Abstract
Abstract We investigated regional gray matter (GM) reduction as a predictor of judgment ability in 120 non-depressed older adults with varying degrees of cognitive complaints and/or impairment (including those with MCI and mild AD). Participants underwent neuropsychological assessment, including the Test of Practical Judgment (TOP-J), a recently developed instrument that evaluates judgment and problem solving related to safety, medical, social/ethical, and financial issues. Structural MR scanning included T1-weighted SPGR volumes acquired at 1.5 Tesla. We used voxel-based morphometry to analyze the relationship between GM density and TOP-J scores, controlling for age, education, gender, intracranial volume, verbal memory, and crystallized knowledge. Consistent with our hypothesis, judgment ability correlated with GM density in prefrontal regions (left inferior and superior frontal gyri). Findings extend previous observations of frontal involvement in higher-order cognitive abilities/executive functions and provide initial validation of the TOP-J’s sensitivity to the integrity of these brain regions in individuals at risk for dementia.
Keywords: Judgment, Frontal lobe, Aging, Dementia, Voxel-based morphometry
Introduction
Loss of judgment ability is a common diagnostic feature of the dementing process, as executive cognitive functions that permit goal-directed use of existing knowledge progressively decline (Duke and Kaszniak 2000; Karlawish et al. 2005; Knopman et al. 2001; Marson and Harrell 1999). Individuals with compromised executive functioning may exercise poor judgment for various reasons such as failing to develop an appropriate problem definition or formulation, making impulsive decisions, not engaging in efficient search strategies, fixating on a single plan due to compromised mental flexibility, or failing to consider the long-term consequences of solutions (Marsiske and Margrett 2006; Thornton and Dumke 2005; Woods et al. 2000). For individuals in the early stages of dementia, compromised decision making about daily activities, such as meal preparation or financial matters, may lead to dangerous outcomes (Kounti et al. 2006; Nygard 2003). These individuals also may persist in behaviors that are no longer safe such as driving or managing medications without assistance. Given its importance, practitioners often evaluate the judgment ability of older adults with suspected dementia (Rabin et al. 2008). Knowledge derived from the clinical assessment of judgment can inform decisions about diagnosis, functional and cognitive competence, and treatment (Bertrand and Willis 1999; Kim et al. 2002).
Neuroimaging techniques are increasingly used to elucidate the neural circuitry subserving specific cognitive abilities. While research into the neural substrates of judgment and decision making is limited, available functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) findings are largely consistent with behavioral data, highlighting the important role of the frontal cortex including inferior frontal, superior frontal, anterior cingulate, and medial frontal gyri, as well as other areas within the cerebellum, parietal lobes, and associative cortices, depending on the task employed (Bechara et al. 1999; Fukui et al. 2005; Gong et al. 2005; Manes et al. 2002; Menon et al. 2001; Paulus et al. 2002; for review see Roth et al. 2006). To our knowledge, no study has used structural neuroimaging to examine the neural correlates of judgment in older adults, including those at risk for Alzheimer’s disease (AD).
We used voxel-based morphometry (VBM) to analyze the relationship between regional GM density and judgment ability. VBM enables global analysis of brain structures without a priori identification of the region of interest (Ashburner and Friston 2000). This approach is not biased toward any brain region and permits identification of potentially unsuspected changes in regional brain structure. We used the Test of Practical Judgment (TOP-J) to assess participants’ judgment (Rabin et al. 2007). We developed the TOP-J in response to an identified need for assessment instruments for the growing number of elders with cognitive deterioration. At present, there appears to be a lack of clinically useful tools to assess practical judgment (Rabin et al. 2007); in a recent survey of neuropsychologists, approximately 90% of respondents called for additional or improved judgment tests (Rabin et al. 2008).
We previously reported on the development and validation of the TOP-J including item selection, scale development, and preliminary psychometric properties, which showed good reliability, validity, and dimensionality (Rabin et al. 2007). The TOP-J was sensitive to declining judgment skills, with mild AD patients scoring in the clinically impaired range (approximately 2 SDs below the mean of healthy controls, HC) and amnestic mild cognitive impairment (MCI) patients showing an intermediate level of performance (approximately 1 SD below the HC mean, low average range). Older adults with significant cognitive complaints (CC) but intact neuropsychological performance also showed similarly reduced scores (approximately 1 SD below the mean of HC, low average range). The CC group is of special interest in light of recent evidence suggesting that these individuals show brain changes intermediate between those seen in MCI and healthy older adults without complaints; they may represent a “pre-MCI” stage (Saykin et al. 2006).
The goal of the current study was to investigate relations between structural neuroimaging data and judgment ability in a group of older adults in various stages of cognitive impairment and with varying degrees of cognitive complaints. We correlated TOP-J scores with brain morphology data (utilizing VBM with covariates) in our combined sample of participants classified as HC, CC, MCI, or probable AD. Based on previous neuroimaging research on the neural correlates of executive functions in both cognitively healthy and impaired populations (Newman et al. 2007; Roth et al. 2006), we hypothesized that the neural basis for impaired judgment skills would involve predominantly prefrontal cortical regions.
Materials and methods
Participants
Participants included 120 euthymic older adults enrolled in a longitudinal memory and aging study at Dartmouth Medical School. All participants provided written informed consent and were at least 60 years of age (range=63–88), right-handed, and fluent in English. Exclusion criteria included any significant or uncontrolled medical, psychiatric, or neurological condition (other than AD or MCI) that could affect brain structure or cognition, history of head trauma with loss of consciousness lasting more than 5 minutes, and current or past history of substance dependence. Participants underwent neuropsychological assessment and structural neuroimaging. All participants had knowledgeable collateral informants who answered questions about participants’ cognition and general health. Level of cognitive complaint was determined from responses on multiple self- and informant-report measures, and a Cognitive Complaint Index was calculated as the percentage of items endorsed by the participant and/or the informant (see Table 1). Saykin et al. (2006) present more detailed information about study methodology and utilized instruments. A board-certified geropsychiatrist conducted a semi-structured interview to rule out depression or other psychiatric disorders. A board-certified neuroradiologist ruled out incidental pathology on structural MRI including white matter changes greater than expected for age (Fazekas et al. 2002).
Table 1.
Demographic and neuropsychological data
| Participant group |
||||||
|---|---|---|---|---|---|---|
| HC n=39 |
CC n=34 |
MCI n=34 |
AD n=13 |
P | Group differencesa | |
| Age (yrs) | 71.8 (5.1) | 74.1 (6.2) | 73.6 (6.1) | 75.2 (7.0) | NS | – |
| Education (yrs) | 16.8 (2.6) | 16.6 (2.8) | 16.6 (2.9) | 14.4 (3.5) | NS | – |
| Gender: M/F | 12/27 | 10/24 | 17/17 | 5/8 | NS | – |
| CCI (%) | 7.2 (5.5) | 23.8 (8.5) | 30.0 (12.1) | 36.7 (10.3) | <.001 | MCI, AD>CC>HC |
| MMSE | 29.0 (1.1) | 28.9 (1.3) | 26.8 (1.5) | 22.4 (3.9) | <.001 | HC, CC>MCI>AD |
| DRS-2 | 141.6 (1.7) | 141.0 (2.1) | 137.7 (3.9) | 123.2 (12.1) | <.001 | HC, CC>MCI>AD |
| CVLT-II | ||||||
| Learning | 50.4 (8.4) | 46.3 (8.4) | 33.9 (6.6) | 24.1 (6.5) | <.001 | HC, CC>MCI>AD |
| Short delay | 11.2 (3.0) | 10.1 (2.8) | 6.1 (2.6) | 1.8 (1.9) | <.001 | HC, CC>MCI>AD |
| Long delay | 11.9 (2.8) | 10.9 (2.7) | 6.2 (3.1) | 0.9 (1.7) | <.001 | HC, CC>MCI>AD |
| TOP-J | 37.4 (3.9) | 33.7 (3.9) | 34.2 (4.4) | 29.6 (5.2) | <.001 | HC>CC, MCI>AD |
Data are Mean (SD) except where otherwise noted.
CCI=Cognitive Complaint Index (Saykin et al. 2006); MMSE=Mini Mental State Exam, raw score (n/30); DRS-2=Mattis Dementia Rating Scale-2, raw score (n/144); CVLT-II=California Verbal Learning Test, Second Edition, total learning score (n/80), short delay free recall score (n/16), and long delay free recall score (n/16); TOP-J=Test of Practical Judgment, total raw score (n/45).
Posthoc analyses were conducted using Tukey’s procedure.
Participants diagnosed as AD (n=13) defined by NINCDS-ADRDA criteria (McKhann et al. 1984) had: 1) impaired general cognitive functioning as determined by cutoff scores on the Mattis Dementia Rating Scale-2 (Jurica et al. 2001) and Mini Mental State Examination (Folstein et al. 1975); 2) impaired activities of daily living (as reported on self and informant versions of Neurobehavioral Function/Activities of Daily Living Scale (NBF-ADL; Saykin 1992); 3) significant memory complaints (endorsed at least 20% of possible complaints across all inventories or complaints were deemed significant by clinical consensus); and 4) impaired memory (memory scores >2 SD below the mean established for age- and education-matched controls). Participants diagnosed as amnestic MCI (n=34; Petersen 2004; Petersen et al. 2001) had: 1) preserved general cognitive functioning; 2) generally normal activities of daily living; 3) no dementia; 4) significant memory complaints; and 5) impaired memory (memory scores 1.5 SD below the mean established for age- and education-matched controls). Notably, all MCI participants had isolated memory problems; those with primary impairments in other domains such as executive functioning or language were excluded. Participants classified as CC (n=34) had: 1) preserved general cognitive functioning; 2) generally normal activities of daily living; 3) no dementia; 4) significant memory complaints; and 5) no significant memory deficit on cognitive testing. Participants classified as HC (n=39) had: 1) preserved general cognitive functioning; 2) generally normal activities of daily living; 3) no dementia; 4) no significant cognitive complaints; and 5) intact memory ability.
Test of Practical Judgment (TOP-J)
The TOP-J is a 15-item, open-ended questionnaire in which participants listen to brief scenarios about everyday problems and report aloud their proposed solutions. These scenarios were designed to be easily understood and representative of the types of judgment problems faced by older adults, yet complex enough to require higher-order cognitive abilities. A survey of practicing neuropsychologists established that formal assessment of everyday judgment should incorporate issues related to the identified content domains (i.e., safety, social/ethical, financial, and medical issues). During the item development process, we considered the possibility that individuals would draw upon crystallized information and personal/social knowledge when responding to questions. Therefore, we strove to create scenarios for which the generation of successful solutions would also require executive cognitive abilities. A related goal was to develop items appropriate for both high- and low- functioning individuals to avoid ceiling and floor effects. A panel of 16 neuropsychology experts verified the content validity of TOP-J items (Rabin et al. 2007, 2008).
TOP-J responses were recorded verbatim and scored on a 4-point scale (scores per item range from 0–3), with a higher value indicating better judgment. Unclear or ambiguous responses were queried in a neutral manner. Given the relatively small number of questions and the overlap of content domains for many scenarios, all TOP-J items were combined into a single, overall composite score (ranging from 0–45), rather than dividing the test into subscales.1 Detailed examiner instructions were included on the protocol, and the TOP-J took approximately 10 minutes to administer and score. As reported by Rabin et al. (2007), initial findings in 134 older adults indicated that test-retest stability was .86, inter-rater reliability was .96, and internal consistency was .68. Evidence for convergent validity was demonstrated by moderate correlations with related constructs (e.g., tests of executive functioning, crystallized knowledge, language, and memory), whereas discriminant validity was demonstrated by low correlations with measures involving minimal executive demands (e.g., rote attention, visuoconstruction, and emotional functioning). We also found a moderate association between the TOP-J and relevant items from the informant version of the activities of daily living scale (i.e,. NBF-ADL; r=−.40, p<.001).
Voxel-based morphometry
Imaging was completed on a GE Signa 1.5 Tesla Horizon LX magnet with echo speed gradients using a standard head RF coil. The structural MR was a T1-weighted 3-dimensional spoiled gradient echo (SPGR) coronal volume. Parameters were: TR=25, TE=3, flip angle=40, 1 NEX, and slice thickness=1.5 mm (no skip), yielding 124 contiguous slices with a 24 cm field of view and 256×256 matrix with 0.9375 mm in-plane resolution. We also acquired T2-weighted 3 mm axial survey images as a screen for focal lesions. VBM was performed on the SPGR images using the SPM5 package (Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/). Briefly, the SPGR volumes were reconstructed from dicom images, registered to the T1 template, segmented in native space, and the GM maps were normalized and re-sampled to 1 mm3. The maps were then spatially smoothed (10 mm full width half maximum) and subjected to statistical parametric mapping on a voxel-by-voxel basis using the General Linear Model approach implemented in SPM5.
We used SPM5 to identify brain areas where GM was correlated with TOP-J scores in the entire sample, adjusting for age, education, gender, and total intracranial volume (ICV). ICV was obtained by manually tracing the outer boundary of the entire brain, including the cortical CSF, using the BRAINS software package (Andreasen et al. 1992). We controlled for the effects of memory using a combination of short and long delay free recall scores on the California Verbal Learning Test, Second Edition (CVLT-II, Delis et al. 2000) given the observed relationship between the TOP-J and these CVLT-II scores in our participants with memory complaints and/or dysfunction (r=.33, p<.001). We controlled for the effects of crystallized verbal knowledge using participants’ raw scores on the Information subtest of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III Info; Wechsler 1997) given the significant relationship between the TOP-J and WAIS-III Info scores (r=.48, p<.001).
Results
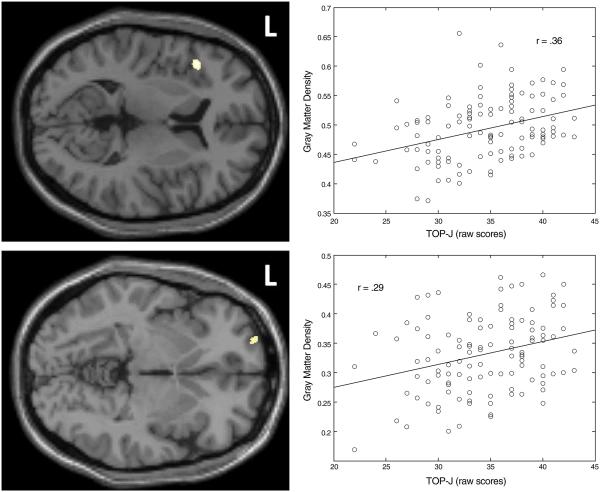
Select demographic and neuropsychological variables are presented in Table 1. Using a critical significance threshold of .001 at the voxel level and a minimum cluster size of 100 voxels, results indicated that decreased judgment was correlated with a reduction in GM density in the left inferior frontal gyrus (r=.36, p=.0001) and left superior gyrus (r=.29, p=.0013) (see Table 2; Fig. 1). Importantly, no region showed an increase in GM density as TOP-J scores decreased. As mentioned previously, age, years of education, gender, ICV, CVLT-II memory scores (short delay free recall score + long delay free recall score), and WAIS-III Info scores were entered into the statistical model as covariates. As a secondary analysis, we examined the degree to which the observed patterns were present within three subgroups of participants: HC, CC, and MCI/AD combined. Results revealed that the TOP-J was related across the sample to GM density in the left inferior frontal gyrus (HC: r=.45, p=.004; CC: r=.35, p=.045; MCI/AD: r=.26, p=.079) and to a lesser extent in the superior frontal gyrus (HC: r=.32, p=.050; CC: r=.26, p=.141; MCI/AD: r=.15, p=.329).
Table 2.
MNI coordinates, t-values, and cluster sizes for VBM analysis: Regions showing reduced GM density associated with lower judgment
| Regiona | x, y, z | Peak t value |
k |
|---|---|---|---|
| Left inferior frontal gyrus (BA45) |
−43, 20, 8 | 3.73 | 126 |
| Left superior frontal gyrus (BA10) |
−24, 63, −4 | 3.36 | 102 |
All t-statistics are significant at p<.001 uncorrected, corresponding to critical t-value of 3.16.
MNI=Montreal Neurological Institute atlas coordinates; VBM=Voxel-based morphometry; judgment=TOP-J total score; BA=Brodmann area.
There were no regions in which GM density was negatively correlated with TOP-J total score.
Fig. 1.
Locations and plots of the two significant clusters for which a relationship exists between judgment and GM density at a threshold of punc<.001 (Top=Left inferior frontal gyrus; Bottom=Left superior frontal gyrus). The respective plots represent the linear relationship between TOP-J scores and the first eigenvariate of the plotted cluster. Covariates were age, years of education, gender, ICV, CVLT-II memory scores, and WAIS-III Info scores
Discussion
This quantitative study examined the relationship between structural neuroimaging data and judgment ability in older adults. We used VBM to identify brain areas where GM density reduction was associated with scores on the TOP-J, an objective performance instrument that assesses practical judgment and problem-solving skills. Our sample included 120 euthymic elders with intact cognition and varying degrees of neuropsychological impairment and/or complaints about cognition. From a neuropsychological perspective, judgment falls under the domain of executive functioning and typically requires the coordination of numerous higher-order cognitive processes including the ability to generate strategies to approach a problem, identify goals, evaluate the consequences of various courses of action, balance competing priorities, inhibit inappropriate responses, and monitor the effectiveness of a chosen plan (Marsiske and Margrett 2006; Thornton and Dumke 2005). Judgment also relies upon other cognitive processes including aspects of memory (e.g., calling to mind relevant past experiences and practical knowledge), crystallized knowledge (e.g., ingrained scripts that result in effective solutions), and language (e.g., understanding the problem being presented and communicating a decision to others).
As hypothesized, TOP-J scores correlated with GM density in prefrontal regions involved in a range of higher-order cognitive processes such as selecting among competing responses and representations, decision making, response inhibition, cognitive flexibility, processing and retrieval of semantic knowledge, and working memory (i.e., left inferior and left superior frontal gyri) (du Boisgueheneuc et al. 2006; Roth et al. 2006; Talati and Hirsch 2005; Thompson-Schill 2003; Thompson-Schill et al. 1997; Uchiyama et al. 2008; Zhang et al. 2004). Our results extend previous validation of the TOP-J by providing evidence of its selective relationship with prefrontal integrity, excluding variance that could be explained by other variables often associated with cognitive functioning and/or GM volume such as age, gender, and education (Buckner 2004; Coffey et al. 1998, 2001; Lemaitre et al. 2005; Manly 2003; Salat et al. 1999; Smith et al. 2007; Sowell et al. 2003; Thompson et al. 2003). We also covaried for performance on tests of verbal memory and crystallized verbal information (given their conceptual association and observed relation with judgment ability) and ICV (to adjust for brain size and global atrophy) (Pell et al. 2008). These findings are consistent with cognitive, behavioral, and functional neuroimaging studies implicating the prefrontal cortex in practical judgment/problem solving.
Dementia typically has a gradual onset, and the transition phase from normal cognition to impairment represents a critical window of opportunity for the initiation of treatment, particularly any future treatments that affect the underlying disease process. While episodic memory is the primary early cognitive deficit, initial symptoms of memory loss may be accompanied by subtle changes in other neuropsychological domains, and research suggests that executive function tests can help predict later conversion to AD (Albert et al. 2001, 2007; Ready et al. 2003). Additionally, functional neuroimaging studies have shown abnormal brain activation of frontal lobe systems during MCI patients’ performance of executive function tasks, and positive effects of psychopharmacological treatments such as cholinesterase inhibitors may be mediated via frontal systems (Rosano et al. 2005; Saykin et al. 2004). Identifying MCI patients who show declines in specific aspects of executive functioning, such as judgment, may be useful for studies investigating how such declines reflect changes in brain function over the course of illness. This could provide a valuable subcategory of participants to be followed in longitudinal studies or treatment trials targeting a personalized therapeutic approach. Tests like the TOP-J also may be used as outcome variables in trials of cognitive-enhancing interventions for which improvements in judgment and/or functional skills are expected and must be demonstrated objectively. Further, as disease modifying agents for AD and other dementias are developed it will be important to determine if degree of parenchymal sparing is associated with preserved judgment.
While the cross-sectional design of the present study restricted conclusions about the ability of mild judgment problems to predict diagnostic conversion, longitudinal assessments are being performed to examine the predictive validity of the TOP-J for clinical progression in our participant groups. This research may have implications for early detection of dementia and intervention. It is important to note that study results were correlational and therefore cannot demonstrate causal relationships. Additionally, due to limited statistical power, we were unable to explore the relationship between TOP-J performance and GM density in the entire brain among our four subgroups. However, when we interrogated the two clusters extracted from the overall analysis by participant status (HC, CC, and MCI/AD), the TOP-J was related to GM density in the left inferior frontal gyrus and to a lesser extent in the superior frontal gyrus. The correlation was relatively strongest in the HC group, suggesting that the relationship was not the unique result of a threshold effect of volume loss in the disease state. In future work we hope to clarify the relationship between TOP-J performance and left frontal GM for individuals in various stages of neurodegenerative decline and evaluate the specificity of the observed relationships. Additionally, while we did not have sufficient power for our findings to withstand a very stringent whole-brain search FDR adjustment, the results we reported using an uncorrected, but reasonably stringent, threshold of p<.001 are meaningful and in regions implicated in the neural circuitry of executive functions. These findings require replication in a larger sample.
Although VBM is advantageous for detecting subtle, localized structural brain changes in large areas of cortex, future research might also employ ROI-based methods to determine whether TOP-J scores correlate with volumes of brain regions identical to those observed in the current study. Another approach would utilize functional neuroimaging techniques such as fMRI with TOP-J-like probes or other decision-making tasks (adapted for in-scanner administration) to examine the degree to which activated sites and sites that show GM density-performance correlations correspond. Higher-order cognitive abilities are the result of the integrated activity in networks of regions, as opposed to activity in any one isolated region (Friston et al. 1993; Luria 1966). The addition of connectivity approaches represents another future direction that would enable investigation of functional interactions between brain regions engaged during judgment/decision making. Together, these structural and functional approaches would provide convergent evidence for how the brain supports judgment ability in older adults, in both health and neurodegenerative disease.
Acknowledgments
This research was supported by grants from the National Institute on Aging (R01 AG19771), Alzheimer’s Association (IIRG-94-133; IIRG-99-1653, sponsored by the Hedco Foundation) and Ira DeCamp Foundation, as well as the Brooklyn College Tow Faculty Fellowship Award and the Indiana Alzheimer’s Disease Center, Indiana Economic Development Corporation (#87884). The authors are grateful to the following individuals for their help with this study: Heather Pixley, Paul Wang, Nadia Paré, Alice Davison, Robert Ferranti, and Alex Mamourian.
Footnotes
The TOP-J also can be administered in a 9-item version. See Rabin et al. (2007) for more detailed information about the test development and validation processes, including sample items. The 15- and 9-item TOP-J protocols are available upon request from the corresponding author.
References
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. doi:10.1017/S1355617701755105. [DOI] [PubMed] [Google Scholar]
- Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. doi:10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Cohen GC, Harris G, Cizaldo T, Parkkinen J, Rezai K, et al. Image processing for the study of brain structure and function: Problems and programs. The Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. doi:10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. The Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand RM, Willis SL. Everyday problem solving in Alzheimer’s patients: A comparison of subjective and objective assessments. Aging & Mental Health. 1999;3:281–293. doi:10.1080/13607869956055. [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. doi:10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billing B, et al. Sex differences in brain aging: A quantitative magnetic resonance imaging study. Archives of Neurology. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. doi:10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JF. Cognitive correlates of human brain aging: A quantitative magnetic resonance imaging investigation. The Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:471–485. doi: 10.1176/jnp.13.4.471. doi:10.1176/appi.neuropsych.13.4.471. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test-second edition: Adult version manual. Psychological Corporation; San Antonio: 2000. [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. Functions of the left superior frontal gyrus in humans: A lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. doi:10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Duke LM, Kaszniak AW. Executive control functions in degenerative dementias: A comparative review. Neuropsychology Review. 2000;10:75–99. doi: 10.1023/a:1009096603879. doi:10.1023/A:1009096603879. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovascular Diseases (Basel, Switzerland) 2002;13:31–36. doi: 10.1159/000049147. doi:10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: The principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow and Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa gambling task. NeuroImage. 2005;24:253–259. doi: 10.1016/j.neuroimage.2004.08.028. doi:10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Gong QY, Sluming V, Mayes A. Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. NeuroImage. 2005;25:1175–1186. doi: 10.1016/j.neuroimage.2004.12.044. doi: 10.1016/j.neuroimage.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Jurica P, Leitten C, Mattis S. Dementia rating scale-2. Psychological Assessment Resources; Lutz: 2001. [Google Scholar]
- Karlawish JHT, Casarett DJ, James BD, Xie SX, Kim SYH. The ability of persons with Alzheimer disease (AD) to make a decision about taking an AD treatment. Neurology. 2005;64:1514–1519. doi: 10.1212/01.WNL.0000160000.01742.9D. doi:10.1212/01.WNL.0000160000.01742.9D. [DOI] [PubMed] [Google Scholar]
- Kim SYH, Karlawish JHT, Caine ED. Current state of research on decision-making competence of cognitively impaired elderly persons. The American Journal of Geriatric Psychiatry. 2002;10:151–165. doi:10.1176/appi.ajgp. 10.2.151. [PubMed] [Google Scholar]
- Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: Diagnosis of dementia (an evidence-based review): Report on the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- Kounti F, Tsolaki M, Kiosseoglou G. Functional cognitive assessment scale (FUCAS): A new scale to assess executive cognitive function in daily life activities in patients with dementia and mild cognitive impairment. Human Psychopharmacology: Clinical and Experimental. 2006;21:305–311. doi: 10.1002/hup.772. doi:10.1002/hup.772. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. NeuroImage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. doi:10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher cortical functions in man. Basic Books; New York: 1966. [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125(Pt 3):624–639. doi: 10.1093/brain/awf049. doi:10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Marsiske M, Margrett JA. Everyday problem solving and decision making. In: Birren JE, Warner Schaie K, editors. Handbook of the psychology of aging. 6th Academic; New York: 2006. pp. 315–342. [Google Scholar]
- Marson D, Harrell L. Executive dysfunction and loss of capacity to consent to medical treatment in patients with Alzheimer’s disease. Seminars in Clinical Neuropsychiatry. 1999;4:41–49. doi: 10.1053/SCNP00400041. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGO response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. doi:10.1002/1097-0193(200103) 12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC. The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging and Behavior. 2007;1:3–10. doi: 10.1007/s11682-007-9000-5. doi:10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard L. Instrumental activities of daily living: A stepping-stone towards Alzheimer’s disease diagnosis in subjects with mild cognitive impairment? Acta Neurologica Scandinavica. 2003;107(Suppl179):42–46. doi:10.1034/j.1600-0404.107.s179.8.x. [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG. Error rate and outcome predictability affect neural activation in prefrontal cortex and anterior cingulate during decision making. NeuroImage. 2002;15:836–846. doi: 10.1006/nimg.2001.1031. doi:10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- Pell GS, Briellmann RS, Chan CHP, Pardoe H, Abbott DF, Jackson GD. Selection of the control group for VBM analysis: Influence of covariates, matching and sample size. NeuroImage. 2008;41:1324–1335. doi: 10.1016/j.neuroimage.2008.02.050. doi:10.1016/j.neuroimage.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review): Report of the quality standards subcommittee of the American academy of neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Borgos MJ, Saykin AJ, Wishart HA, Crane PK, Nutter-Upham KE, et al. Judgment in older adults: Development and psychometric evaluation of the Test of Practical Judgment (TOP-J) Journal of Clinical and Experimental Neuropsychology. 2007;29:752–767. doi: 10.1080/13825580601025908. doi:10.1080/13825580601025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Borgos MJ, Saykin AJ. A survey of neuropsychologists’ practices and perspectives regarding the assessment of judgment ability. Applied Neuropsychology. 2008. in press. [DOI] [PMC free article] [PubMed]
- Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. The American Journal of Geriatric Psychiatry. 2003;11:222–228. doi:10.1176/appi.ajgp. 11.2.222. [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Cochran JL, Saxton JA, De Kosky ST, Newman AB, et al. Event-related functional magnetic resonance imaging investigation of executive control in very old individuals with mild cognitive impairment. Biological Psychiatry. 2005;57:761–767. doi: 10.1016/j.biopsych.2004.12.031. doi:10.1016/j.biopsych.2004.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Randolph JJ, Koven NS, Isquith PI. Neural substrates of executive functions: Insights from functional neuroimaging. In: Dupri JR, editor. Focus on neuropsychology research. Nova Science Publishers; Hauppauge: 2006. pp. 1–36. [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Archives of Neurology. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. doi:10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Saykin AJ. Neurobehavioral function and activities of daily living rating scale. Dartmouth Medical School; 1992. (NBFADL-63 item version) (Available from Author) [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Flashman LA, Mamourian AC, Santulli RB. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. doi:10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. doi:10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: A voxel-based morphometric study in healthy elderly. Neurobiology of Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. doi: 10.1016/j. neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human lifespan. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. doi:10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what”, “when”, and “where” related information: An fMRI study. Journal of Cognitive Neuroscience. 2005;17:981–993. doi: 10.1162/0898929054475226. doi:10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. doi:10.1016/S0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. doi:10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray GI, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer’s disease. Journal of Neuroscience. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton WJL, Dumke HA. Age differences in everyday problem-solving and decision-making: A meta-analytic review. Psychology and Aging. 2005;20:85–99. doi: 10.1037/0882-7974.20.1.85. doi:10.1037/0882-7974.20.1.85. [DOI] [PubMed] [Google Scholar]
- Uchiyama Y, Toyoda H, Honda M, Yoshida H, Kochiyama T, Ebe K, et al. Functional segregation of the inferior frontal gyrus for syntactic processes: A functional magnetic-resonance imaging study. Neuroscience Research. 2008;61:309–318. doi: 10.1016/j.neures.2008.03.013. doi:10.1016/j.neures.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-third edition. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Woods DC, Patterson MB, Whitehouse PJ. Utility of the judgment questionnaire subtest of the neurobehavioral cognitive status examination in the evaluation of individuals with Alzheimer’s disease. Clinical Gerontologist. 2000;21:49–66. doi:10.1300/J018v21n04_05. [Google Scholar]
- Zhang JX, Feng C-M, Fox PT, Gao J-H, Tan LH. Is left inferior frontal gyrus a general mechanism for selection? NeuroImage. 2004;23:596–603. doi: 10.1016/j.neuroimage.2004.06.006. doi:10.1016/j.neuroimage.2004.06.006. [DOI] [PubMed] [Google Scholar]