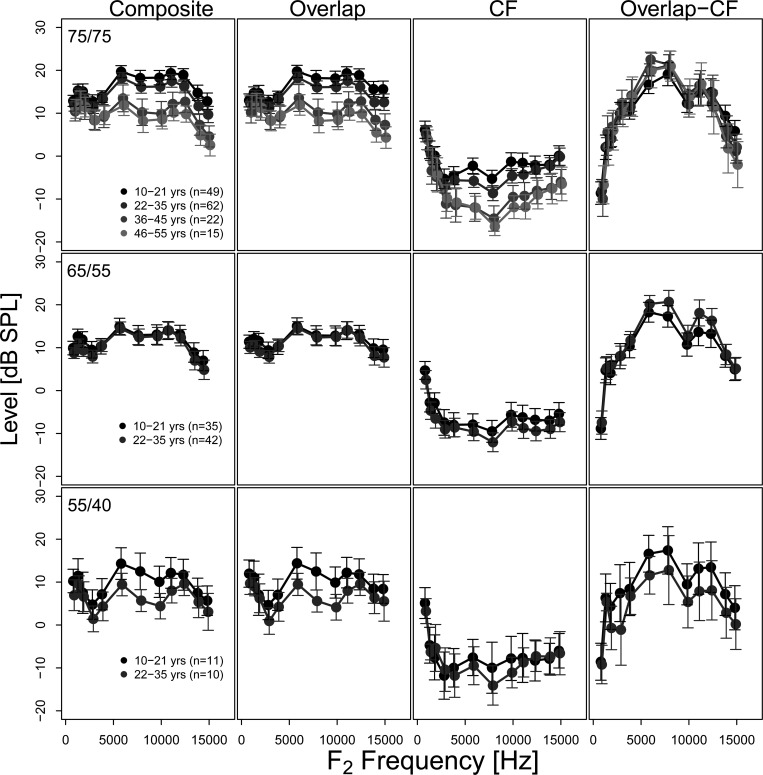
Figure 5 displays the mean composite DPOAE and separated component levels as a function of f2 frequency, for the different age groups in the subset. Measurements made using different stimulus level combinations are presented in the three rows. Average noise floor estimates were similar across age groups (never exceeding −15 dB SPL) and were not included in Fig. 5 for clarity. The composite and individual component levels decrease with increasing age for all stimulus level conditions. The composite DPOAE level could be compared across the four youngest age groups for the stimulus level combination of 75/75 dB SPL. This comparison shows a gradual decrease in the composite DPOAE level with increasing age, in agreement with the data from the full dataset presented in Fig. 1. A similar effect of age is seen in the overlap and CF components in the middle columns of Fig. 5. Data from the two youngest age groups were available for the stimulus levels of 55/40 and 65/55 dB SPL. At these stimulus levels composite and overlap component levels are generally indistinguishable. However, differences can be observed in the CF component level with higher CF component levels observed for the youngest age group. On average the overlap component was larger than the CF level for all age groups and stimulus level combinations.
Figure 5.
Mean composite and component levels and difference scores (overlap-CF component level) as a function of frequency for different age groups. The three stimulus level conditions of 75/75, 65/55, and 55/40 dB SPL are displayed in the three rows. The error bars represent 95% CI. Note that only the data subset where information was available at least every 6 Hz was included. This resulted in only the youngest two age groups (10–21 yr and 22–35 yr) represented in all three stimulus level conditions. Average noise floor estimates were similar across age groups (never exceeding −15 dB SPL) and were not included here for clarity.
A mixed effects model, which accounts for the correlation of individual subjects having repeated measurements for different conditions, was used to examine the composite and component DPOAE levels. Post hoc multiple comparisons using a Tukey adjustment was conducted to determine the proportion of significant pairwise comparisons for composite and component characteristics at each stimulus level. In order to investigate if differences in age groups could be more observable by examining individual DPOAE components, age group separation summary (AGSS) scores representing the proportion of significant pairwise comparisons (number significant pairs divided by the number total comparisons) were derived (Table TABLE III.). Data are presented only for the stimulus level of 75/75 dB SPL, as only two age groups were available for comparison for the other stimulus levels. An AGSS score of 1 would indicate significant differences in all possible comparisons. This was examined for three conditions where composite and component levels were averaged across: (1) all frequencies (1–15 kHz), (2) frequencies less than 6 kHz, and (3) frequencies equal to or greater than 6 kHz. In general, the CF component allowed the most robust separation of age groups at 75/75 dB SPL.
TABLE III.
Age group separation summary (AGSS) scores for composite, overlap, and CF components averaged across: frequencies less than 6 kHz, all frequencies, or frequencies equal or greater than 6 kHz. Data for stimulus levels of 75/75 dB SPL are presented as only two groups were available for comparison for the other stimulus levels. AGSS scores computed using level and phase are presented separately. An AGSS score of 1 would indicate significant differences in all possible comparisons.
| Composite | Overlap | CF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <6 kHz | ALL | ≥6 kHz | <6 kHz | ALL | ≥6 kHz | <6 kHz | ALL | ≥6 kHz | |
| Level | 0.60 | 0.76 | 0.76 | 0.63 | 0.80 | 0.80 | 0.91 | 0.94 | 0.94 |
| Phase | 0.36 | 0.36 | 0.36 | 0.39 | 0.39 | 0.39 | 0.68 | 0.72 | 0.75 |
A secondary analysis, using a mixed effects model, was done to explore the effect of sex on DPOAE component levels. Post hoc multiple comparison using a Tukey adjustment was conducted to determine the proportion of significant pairwise comparisons for each stimulus level and component condition based on sex. AGSS scores were calculated separately for males and females. Both DPOAE components were equivalently dependent on sex, with AGSS scores in 99% agreement (on average) between males and females.
In order to explore whether the relative contribution of the two components changed as a function of stimulus level or frequency, the difference between the overlap and CF component levels was calculated for each subject at each frequency and then averaged across 1/3-octave bands within each subject and across subjects within each age group. Mean difference scores as a function of frequency for the three stimulus levels are shown in Fig. 5 (right column). Difference scores of zero indicate no difference in level between the overlap and CF components. Positive and negative difference scores indicate larger overlap and CF components, respectively. The difference in level between the overlap and CF components was most pronounced in the mid-frequency region between approximately 5 and 10 kHz.
A statistical examination using the mixed effects model revealed no significant effects of stimulus level or frequency on the difference score. Within specific age groups, significant differences in the difference score were only found for the following: between 75/75 and 55/40 dB SPL in 10–21 yr (p = 0.0035) and between 75/75, 65/55, and 55/40 dB SPL in 22–35 yr (p = <0.0001). In general, the difference between component levels increased with increasing stimulus level and was primarily a consequence of increasing overlap component levels.
Component phase
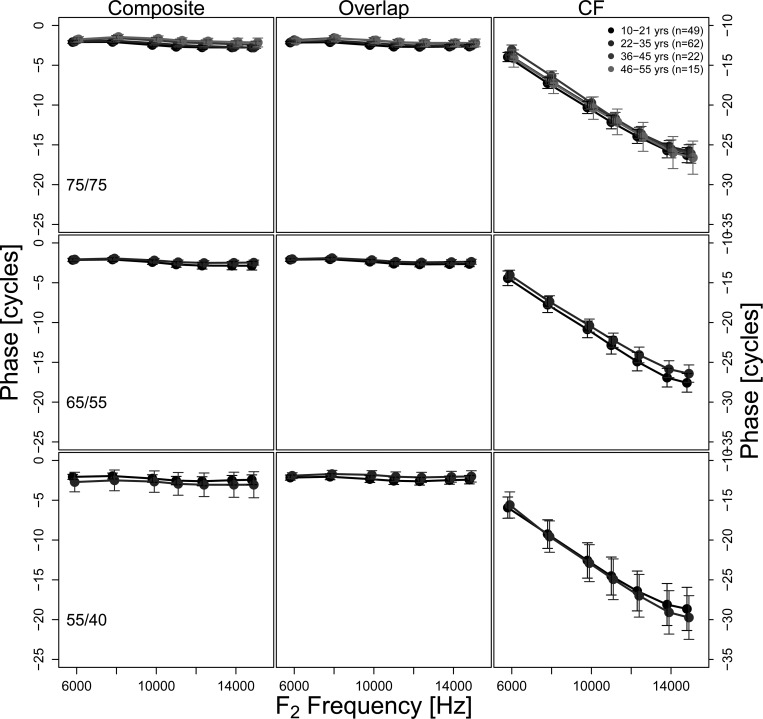
Mean composite DPOAE and component phase as a function of f2 frequency for the different age groups are shown in Fig. 6 for frequencies ≥6 kHz (1–4 kHz was also examined and demonstrated similar results; results between 1 and 4 kHz are not presented graphically as they replicate what is available in the archival literature). Measurements made using different stimulus level combinations are presented in the three rows, with the error bars representing the 95% confidence intervals. Three columns represent phase of the composite DPOAE, overlap component, and CF component. Results demonstrate the expected approximately invariant phase (shallow slope) for the composite DPOAE and the overlap component. The phase of the CF component falls through approximately 30 cycles between 0.75 and 15 kHz, in keeping with its expected phase behavior. No observable differences in either composite or component phase behavior can be noted across age groups or stimulus levels.
Figure 6.
Composite and component phase accumulation for different age groups as a function of frequency above 4 kHz. The three stimulus level conditions of 75/75, 65/55, and 55/40 dB SPL are displayed in the three rows. The error bars represent 95% CI. Only data sets where information was available at least every 6 Hz were included and are represented here.
There was no difference in mean DPOAE phase between age groups for each component and/or stimulus level using the mixed effects model and post hoc multiple pairwise comparisons with Tukey adjustment (detailed above). AGSS scores derived as described above were small in comparison to the level scores, but similar across conditions consistent with the similar phase patterns observed across age groups (Table TABLE III.). Larger AGSS scores were noted for the CF component as compared to the phase of the composite or overlap component. No reliable differences were observed between age groups or between male and female subjects.
Validity of results from the subset
Data density requirements for the signal processing technique used to separate DPOAE components forced us to use only a subset of our full data set (Table TABLE I.). The similarity of this subset of data to the full data set was examined by comparing the proportion of subjects with fine structure and characteristics (depth, spacing, number of periods per 1/3 octave) between the subset and the full data set. No statistical differences in fine structure characteristics were noted between the complete data set and the subset used for the component analyses. As an example, the proportion of subjects with fine structure in the subset is presented beside the same data from the full data set in Fig. 2. All other measures showed a similar degree of agreement between the subset and the full data set.
DISCUSSION
In this paper we build on the current knowledge of DPOAE fine structure and components by extending observations beyond ∼6 kHz and by reporting fine structure and component characteristics in a large clinically normal population without regard to their DPOAE status or presence of fine structure. Studying carefully pre-screened subjects with prominent fine structure and recognizable DPOAE components has been important to understand the mechanics related to DPOAE generation and propagation. However, data sets like the one presented here are better suited to understanding population behavior and developing clinical norms.
In this group of 356 individuals between 10 and 65 yr of age with behavioral hearing thresholds of 20 dB HL or better up to 4 kHz: (1) fine structure depth did not vary systematically with age or stimulus level; (2) the number of recognizable fine structure periods declined dramatically above 12 kHz in all ages, but the oldest subjects demonstrated the most fine structure at stimulus levels of 75/75 dB SPL; (3) fine structure spacing was narrowest in the youngest age group and widest in the oldest age group between 5 and 12 kHz; (4) whenever gender differences were observed, female subjects had more, deeper, and narrower fine structure; (5) relatively speaking the levels of the CF component allowed better distinction between different age groups than the levels of the overlap component or the composite DPOAE at 75/75 dB SPL; and (6) phase behavior of the composite or DPOAE components failed to distinguish between the age groups examined here.
DPOAE fine structure and relation to DPOAE components
Across different stimulus levels fine structure was observed in approximately 57% of the subjects. However, some fine structure was observed in virtually all subjects between 1 and 4 kHz. In that regard these results are in agreement with those of He and Schmiedt (1996) who observed fine structure in subjects between 21 and 84 yr old whenever DPOAEs were recordable above the noise floor. On average, between one and two fine structure periods were observed per 1/3-octave band in agreement with previous reports (Reuter and Hammershøi, 2006; Abdala and Dhar, 2012). Also in agreement with Abdala and Dhar (2012) fine structure was more frequently observed in the oldest age group between 5 and 12 kHz (56–65 yr here, >65 yr in Abdala and Dhar), in this case for the stimulus levels of 75/75 dB SPL (see left panel in top row of Fig. 2). Abdala and Dhar (2012) were able to document that the reemergence of fine structure in the older subjects was a consequence of a relatively smaller reduction in the level of the CF component compared to that of the overlap component. That is, greater equality between the magnitudes of the overlap and CF components led to more complete cancellation when the two were in phase opposition consequently increasing fine structure depth. We suspect the same interplay between the two DPOAE components to be responsible for our finding. However, we are unable to provide validation as data density requirements precluded the separation of DPOAE components in the older age group in this data set.
Mean fine structure depth varied between 3 and 7 dB depending on stimulus level and frequency, in agreement with previous findings (e.g., Abdala and Dhar, 2012, –4 dB; Reuter and Hammershøi, 2006, −6 to 9 dB). Also consistent with previous findings (Abdala and Dhar, 2012), no influence of age on fine structure depth was observed. Fine structure depth gradually increases with increasing frequency above 5 kHz for the 65/55 and 75/75 dB SPL stimulus levels (Fig. 3). Given that DPOAE fine structure above 5 kHz has not been reported before, this increase in depth with frequency has also not been reported. The increase in depth can be related to the changing level relationship between the overlap and CF components. A gradual decline in the level of the overlap component and an increase in the level of the CF component is observed with increasing frequency in Fig. 5. Consequently the difference in level between the two components diminishes with increasing frequency (Fig. 5; right panel) leading to deeper fine structure. This relationship is also reflected in the lowest frequencies (<2 kHz) examined, where a reduced difference in level between the two components is also observed in the difference scores in Fig. 5 (right panel) at all three stimulus levels. In this case, as frequency decreased from 2 kHz toward lower frequencies a sharp increase in the CF component level is observed while the level of the overlap component remains relatively stable (Fig. 5; middle panels) contributing to the observed peak in depth at 1 kHz in Fig. 3.
Our results on fine structure spacing or width are also consistent with previous reports (Abdala and Dhar, 2012—average spacing ratio of 13.1) with average spacing ratios (f/Δf) between 15 and 25 (∼1/10 to 1/17 octave) depending on frequency. Indeed, others (Reuter and Hammershøi, 2006; Abdala and Dhar, 2012) have reported an increase in the spacing ratio (narrowing of fine structure) with increasing frequency. This effect of frequency is clear above 8 kHz in Fig. 4. Also evident in the two lower panels of Fig. 4 is a systematic decrease in spacing ratio (widening fine structure) with increasing age above 8 kHz. While the trend is clear, statistically significant differences in spacing ratio were only found between the two youngest and the oldest age group. Fine structure spacing is determined by the phase relationship between the two components. Abdala and Dhar (2012) have recently shown the phase slope of the CF component to be shallower in older adults as compared to younger subjects. A gradual reduction in the slope of the CF component phase with age would account for the observation of gradually widening fine structure. However, this cannot be explicitly verified in the current data set as components could be segregated only for the two youngest age groups for the two stimulus levels (55/40 and 65/55 dB SPL) where this trend in spacing is seen. The functional consequence or correlate of a reduction in the slope of the CF component is yet to be identified. Proponents of the coherent reflection filtering model (Shera and Guinan, 2003) have demonstrated a theoretical and experimental relationship between the phase slope of the stimulus frequency OAE and cochlear tuning in variety of mammals at low to moderate sound levels (Shera et al., 2002, 2010; Bergevin et al., 2008). Models of DPOAEs as well as experimental support (e.g., Kalluri and Shera, 2001) attribute the CF component to a stimulus frequency OAE-like generation mechanism (Talmadge et al., 1998; Shera and Guinan, 1999). Therefore, changes in CF component phase with age could be reflective of changes in cochlear tuning. However, the link between stimulus frequency OAE phase and tuning is currently debated (Siegel et al., 2005; Ruggero and Temchin, 2005; Ruggero and Temchin, 2007).
DPOAE components
DPOAE components were separable only in 41.6, 21.6, and 5.9 % of the subject pool for stimulus levels of 75/75, 65/55, and 55/40 dB SPL, respectively. This resulted in usable comparisons only in the two youngest age groups for the two lower stimulus levels. The availability of component information in such a small proportion of the “normal-hearing” population raises questions about the applicability of DPOAE component behavior as a clinical metric (clinical application of DPOAE components and fine structure is discussed later). The strict requirement of the IFFT algorithm for continuous data is a procedural limitation that could be bypassed by applying alternate techniques such as a suppressor tone (e.g., Heitmann et al., 1998), analysis in the true time domain (Vetesnik et al., 2009), or time-frequency analysis using wavelets (Moleti et al., 2012). Further, since this is the first report exploring component analysis to frequencies beyond 6 kHz, a comparison of alternate techniques (in particular a suppression method) in this frequency region would permit further validation and interpretation of findings.
When DPOAE component information was available, the CF component level allowed the greatest separation of the age groups (see bolded text in Table TABLE III.). Composite or component phase characteristics were not as useful in segregating the age groups by this comparison. It should be noted that the slope of the CF component phase has recently been reported to be steeper in newborn humans and shallower in older adults (>65 yr old, Abdala and Dhar, 2012). Neither of these age groups is represented here. It is remarkable that the age groups can be segregated with greater than 90% accuracy even <6 kHz (refer to Table TABLE III.) where these subjects have, on average, similar hearing thresholds (see Fig. 2 of Lee et al., 2012).
Using stimulus levels of 75/75 dB SPL facilitated the extraction of the CF component in subjects older than 35 yr. However, these high stimulus levels may not be conducive for inducing a significant contribution of the CF component to the ear canal DPOAE. It is likely that the amount of suppression of the DPOAE CF region by the lower frequency stimulus tone will increase with increasing stimulus levels. Using higher stimulus levels may also alter the proportion of energy that propagates towards the DPOAE CF region from the overlap region. It has indeed been shown that the contribution of the CF component to the ear canal DPOAE generally decreases with increasing stimulus levels (e.g., Dhar et al., 2005). These limitations notwithstanding, the CF component level appears to be an indicator of aging processes in the cochlea. Finally, DPOAE component characteristics are expected to determine fine structure behavior and that expectation is fulfilled when both component characteristics and fine structure behavior are available for comparison. For example, the difference between DPOAE component levels is found to decrease with increasing frequency in Fig. 5 and fine structure depth is found to increase with frequency in Fig. 3.
Applications
The utility of DPOAE fine structure has long been of interest for both scientific and clinical purposes. Changes in fine structure have been documented after acute noise exposure (Engdahl and Kemp, 1996), after excessive salicylate consumption (Rao and Long, 2011), and after cisplatin exposure (McMillan et al., 2012). More recently focus appears to have shifted from general fine structure to DPOAE components (e.g., Deeter et al., 2009; Abdala and Dhar, 2010, 2012; Rao and Long, 2011). The argument in favor of focusing on DPOAE components is to eliminate the uncertainty related to DPOAE fine structure. The presence of fine structure and its depth is dependent on the relative levels of the two components. Therefore changes in this level relationship could result in either an increase or decrease of fine structure depth, making it an unreliable clinical metric. However, fine structure characteristics were observable in a greater number of our subjects than were DPOAE component characteristics. While an isolated fine structure period in a frequency zone allowed the inclusion of that frequency zone in further analyses, we required a high standard of data density over the entire frequency range for a subject's inclusion in the component analysis. Perhaps this requirement can be relaxed by using alternate methods of component segregation or segregating components over more limited frequency ranges. The accuracy and appropriateness of various signal processing methods for separating DPOAE components is being actively explored by various research groups. Thus, one can hope that the clinical utility of DPOAE components will improve with the discovery and refinement of these strategies. One could also envision a metric of cochlear health that quantifies the patches of the cochlea from which both DPOAE components are recordable. Much like a hair cell count as a function of distance from the base of the cochlea, this metric could provide a detailed differential view of cochlear health along its length.
Among all the features of DPOAE fine structure and components that were evaluated here, the most successful in exposing an effect of age was the CF component level at 75/75 dB SPL. This evaluation could only be made for stimulus levels of 75/75 dB SPL—not thought to be ideal for detecting small changes in cochlear function. This makes CF component level (and stimulus frequency OAEs by extension) a front-runner as a viable tool that could be sensitive to early changes in cochlear function in certain applications. It is remarkable that the four youngest age groups were separable using this metric even at frequencies below 6 kHz, where little differences in average hearing thresholds existed between these groups. The trend of widening fine structure with age above 8 kHz is also intriguing, but will have to be evaluated further.
CONCLUSIONS
Data sets such as the one presented here mark the beginning of a process of evaluating clinical utility of a phenomenon or measure. By documenting both DPOAE fine structure and DPOAE component characteristics in a large sample of normal hearing individuals we hope to have provided the baseline for future studies. The extension to frequencies up to 16 kHz and the use of various stimulus levels under carefully calibrated conditions should add to the utility of the data. These data also suggest that fine structure spacing and CF component level at 75/75 dB SPL may expose age-related differences in cochlear function that are not observable in behavioral hearing thresholds.
ACKNOWLEDGMENTS
The authors wish to thank Rebekah Abel, Renee Banakis, Erica Choe, Helen Han, Lauren Hardies, Evan Grolley, Kelly Waldvogel, Coryn Weissinger, Darrin Worthington, and Wei Zhao for help in data collection. Victoria Hellyer managed research subject recruitment and participation. Portions of this study were presented at the American Auditory Society Conference (2011), the Association for Research in Otolaryngology (2012), and the Acoustical Society of America (2013). This research was supported by NIH/NIDCD Grants Nos. R01DC008420, T32DC009399, American-Speech-Language Hearing Foundation, and Northwestern University.
Footnotes
The component from the DPOAE CF region often retains some structure in amplitude. This complexity is ignored in this elementary example.
References
- Abdala, C., and Dhar, S. (2010). “Distortion product otoacoustic emission phase and component analysis in human newborns,” J. Acoust. Soc. Am. 127, 316–325. 10.1121/1.3268611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala, C., and Dhar, S. (2012). “Maturation and aging of the human cochlear: A view through the DPOAE looking glass,” J. Assoc. Res. Otoalyngol. 13, 403–421. 10.1007/s10162-012-0319-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti, A., and Coull, B. A. (1998). “Approximate is better than ‘exact’ for interval estimation of binomial proportions,” Am. Stat. 52, 119–126. [Google Scholar]
- ANSI. (1996). S3.6-1996, Specifications for Audiometers (American National Standards Institute, New York: ). [Google Scholar]
- ANSI. (2008). S3.1-1999-R2008, Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms (American National Standards Institute, New York: ). [Google Scholar]
- Bergevin, C., Freeman, D. M., Saunders, J. C., and Shera, C. A. (2008). “Evidence of multiple generation mechanisms,” J. Comp. Physiol., A 194, 665–683. 10.1007/s00359-008-0338-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. M., Williams, D. M., and Gaskill, S. A. (1993). “The effect of aspirin on cochlear mechanical tuning,” J. Acoust. Soc. Am. 93, 3298–3307. 10.1121/1.405714 [DOI] [PubMed] [Google Scholar]
- Deeter, R., Abel, R., Calandruccio, L., and Dhar, S. (2009). “Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 126, 2413–2424. 10.1121/1.2770544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar, S., Long, G. R., Talmadge, C. L., and Tubis, A. (2005). “The effect of stimulus frequency ratio on distortion product otoacoustic emission components,” J. Acoust. Soc. Am. 117, 3766–3776. 10.1121/1.1903846 [DOI] [PubMed] [Google Scholar]
- Dhar, S., Talmadge, C. L., Long, G. R., and Tubis, A. (2002). “Multiple internal reflections in the cochlea and their effect on DPOAE fine structure,” J. Acoust. Soc. Am. 112, 2882–2897. 10.1121/1.1516757 [DOI] [PubMed] [Google Scholar]
- Dorn, P. A., Piskorski, P., Gorga, M. P., Neely, S. T., and Keefe, D. H. (1999). “Predicting audiometric status from distortion product otoacoustic emissions using multivariate analyses,” Ear. Hear. 20, 149–163. 10.1097/00003446-199904000-00006 [DOI] [PubMed] [Google Scholar]
- Engdahl, B., and Kemp, D. T. (1996). “The effect of noise exposure on the details of distortion product otoacoustic emissions in humans,” J. Acoust. Soc. Am. 99, 1573–1587. 10.1121/1.414733 [DOI] [PubMed] [Google Scholar]
- Gaskill, S. A., and Brown, A. M. (1996). “Suppression of human acoustic distortion product: Dual origin of 2f1-f2,” J. Acoust. Soc. Am. 100, 3268–3274. 10.1121/1.417210 [DOI] [PubMed] [Google Scholar]
- Greenwood, D. D. (1990). “A cochlear frequency-position function for several species—29 years later,” J. Acoust. Soc. Am. 87, 2592–2605. 10.1121/1.2172169 [DOI] [PubMed] [Google Scholar]
- He, N., and Schmiedt, R. A. (1996). “Effects of aging on the fine structure of the 2f1-f2 acoustic distortion product,” J. Acoust. Soc. Am. 99, 1002–1015. 10.1121/1.414629 [DOI] [PubMed] [Google Scholar]
- Heitmann, J., Waldmann, B., Schnitzler, H. U., Plinkert, P. K., and Zenner, H. P. (1998). “Suppression of distortion product otoacoustic emissions (DPOAE) near f1-f2 removes DP gram fine structure—Evidence for a secondary generator,” J. Acoust. Soc. Am. 103, 1527–1531. 10.1121/1.421290 [DOI] [Google Scholar]
- Kalluri, R., and Shera, C. A. (2001). “Distortion-product source unmixing: A test of the twomechanism model for DPOAE generation,” J. Acoust. Soc. Am. 109, 622–637. 10.1121/1.1334597 [DOI] [PubMed] [Google Scholar]
- Kemp, D. T. (1979). “Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlear,” Arch. Oto-Rhino-Laryngol. 224, 37–45. 10.1007/BF00455222 [DOI] [PubMed] [Google Scholar]
- Kim, D. O. (1980). “Cochlear mechanics: Implications of electrophysiological and acoustical observations,” Hear. Res. 2, 297–317. 10.1016/0378-5955(80)90064-7 [DOI] [PubMed] [Google Scholar]
- Lee, J., Dhar, S., Abel, R., Banakis, R., Grolley, E., Lee, J., Zecker, S., and Siegel, J. (2012). “Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration,” Ear. Hear. 33, 315–329. 10.1097/AUD.0b013e31823d7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, G. R., and Talmadge, C. L. (1997). “Spontaneous otoacoustic emission frequency is modulated by heartbeat,” J. Acoust. Soc. Am. 102, 2831–2848. 10.1121/1.420339 [DOI] [PubMed] [Google Scholar]
- Long, G. R., Talmadge, C. L., and Lee, J. (2008). “Measuring distortion product otoacoustic emissions using continuously sweeping primaries,” J. Acoust. Soc. Am. 124, 1613–1626. 10.1121/1.2949505 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Stagner, B. B., Chung, Y. S., and Lonsbury-Martin, B. L. (2011). “Characterizing distortion-product otoacoustic emissions components across four species,” J. Acoust. Soc. Am. 129, 3090–3103. 10.1121/1.3560123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauermann, M., Long, G. R., and Kollmeier, B. (2004). “Fine structure of hearing threshold and loudness perception,” J. Acoust. Soc. Am. 116, 1066–1080. 10.1121/1.1760106 [DOI] [PubMed] [Google Scholar]
- Mauermann, M., Uppenkamp, S., van Hengel, P. W. J., and Kollmeier, B. (1999). “Evidence for the distortion product frequency place as a source of distortion product otoacoustic emission (DPOAE) fine structure in humans. I. Fine structure and higher-order DPOAE as a function of the frequency ratio f2/f1,” J. Acoust. Soc. Am. 106, 3473–3483. 10.1121/1.428200 [DOI] [PubMed] [Google Scholar]
- McMillan, G. P., Konrad-Martin, D., and Dille, M. F. (2012). “Accuracy of distortion-product otoacoustic emissions-based ototoxicity monitoring using various primary frequency stepsizes,” Int. J. Audiol. 51, 689–696. 10.3109/14992027.2012.688143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleti, A., Longo, F., and Sisto, R. (2012). “Time-frequency domain filtering of evoked otoacoustic emissions,” J. Acoust. Soc. Am. 132, 2455–2467. 10.1121/1.4751537 [DOI] [PubMed] [Google Scholar]
- Rao, A., and Long, G. R. (2011). “Effects of aspirin on distortion product fine structure: Interpreted by the two-source model for distortion product otoacoustic emissions generation,” J. Acoust. Soc. Am. 129, 792–800. 10.1121/1.3523308 [DOI] [PubMed] [Google Scholar]
- Reuter, K., and Hammershøi, D. (2006). “Distortion product otoacoustic emission fine structure analysis of 50 normal-hearing humans,” J. Acoust. Soc. Am. 120, 270–279. 10.1121/1.2205130 [DOI] [PubMed] [Google Scholar]
- Reuter, K., and Hammershøi, D. (2007). “Distortion product otoacoustic emission of symphony orchestra musicians before and after rehearsal,” J. Acoust. Soc. Am. 121, 327–336. 10.1121/1.2395915 [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A., and Temchin, A. N. (2005). “Unexceptional sharpness of frequency tuning in the human cochlea,” Proc. Natl. Acad. Sci. U.S.A. 102, 18614–18619. 10.1073/pnas.0509323102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., and Temchin, A. N. (2007). “Similarity of traveling-wave delays in the hearing organs of humans and other tetrapods,” J. Assoc. Res. Otolaryngol. 8, 153–166. 10.1007/s10162-007-0081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperle, R. A., Neely, S. T., Kopun, J. G., and Gorga, M. P. (2008). “Influence of in situ, soundlevel calibration on distortion-product otoacoustic emission variability,” J. Acoust. Soc. Am. 124, 288–300. 10.1121/1.2931953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera, C. A. (2003). “Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves,” J. Acoust. Soc. Am. 114, 244–262. 10.1121/1.1575750 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J. (1999). “Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs,” J. Acoust. Soc. Am. 105, 782–798. 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J., Jr. (2003). “Stimulus-frequency-emission group delay: A test of coherent reflection filtering and a window on cochlear tuning,” J. Acoust. Soc. Am. 113, 2762–2772. 10.1121/1.1557211 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., Guinan, J. J., Jr., and Oxenham, A. J. (2002). “Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements,” Proc. Natl. Acad. Sci. U.S.A. 99, 3318–3323. 10.1073/pnas.032675099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera, C. A., Guinan, J. J., Jr., and Oxenham, A. J. (2010). “Otoacoustic estimation of cochlear tuning: Validation in the chinchilla,” J. Assoc. Res. Otolaryngol. 11, 343–365. 10.1007/s10162-010-0217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, J. H., Cerka, A. J., Recio-Spinoso, A., Temchin, A. N., van Dijk, P., and Ruggero, M. A. (2005). “Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering,” J. Acoust. Soc. Am. 118, 2434–2443. 10.1121/1.2005867 [DOI] [PubMed] [Google Scholar]
- Siegel, J. H., and Kim, D. O. (1982). “Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity,” Hear. Res. 6, 171–182. 10.1016/0378-5955(82)90052-1 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Long, G. R., Tubis, A., and Dhar, S. (1999). “Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 105, 275–292. 10.1121/1.424584 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Tubis, A., Long, G. R., and Piskorski, P. (1998). “Modeling otoacoustic emission and haring threshold fine structures,” J. Acoust. Soc. Am. 104, 1517–1543. 10.1121/1.424364 [DOI] [PubMed] [Google Scholar]
- Vetesnik, A., Turcanu, D., Dalhoff, E., and Gummer, A. W. (2009). “Extraction of sources of distortion product otoacoustic emissions by onset-decomposition,” Hear. Res. 256, 21–38. 10.1016/j.heares.2009.06.002 [DOI] [PubMed] [Google Scholar]